
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Qiang-Sheng Wu | + 1940 word(s) | 1940 | 2021-09-26 10:36:50 | | | |
| 2 | Conner Chen | Meta information modification | 1940 | 2021-10-09 02:24:23 | | | | |
| 3 | Conner Chen | + 2 word(s) | 1942 | 2021-10-09 02:33:26 | | | | |
| 4 | Conner Chen | Meta information modification | 1942 | 2021-10-11 04:16:38 | | | | |
| 5 | Conner Chen | + 15 word(s) | 1957 | 2021-10-11 04:18:03 | | |
Video Upload Options
Camellia is a genus of evergreen shrubs or trees, such as C. japonica, C. sinensis, C. oleifera, etc. A group of beneficial soil microorganisms, arbuscular mycorrhizal fungi (AMF), inhabit the rhizosphere of these Camellia spp. A total of eight genera of Acaulospora, Entrophospora, Funneliformis, Gigaspora, Glomus, Pacispora, Scutellospora, and Sclerocystis were found to be associated with Camellia plants with Glomus and/or Acaulospora being most abundant. These mycorrhizal fungi can colonize the roots of Camellia spp. and thus form arbuscular mycorrhizal symbionts. AMF is an important partner of Camellia spp. in the field of physiological activities. Studies indicated that AMF inoculation has been shown to promote plant growth, improve nutrient acquisition and nutritional quality, and increase resistance to drought, salinity and heavy metal contamination in potted Camellia.
1. Introduction
2. AMF Diversity in Rhizosphere of Camellia spp.
2.1. Morphological Identification
| Camellia Plants | Sampling Regions |
Identification Method | Identified Genena of AMF | Dominant Genus of AMF | Reference |
|---|---|---|---|---|---|
| C. sinensis | Uttaranchal Himalaya (India) | Morphology | Acaulospora; Gigaspora; Glomus; Scutellospora | Glomus | [18] |
| Dehradun District (India) | Morphology | Glomus | Glomus | [19] | |
| Dehradun Himalaya (India) | Morphology | Acaulospora; Glomus; Gigaspora | Acaulospora and Glomus | [20] | |
| Henan (China) | Morphology | Acaulospora; Gigaspora; Glomus; Scutellospora | Acaulospora and Glomus | [21] | |
| Qingdao (China) | Morphology | Acaulospra; Gigaspora; Glomus | Acaulospra and Glomus | [22] | |
| Guizhou (China) | Morphology | Acaulospora; Entrophospora; Gigaspora; Glomus | Acaulospra and Glomus | [23] | |
| C. oleifera | Hunan (China) | Morphology | Acaulospora; Glomus; Scutellospora | Glomus | [24] |
| Wuhan (China) | High-throughput sequencing of 18S rRNA gene | Acaulospora; Ambispora; Archaeospora; Claroideoglomus; Diversispora; Gigaspora; Glomus; Paraglomus; Redeckera; Scutellospora | Glomus | [25] | |
| Jiangxi (China) | High-throughput sequencing of 18S rRNA gene | Acaulospora; Ambispora; Archaeospora; Claroideoglomus; Diversispora; Geosiphon; Gigaspora; Glomus; Pacispora; Paraglomus; Scutellospora; Septoglomus | Glomus | [26] | |
| Guiyang (China) | High-throughput sequencing of 18S rRNA gene | Acaulospora; Archaeospora; Claroideoglomus; Diversispora; Glomus; Paraglomus | Glomus | [27] | |
| C. japonica | Fanjing Mountain (China) | Morphology | Acaulospora; Funneliformis; Glomus; Pacispora; Scutellospora; | Glomus | [28] |
| Chongqing (China) | Morphology | Acaulospora; Gigaspora; Glomus; Scutellospora | Acaulospora and Glomus | [29] | |
| Shimane prefecture (Japan) | High-throughput sequencing of 18S rRNA gene | Acaulospora; Ambispora; Archaeospora; Claroideoglomus; Diversispora; Funneliformis; Geosiphon; Gigaspora; Glomus; Paraglomus; Redeckera; Rhizophagus; Scutellospora | Glomus and Rhizophagus | [30] | |
| Diankwan Island (Korea) | High-throughput sequencing of 18S rRNA gene | Acaulospora; Ambispora; Claroideoglomus; Glomus; Rhizophagus; Scutellospora | Acaulospora and Rhizophagus | [31] |
2.2. Molecular Identification
3. AMF Colonization of Camellia Plants and Its Influencing Factors
3.1. Root AMF Colonization of Camellia Plants
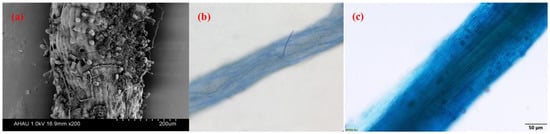
3.2. Factors Affecting AMF Colonization
3.2.1. Seasonal Variations
3.2.2. Soil Factors
References
- Yang, L.; Zou, Y.N.; Tian, Z.H.; Wu, Q.S.; Kuča, K. Effects of beneficial endophytic fungal inoculants on plant growth and nutrient absorption of trifoliate orange seedlings. Sci. Hortic. 2021, 277, 109815.
- Meng, L.L.; He, J.D.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhiza-released glomalin-related soil protein fractions contribute to soil total nitrogen in trifoliate orange. Plant Soil Environ. 2020, 66, 183–189.
- Wu, Q.S.; Gao, W.Q.; Srivastava, A.K.; Zhang, F.; Zou, Y.N. Nutrient acquisition and fruit quality of Ponkan mandarin in response to AMF inoculation. Ind. J. Agric. Sci. 2020, 90, 1563–1567.
- Zou, Y.N.; Zhang, F.; Srivastava, A.K.; Wu, Q.S.; Kuča, K. Arbuscular mycorrhizal fungi regulate polyamine homeostasis in roots of trifoliate orange for improved adaptation to soil moisture deficit stress. Front. Plant Sci. 2021, 11, 600792.
- Eid, K.E.; Abbas, M.H.H.; Mekawi, E.M.; EINagar, M.M.; Abdelhafez, A.A.; Amin, B.H.; Mohamed, I.; Ali, M.B. Arbuscular mycorrhiza and environmentally biochemicals enhance the nutritional status of Helianthus tuberosus and induce its resistance against Sclerotium rolfsii. Ecotox. Environ. Saf. 2019, 186, 109783.
- Karthikeyan, A.; Muthukumar, T.; Udaiyan, K. Response of tea (Camellia sinensis (L). Kuntze) to arbuscular mycorrhizal fungi under plantation nursery conditions. Biol. Agric. Hortic. 2005, 22, 305–319.
- Zhang, F.; Zou, Y.N.; Wu, Q.S. Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci. Hortic. 2018, 229, 132–136.
- Wu, Q.S.; Srivastava, A.K.; Zou, Y.N. AMF-induced tolerant to drought stress in citrus: A review. Sci. Hortic. 2013, 164, 77–87.
- González-Chávez, M.C.; Carrillo-González, R.; Wright, S.F.; Nichols, K.A. The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environ. Pollut. 2004, 130, 317–323.
- Cornejo, P.; Meier, S.; Borie, G.; Rillig, M.C.; Borie, F. Glomalin related soil protein in a Mediterranean ecosystem affected by a copper smelter and its contribution to Cu and Zn sequestration. Sci. Total Environ. 2008, 406, 154–160.
- He, J.D.; Chi, G.G.; Zou, Y.N.; Shu, B.; Wu, Q.S.; Srivastava, A.K.; Kuča, K. Contribution of glomalin-related soil proteins to soil organic carbon in trifoliate orange. Appl. Soil Ecol. 2020, 154, 103592.
- Cheng, H.Q.; Giri, B.; Wu, Q.S.; Zou, Y.N.; Kuča, K. Arbuscular mycorrhizal fungi mitigate drought stress in citrus by modulating root microenvironment. Arch. Agron. Soil Sci. 2021.
- Luo, C.Q.; Tan, X.F.; Ling, L.L. A Classification summary on plant of genus Camellia. J. Cent. South For. Univ. 1999, 19, 78–81.
- Mondal, T.K.; Bhattacharya, A.; Laxikumaran, M.; Ahuja, P.S. Recent advance of tea (Camellia sinensis) biotechnology. Plant Cell Tiss. Org. 2004, 76, 195–254.
- Singh, S.; Pandey, A.; Kumar, B.; Palni, L.M.S. Enhancement in growth and quality parameters of tea through inoculation with arbuscular mycorrhizal fungi in an acid soil. Biol. Fert. Soils 2010, 46, 427–433.
- Lin, X.G.; Hao, W.Y. Mycorrhizal dependency of various kind of plants. Acta Bot. Sin. 1989, 31, 721–725.
- Tunstall, A.C. Mycorrhiza in tea plants. Quart. J. Indian Tea Assoc. Indian 1926, 159.
- Singh, S.; Pandey, A.; Chaurasia, B.; Palni, L.M.S. Diversity of arbuscular mycorrhizal fungi associated with the rhizosphere of tea growing in ‘natural’ and ‘cultivated’ ecosites. Biol. Fert. Soils 2008, 44, 491–500.
- Gupta, R.K.; Sharma, C. Diversity of arbuscular mycorrhizal fungi in Camellia sinensis in Uttarakhand State, India. Afr. J. Biotechnol. 2013, 9, 5313–5319.
- Sharma, C.; Gupta, R.K.; Pathak, R.K.; Choudhary, K.K. Seasonal colonization of arbuscular mycorrhiza fungi in the roots of Camellia sinensis (tea) in different tea gardens of India. ISRN Biodivers. 2015, 2013, 593086.
- Lu, D.S.; Wu, X.Q. Species of VAM fungi around tea roots in the southern area of Henan province. J. Nanjing For. Univ. 2005, 29, 33–36.
- Wu, L.S.; Wang, Y.; Li, M.; Liu, R.J.; Ding, Z.T. A survey of arbuscular mycorrhizal fungi in the rhizosphere of Camellia sinensis in Laoshan. J. Qingdao Agric. Univ. 2009, 26, 171–173.
- Xing, D.; Zhang, A.M.; Li, Z.; Chen, J.; Wang, Z.X.; Tu, Y.Y.; Gao, X.B. Resources and morphological characteristics of arbuscular mycorrhiza fungi around tea rhizosphere in Guizhou. Guizhou Agric. Sci. 2015, 43, 102–106.
- Deng, X.J.; Zhou, G.Y.; Liu, J.A.; Li, L.; Bu, T.T. Diversity and community structure of arbuscular mycorrhizal fungi in Camellia oleifera stands in Hunan. J. Cent. South Univ. For. Tech. 2011, 31, 38–42.
- Liu, R.C.; Xiao, Z.Y.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Mycorrhizal fungal diversity and its relationship with soil properties in Camellia oleifera. Agriculture 2021, 11, 470.
- Lin, Y.L.; Li, Z.Y.; Wu, F.; Pei, Y.; Zhang, Y.; Zhang, L.P.; Yang, T.; Tan, M.X. Community structure characteristics of arbuscular mycorrhizal fungi among Camellia oleifera cultivars. For. Res. 2020, 33, 163–169.
- Zhou, G.R.; Shang, K.; Jiang, L. Diversity survey of AM fungi in rhizosphere soil of wild Camellia oleifera. J. Guizhou Univ. 2019, 36, 26–31.
- Yuan, T.; Tao, G.Y.; Jiang, L. Arbuscular mycorrhizal fungi in the rhizospheric soil of four forest types in Fanjingshan national nature reserve. J. Northeast For. Univ. 2018, 46, 83–86.
- He, W. AM Fungi Diversity in the Main Ornamental Gardens of Chongqing Nanshan Botanical Park. Master’s Thesis, Southwest University, Chongqing, China, 2009.
- Berruti, A.; Demasi, S.; Lumini, E.; Kobayashi, N.; Bianciotto, V.; Bianciotto, V. Wild Camellia japonica specimens in the Shimane prefecture (Japan) host previously undescribed AMF diversity. Appl. Soil Ecol. 2017, 115, 10–18.
- Lee, E.H.; Ka, K.H.; Eom, A.H. Diversity of arbuscular mycorrhizal fungi in rhizospheres of Camellia japonica and neighboring plants inhabiting Wando of Korea. Korean J. Mycol. 2014, 42, 34–39.
- Wu, Q.S.; Srivastava, A.K. AMF diversity in citrus rhizosphere. Ind. J. Agric. Sci. 2017, 87, 653–659.
- Gai, J.P.; Feng, G.; Li, X.L. Review of researches on biodiversity of arbusculay mycorrhizal fungi. Soils 2005, 37, 236–242.
- Yang, C.X.; Li, L.L. Research progress in arbuscular mycorrhizal fungi identification method application. Guizhou Agric. Sci. 2014, 42, 93–97.
- Guo, X.H.; Gong, J. Differential effects of abiotic factors and host plant traits on diversity and community composition of root-colonizing arbuscular mycorrhizal fungi in a salt-stressed ecosystem. Mycorrhiza 2014, 24, 79–94.
- Yang, F.; Cao, J.M.; Chen, Y.Y.; Wang, J.L. Research progress on structure and identification method of arbusular mycorrhizal fungi. Mod. Agric. Sci. Tech. 2019, 17, 152–154+157.
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250.
- Morita, A.; Konishi, S. Relationship between vesicular-arbuscular mycorrhizal infection and soil phosphorus concentration in tea fields. Soil Sci. Plant Nutr. 1989, 35, 139–143.
- Gao, X.B.; Chen, J.; Zhao, J.F.; Li, Z.; Guo, C.; Zhou, F.Y.; Wang, Z.X.; Tu, Y.Y.; Zhou, Y.F. Colonization characteristics of arbuscular mycorrhiza fungi in rhizosphere of local tea trees in Guizhou. Southwest China J. Agric. Sci. 2016, 29, 1328–1335.
- Ren, M.X.; Luo, Y.P. Advances in the study of VA mycorrhizae of tea trees. J. Tea 2005, 31, 28–31.
- Mejstrik, V. The frequency of vesicular-arbuscular mycorrhizae in the roots of Camellia japonica L. from different sites in New Zealand. Pac. Sci. 1974, 28, 73–77.
- Borriello, R.; Berruti, A.; Lumini, E.; Beffa, M.T.D.; Scariot, V.; Bianciotto, V. Edaphic factors trigger diverse AM fungal communities associated to exotic camellias in closely located Lake Maggiore (Italy) sites. Mycorrhiza 2015, 25, 253–265.
- Bencherif, K.; Boutekrabt, A.; Dalpé, Y.; Sahraoui, A.L.H. Soil and seasons affect arbuscular mycorrhizal fungi associated with Tamarix rhizosphere in arid and semi-arid steppes. Appl. Soil Ecol. 2016, 107, 182–190.
- Varela-Cervero, S.; López-García, A.; Barea, J.M.; Azcón-Aguilar, C. Spring to autumn changes in the arbuscular mycorrhizal fungal community composition in the different propagule types associated to a Mediterranean shrubland. Plant Soil 2016, 408, 1–14.
- Chandra, K.K.; Jamaluddin, A. Seasonal variation of VAM fungi in tree species planted in coalmine overbunden of Kusmunda (MP). J. Trop. For. 1998, 14, 118–123.
- Gould, A.B.; Hendrix, J.W.; Ferriss, R.S. Relationship of mycorrhizal activity to time following reclamation of surface mine land in western Kentucky. I. Propagule and spore population densities. Can. J. Bot. 1996, 74, 247–261.
- Liang, S.M.; Zheng, F.L.; Abd_Allah, E.F.; Muthuramalingam, P.; Wu, Q.S.; Hashem, A. Spatial changes of arbuscular mycorrhizal fungi in peach and their correlation with soil properties. Soudi J. Biol. Sci. 2021.
- Lin, Z. Effect of VA mycorrhizal species on tea tree growth and absorption of mineral elements. J. Tea Sci. 1993, 13, 15–20.
- Pandey, A.; Palni, L.M.S. Bacillus species: The dominant bacteria of the rhizosphere of established tea bushes. Microbiol. Res. 1997, 152, 359–365.
- Avio, L.; Castaldini, M.; Fabiani, A.; Bedini, S.; Sbrana, C.; Turrini, A.; Giovannetti, M. Impact of nitrogen fertilization and soil tillage on arbuscular mycorrhizal fungal communities in a Mediterranean agroecosystem. Soil Biol. Biochem. 2013, 67, 285–294.




