
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kira Zeevaert | + 9111 word(s) | 9111 | 2021-07-15 06:22:56 | | | |
| 2 | Nora Tang | -2327 word(s) | 6784 | 2021-10-08 05:18:27 | | |
Video Upload Options
Embryoid bodies (EBs) resemble self-organizing aggregates of pluripotent stem cells that recapitulate some aspects of early embryogenesis. Within few days, the cells undergo a transition from rather homogeneous epithelial-like pluripotent stem cell colonies into a three-dimensional organization of various cell types with multifaceted cell–cell interactions and lumen formation—a process associated with repetitive epithelial-mesenchymal transitions. In the last few years, culture methods have further evolved to better control EB size, growth, cellular composition, and organization—e.g., by the addition of morphogens or different extracellular matrix molecules. There is a growing perception that the mechanical properties, cell mechanics, and cell signaling during EB development are also influenced by physical cues to better guide lineage specification; substrate elasticity and topography are relevant, as well as shear stress and mechanical strain. Epithelial structures outside and inside EBs support the integrity of the cell aggregates and counteract mechanical stress. Furthermore, hydrogels can be used to better control the organization and lineage-specific differentiation of EBs. In this review, we summarize how EB formation is accompanied by a variety of biomechanical parameters that need to be considered for the directed and reproducible self-organization of early cell fate decisions.
1. Introduction
2. Methods of Embryoid Body Formation
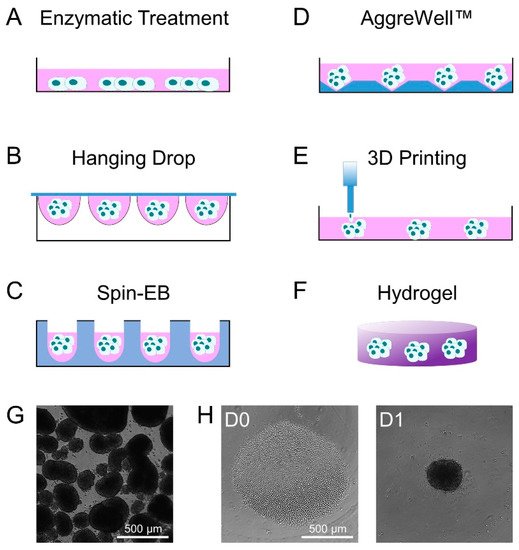
3. Molecular Changes during Embryoid Body Formation
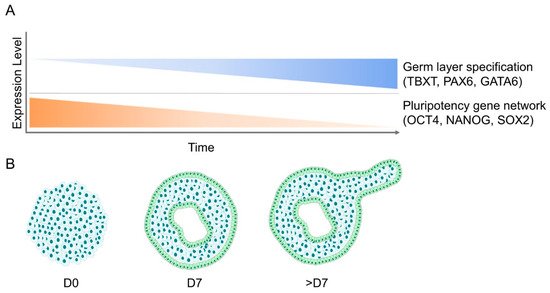
4. Cell Mechanics and Lineage Specification in Embryoid Bodies
4.1. Modulation of Cell–Cell and Cell–Matrix Interaction during Embryoid Body Formation
4.2. Epithelial Cell to Mesenchymal Cell Transition (EMT) in Embryoid Bodies
4.3. Cellular Patterning and Germ Layer Specification during Embryoid Body Development
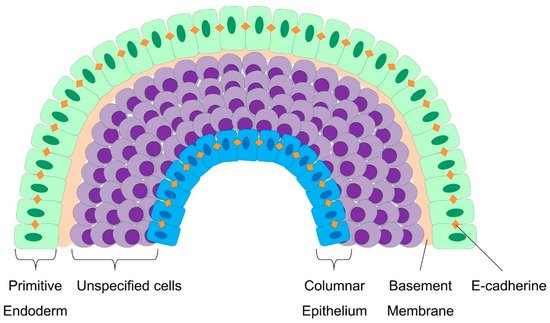
4.4. Molecular Pathways that Trigger the Cell-Mechanic Changes during EBs
5. Impact of Size and Mechanical Stimulation on Differentiation of EBs
5.1. The Size of Stem Cell Aggregates Modulates Their Differentiation Potential
5.2. Effect of Shear Stress on Embryoid Body Differentiation
5.3. Impact of Mechanical Strain on EBs
5.4. Hydrogels for EB Differentiation
| Species | Cell Type | Mechanical Stimulus | Parameters Tested | Duration | Readout | Ref. | |
|---|---|---|---|---|---|---|---|
| Substrate Elasticity | Human | ESC | Rigidity of PDMS micropost arrays | 1.92–1218.4 kPa | 24 hours | Traction force measurements; pluripotency and cytoskeletal changes (IF) | [93] |
| Human | iPSC | Elastomer pillars of different heights on a rigid substrate | 3–168 kPa | Up to 8 days | Morphology; viability; proliferation; pluripotency (IF); cardiac differentiation (IF, FACS, Ca2+ assays, electrical stimulation) | [85] | |
| Substrate Topography | Human | iPSC | Polyimide substrate with submicrometer groove-ridge structure | 340 nm, 650 nm, 1400 nm periodicity, 200 nm depth; compared to unstructured | 3 days | Morphology; pluripotency (IF, qRT-PCR); YAP/TAZ expression (WB), gene expression profiles | [94] |
| Colony Size | Human | ESC, EB | Size of initial 2D colony and EB size via micropatterning | 200, 400, 800 µm | 4–22 days | Morphology; pluripotency (FACS) and germ layer differentiation (qRT-PCR, IF) | [70] |
| Mouse | ESC, EB | Size of initial 2D colony and cell density via laser direct-write cell printing | Colony size: 200–3000 µm; cell density: <25,000 cells, 25,000–125,000 cells, >125,000 cells | 3–8 days | Morphology | [71] | |
| Mouse, human | ESC, EB | Size of EBs; substrate hydrophobicity | Colony size: <100 µm, 100–300 µm, >300 µm; surface chemical properties: agarose, PEG, pHEMA, PDMS, TCP, LAC | 4–20 days | Morphology; viability; proliferation; (germ layer) differentiation potential (qRT-PCR, FACS) | [72] | |
| Mouse | ESC, EB | Size of EBs via adhesive stencils with different diameter | 100–500 µm diameter | 20 days | Germ layer differentiation (IF, qRT-PCR) | [73] | |
| Shear Stress | Mouse | ESC | Laminar shear stress | 1.5–10 dyn/cm2 | 3 days | Cell density; cell cycle (FACS, ELISA); endothelial differentiation (IF, WB, qRT-PCR) | [75] |
| Mouse | ESC, EB | Shear stress in rotary suspension culture | Comparison of static and rotary suspension culture (25–55 rpm) | 12 hours to 7 days | Morphology; viability; proliferation; cyst formation; germ layer formation (qRT-PCR) | [76] | |
| Mouse | ESC, EB | Y-channel microfluidic system with two different media | Laminar flow 50–200 µL/min | 5 days | Differentiation potential (WB, IF) | [78] | |
| Mouse | EB | Microfluidic chip system with continuous laminar flow and shear stress | Comparison of static culture and laminar flow 2 µL/min | 21 days | Viability; proliferation; steroidogenic differentiation (hormone release, IF, ELISA) | [77] | |
| Mouse | ECS, EB | Comparison of static conditions and shear stress as pre-condition | 0–5 dyn/cm2 | 48 hours before EB formation; up to 10 days of EB culture | Morphology; pluripotency and endothelial differentiation (IF, qRT-PCR, FACS) cellular organization | [79] | |
| Mouse | ESC, EB | Shear stress in rotary orbital suspension culture | 0.7–2.5 dyn/cm2; 20–60 rpm | EB culture for 7 days; up to day 12 of differentiation | Morphology (IF); pluripotency and germ layer differentiation (qRT-PCR, FACS); global gene expression (PCR array analysis) | [80] | |
| Mechanical Strain | Mouse | ESC, EB | Mechanical strain by short-term magnetization via incorporated RGD-conjugated paramagnetic beads | Stimulation pulses using short-term magnetization; 0.128–0.4 Tesla | 1 hour stimulation for up to 7 days | Morphology; viability; protein expression and cardiomyogenesis (β1 integrin inhibition, FACS, WB, IF) | [82] |
| Mouse | ESC, EB | Mechanical strain by (cyclic) stretching and compression between two microtips with a magnetic tissue stretcher | Stretching amplitude of 50% of original size (+ cyclic stretching 1 Hz, 10% amplitude, twice daily for 2 hours) | 3 days; with three additional days of cyclic stretching | Morphology; viability; proliferation; pluripotency and germ layer differentiation (qRT-PCR, IF) | [39] | |
| Mouse | ESC, EB | Mechanical strain by stretching device (Flexercell Strain Unit) | 10% elongation of undifferentiated EBs | 2 hours stretching on day 4 of EB generation | Monitoring of intracellular [Ca2+]i; Expression of angiogenesis guidance molecules; Expression of pro-angiogenic growth factors; ROS generation | [83] | |
| Mouse | ESC, EB | Mechanical strain by stretching device (Flexercell Strain Unit) | 5%, 10%, or 20% elongation of undifferentiated EBs | 2 hours stretching on day 4 of EB generation | Staining of capillary-like structures; counting of beating bodies; staining of sarcomeric α-actinin; upregulation of NADPH oxidase subunits; ROS generation; inhibition of mechanical-strain stimulated MAPKs | [84] | |
| 3D Culture in Hydrogel | Human | ESC, EB | Agarose 3D culture system | Comparison of agarose 3D culture and suspension and hanging droplet culture | 7 days up to 8 weeks | In vivo teratoma assay; morphology; germ layer differentiation (IF) | [95] |
| Human | ESC | Hydrogel based material for switching between alginate and collagen via ionic de-crosslinking | Change hydrogel composition and in matrix elasticity from 21.37 ± 5.37 kPa (alginate) to 4.87 ± 1.64 kPa (collagen) | 21 days | Viability; proliferation; pluripotency and germ layer differentiation (qRT-PCR) | [96] | |
| Human | ESC, EB | Dextran-acrylate and PEG hydrogel +/- RGD and VEGF | Comparison of dextran-acrylate and PEG hydrogel +/- RGD and VEGF | 10 days | Viability; vascular differentiation (FACS, IF, qRT-PCR) | [88] | |
| Human | ESC, EB | Biodegradable polymer scaffolds (50:50 PLGA:PLLA) | Medium supplemented with different growth factors | 14 days in vitro; 14 days in vivo | Proliferation; germ layer differentiation (qRT-PCR); transplantation into SCID mice (IF) | [89] | |
| Human | iPSC, EB | Concave PEG hydrogel microstructures via 3D projection printing; initial cell number | Comparison between concave and flat gels; low (250,000/mL) and high (750,000/mL) cell density | Up to 10 days | Morphology; culture duration; pluripotency and germ layer differentiation (IF); cyst formation | [90] | |
| Mouse | ESC, EB | Hybrid hydrogels (GelMA, PEG) with varying matrix elasticity | Analysis of a hybrid GelMA (3 wt%)/PEG (10 wt%) hydrogel | Up to 7 days | Vasculogenic and cardiogenic differentiation (qRT-PCR, IF) | [91] | |
| Mouse | ESC, EB | PEG hydrogel with and without RGD | Comparison of different PEG gels; 150 µm EBs and 450 µm EBs | Up to 15 days | Morphology; endothelial and cardiac differentiation (contraction behavior, IF, qRT-PCR) | [92] |
6. Conclusions
References
- Martin, G.R.; Evans, M.J. Differentiation of clonal lines of teratocarcinoma cells: Formation of embryoid bodies in vitro. Proc. Natl. Acad. Sci. USA 1975, 72, 1441–1445.
- Brickman, J.M.; Serup, P. Properties of embryoid bodies. Wiley Interdiscip. Rev. Dev. Biol. 2017, 6.
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676.
- Puri, M.C.; Nagy, A. Concise review: Embryonic stem cells versus induced pluripotent stem cells: The game is on. Stem Cells 2012, 30, 10–14.
- Pekkanen-Mattila, M.; Pelto-Huikko, M.; Kujala, V.; Suuronen, R.; Skottman, H.; Aalto-Setala, K.; Kerkela, E. Spatial and temporal expression pattern of germ layer markers during human embryonic stem cell differentiation in embryoid bodies. Histochem. Cell Biol. 2010, 133, 595–606.
- Takito, J.; Al-Awqati, Q. Conversion of ES cells to columnar epithelia by hensin and to squamous epithelia by laminin. J. Cell Biol. 2004, 166, 1093–1102.
- Bratt-Leal, A.M.; Carpenedo, R.L.; McDevitt, T.C. Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation. Biotechnol. Prog. 2009, 25, 43–51.
- Liu, J.; He, X.; Corbett, S.A.; Lowry, S.F.; Graham, A.M.; Fassler, R.; Li, S. Integrins are required for the differentiation of visceral endoderm. J. Cell Sci. 2009, 122, 233–242.
- Fuchs, C.; Scheinast, M.; Pasteiner, W.; Lagger, S.; Hofner, M.; Hoellrigl, A.; Schultheis, M.; Weitzer, G. Self-organization phenomena in embryonic stem cell-derived embryoid bodies: Axis formation and breaking of symmetry during cardiomyogenesis. Cells Tissues Organs 2012, 195, 377–391.
- Bruckner, B.R.; Janshoff, A. Elastic properties of epithelial cells probed by atomic force microscopy. Biochim. Biophys. Acta 2015, 1853, 3075–3082.
- Campas, O. A toolbox to explore the mechanics of living embryonic tissues. Semin. Cell Dev. Biol. 2016, 55, 119–130.
- Hsiao, C.; Tomai, M.; Glynn, J.; Palecek, S.P. Effects of 3D microwell culture on initial fate specification in human embryonic stem cells. Aiche J. 2014, 60, 1225–1235.
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156.
- Doetschman, T.C.; Eistetter, H.; Katz, M.; Schmidt, W.; Kemler, R. The in vitro development of blastocyst-derived embryonic stem cell lines: Formation of visceral yolk sac, blood islands and myocardium. J. Embryol. Exp. Morphol. 1985, 87, 27–45.
- Sheridan, S.D.; Surampudi, V.; Rao, R.R. Analysis of embryoid bodies derived from human induced pluripotent stem cells as a means to assess pluripotency. Stem Cells Int. 2012, 2012, 738910.
- Kim, J.M.; Moon, S.H.; Lee, S.G.; Cho, Y.J.; Hong, K.S.; Lee, J.H.; Lee, H.J.; Chung, H.M. Assessment of differentiation aspects by the morphological classification of embryoid bodies derived from human embryonic stem cells. Stem Cells Dev. 2011, 20, 1925–1935.
- Messana, J.M.; Hwang, N.S.; Coburn, J.; Elisseeff, J.H.; Zhang, Z. Size of the embryoid body influences chondrogenesis of mouse embryonic stem cells. J. Tissue Eng. Regen. Med. 2008, 2, 499–506.
- Hong, S.H.; Werbowetski-Ogilvie, T.; Ramos-Mejia, V.; Lee, J.B.; Bhatia, M. Multiparameter comparisons of embryoid body differentiation toward human stem cell applications. Stem Cell Res. 2010, 5, 120–130.
- Cerdan, C.; Hong, S.H.; Bhatia, M. Formation and hematopoietic differentiation of human embryoid bodies by suspension and hanging drop cultures. Curr Protoc Stem Cell Biol 2007, Chapter 1, Unit 1D 2.
- Kurosawa, H. Methods for inducing embryoid body formation: In vitro differentiation system of embryonic stem cells. J. Biosci. Bioeng. 2007, 103, 389–398.
- Ng, E.S.; Davis, R.; Stanley, E.G.; Elefanty, A.G. A protocol describing the use of a recombinant protein-based, animal product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nat. Protoc. 2008, 3, 768–776.
- Kibschull, M. Differentiating Mouse Embryonic Stem Cells into Embryoid Bodies in AggreWell Plates. Cold Spring Harb. Protoc. 2017, 2017, pdb prot094169.
- Ouyang, L.; Yao, R.; Mao, S.; Chen, X.; Na, J.; Sun, W. Three-dimensional bioprinting of embryonic stem cells directs highly uniform embryoid body formation. Biofabrication 2015, 7, 044101.
- Choi, Y.Y.; Chung, B.G.; Lee, D.H.; Khademhosseini, A.; Kim, J.H.; Lee, S.H. Controlled-size embryoid body formation in concave microwell arrays. Biomaterials 2010, 31, 4296–4303.
- Poh, Y.C.; Chen, J.; Hong, Y.; Yi, H.; Zhang, S.; Chen, J.; Wu, D.C.; Wang, L.; Jia, Q.; Singh, R.; et al. Generation of organized germ layers from a single mouse embryonic stem cell. Nat. Commun. 2014, 5, 4000.
- Pettinato, G.; Wen, X.; Zhang, N. Engineering Strategies for the Formation of Embryoid Bodies from Human Pluripotent Stem Cells. Stem Cells Dev. 2015, 24, 1595–1609.
- Mansergh, F.C.; Daly, C.S.; Hurley, A.L.; Wride, M.A.; Hunter, S.M.; Evans, M.J. Gene expression profiles during early differentiation of mouse embryonic stem cells. BMC Dev. Biol 2009, 9, 5.
- Spangler, A.; Su, E.Y.; Craft, A.M.; Cahan, P. A single cell transcriptional portrait of embryoid body differentiation and comparison to progenitors of the developing embryo. Stem Cell Res. 2018, 31, 201–215.
- Argelaguet, R.; Clark, S.J.; Mohammed, H.; Stapel, L.C.; Krueger, C.; Kapourani, C.A.; Imaz-Rosshandler, I.; Lohoff, T.; Xiang, Y.; Hanna, C.W.; et al. Multi-omics profiling of mouse gastrulation at single-cell resolution. Nature 2019, 576, 487–491.
- Sajini, A.A.; Greder, L.V.; Dutton, J.R.; Slack, J.M. Loss of Oct4 expression during the development of murine embryoid bodies. Dev. Biol. 2012, 371, 170–179.
- Kopper, O.; Giladi, O.; Golan-Lev, T.; Benvenisty, N. Characterization of gastrulation-stage progenitor cells and their inhibitory crosstalk in human embryoid bodies. Stem Cells 2010, 28, 75–83.
- Boxman, J.; Sagy, N.; Achanta, S.; Vadigepalli, R.; Nachman, I. Integrated live imaging and molecular profiling of embryoid bodies reveals a synchronized progression of early differentiation. Sci. Rep. 2016, 6, 31623.
- Lian, I.; Kim, J.; Okazawa, H.; Zhao, J.; Zhao, B.; Yu, J.; Chinnaiyan, A.; Israel, M.A.; Goldstein, L.S.; Abujarour, R.; et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010, 24, 1106–1118.
- Hazeltine, L.B.; Badur, M.G.; Lian, X.; Das, A.; Han, W.; Palecek, S.P. Temporal impact of substrate mechanics on differentiation of human embryonic stem cells to cardiomyocytes. Acta Biomater. 2014, 10, 604–612.
- Mohr, J.C.; Zhang, J.; Azarin, S.M.; Soerens, A.G.; de Pablo, J.J.; Thomson, J.A.; Lyons, G.E.; Palecek, S.P.; Kamp, T.J. The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomaterials 2010, 31, 1885–1893.
- Branco, M.A.; Cotovio, J.P.; Rodrigues, C.A.V.; Vaz, S.H.; Fernandes, T.G.; Moreira, L.M.; Cabral, J.M.S.; Diogo, M.M. Transcriptomic analysis of 3D Cardiac Differentiation of Human Induced Pluripotent Stem Cells Reveals Faster Cardiomyocyte Maturation Compared to 2D Culture. Sci. Rep. 2019, 9, 9229.
- Hwang, Y.S.; Chung, B.G.; Ortmann, D.; Hattori, N.; Moeller, H.C.; Khademhosseini, A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc. Natl. Acad. Sci. USA 2009, 106, 16978–16983.
- Ahadian, S.; Yamada, S.; Ramon-Azcon, J.; Estili, M.; Liang, X.; Nakajima, K.; Shiku, H.; Khademhosseini, A.; Matsue, T. Hybrid hydrogel-aligned carbon nanotube scaffolds to enhance cardiac differentiation of embryoid bodies. Acta Biomater. 2016, 31, 134–143.
- Du, V.; Luciani, N.; Richard, S.; Mary, G.; Gay, C.; Mazuel, F.; Reffay, M.; Menasche, P.; Agbulut, O.; Wilhelm, C. A 3D magnetic tissue stretcher for remote mechanical control of embryonic stem cell differentiation. Nat. Commun. 2017, 8, 400.
- Martin, S.; Poppe, D.; Olova, N.; O’Leary, C.; Ivanova, E.; Pflueger, J.; Dechka, J.; Simmons, R.K.; Cooper, H.M.; Reik, W.; et al. Conserved and divergent features of DNA methylation in embryonic stem cell-derived neurons. bioRxiv 2020.
- Brunner, A.L.; Johnson, D.S.; Kim, S.W.; Valouev, A.; Reddy, T.E.; Neff, N.F.; Anton, E.; Medina, C.; Nguyen, L.; Chiao, E.; et al. Distinct DNA methylation patterns characterize differentiated human embryonic stem cells and developing human fetal liver. Genome Res. 2009, 19, 1044–1056.
- Gothard, D.; Roberts, S.J.; Shakesheff, K.M.; Buttery, L.D. Controlled embryoid body formation via surface modification and avidin-biotin cross-linking. Cytotechnology 2009, 61, 135–144.
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26.
- Dang, S.M.; Gerecht-Nir, S.; Chen, J.; Itskovitz-Eldor, J.; Zandstra, P.W. Controlled, scalable embryonic stem cell differentiation culture. Stem Cells 2004, 22, 275–282.
- Larue, L.; Antos, C.; Butz, S.; Huber, O.; Delmas, V.; Dominis, M.; Kemler, R. A role for cadherins in tissue formation. Development 1996, 122, 3185–3194.
- Li, X.; Chen, Y.; Scheele, S.; Arman, E.; Haffner-Krausz, R.; Ekblom, P.; Lonai, P. Fibroblast growth factor signaling and basement membrane assembly are connected during epithelial morphogenesis of the embryoid body. J. Cell Biol. 2001, 153, 811–822.
- Hori, K.; Sen, A.; Artavanis-Tsakonas, S. Notch signaling at a glance. J. Cell Sci 2013, 126, 2135–2140.
- Beckstead, B.L.; Santosa, D.M.; Giachelli, C.M. Mimicking cell-cell interactions at the biomaterial-cell interface for control of stem cell differentiation. J. Biomed. Mater. Res. A 2006, 79, 94–103.
- Quintin, S.; Gally, C.; Labouesse, M. Epithelial morphogenesis in embryos: Asymmetries, motors and brakes. Trends Genet. 2008, 24, 221–230.
- Conti, M.A.; Even-Ram, S.; Liu, C.; Yamada, K.M.; Adelstein, R.S. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J. Biol. Chem. 2004, 279, 41263–41266.
- De Bank, P.A.; Hou, Q.; Warner, R.M.; Wood, I.V.; Ali, B.E.; Macneil, S.; Kendall, D.A.; Kellam, B.; Shakesheff, K.M.; Buttery, L.D. Accelerated formation of multicellular 3-D structures by cell-to-cell cross-linking. Biotechnol. Bioeng. 2007, 97, 1617–1625.
- Petersen, D.R.; Gustavsen, C.; Lindskog, S.R.; Magnuson, M.A.; Zaret, K.S.; Serup, P. Engineering artificial signaling centers to polarize embryoid body differentiation. Stem Cells Dev. 2012, 21, 647–653.
- Kim, D.H.; Xing, T.; Yang, Z.; Dudek, R.; Lu, Q.; Chen, Y.H. Epithelial Mesenchymal Transition in Embryonic Development, Tissue Repair and Cancer: A Comprehensive Overview. J. Clin. Med. 2017, 7.
- Chan, D.N.; Azghadi, S.F.; Feng, J.; Lowry, W.E. PTK7 marks the first human developmental EMT in vitro. PLoS ONE 2012, 7, e50432.
- Skromne, I.; Stern, C.D. Interactions between Wnt and Vg1 signalling pathways initiate primitive streak formation in the chick embryo. Development 2001, 128, 2915.
- ten Berge, D.; Koole, W.; Fuerer, C.; Fish, M.; Eroglu, E.; Nusse, R. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell 2008, 3, 508–518.
- Hrabchak, C.; Ringuette, M.; Woodhouse, K. Recombinant mouse SPARC promotes parietal endoderm differentiation and cardiomyogenesis in embryoid bodies. Biochem. Cell Biol. 2008, 86, 487–499.
- Lei, X.; Deng, Z.; Zhang, H.; Zhao, H.; Zhou, J.; Liu, S.; Chen, Q.; Ning, L.; Cao, Y.; Wang, X.; et al. Rotary suspension culture enhances mesendoderm differentiation of embryonic stem cells through modulation of Wnt/beta-catenin pathway. Stem Cell Rev. Rep. 2014, 10, 526–538.
- Carver, E.A.; Jiang, R.; Lan, Y.; Oram, K.F.; Gridley, T. The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol. Cell Biol. 2001, 21, 8184–8188.
- Karimzadeh, F.; Opas, M. Calreticulin Is Required for TGF-beta-Induced Epithelial-to-Mesenchymal Transition during Cardiogenesis in Mouse Embryonic Stem Cells. Stem Cell Rep. 2017, 8, 1299–1311.
- Azarin, S.M.; Lian, X.; Larson, E.A.; Popelka, H.M.; de Pablo, J.J.; Palecek, S.P. Modulation of Wnt/beta-catenin signaling in human embryonic stem cells using a 3-D microwell array. Biomaterials 2012, 33, 2041–2049.
- Kinney, M.A.; Sargent, C.Y.; McDevitt, T.C. Temporal modulation of beta-catenin signaling by multicellular aggregation kinetics impacts embryonic stem cell cardiomyogenesis. Stem Cells Dev. 2013, 22, 2665–2677.
- Doughton, G.; Wei, J.; Tapon, N.; Welham, M.J.; Chalmers, A.D. Formation of a polarised primitive endoderm layer in embryoid bodies requires fgfr/erk signalling. PLoS ONE 2014, 9, e95434.
- Chen, Y.; Li, X.; Eswarakumar, V.P.; Seger, R.; Lonai, P. Fibroblast growth factor (FGF) signaling through PI 3-kinase and Akt/PKB is required for embryoid body differentiation. Oncogene 2000, 19, 3750–3756.
- Qi, Y.; Liu, J.; Saadat, S.; Tian, X.; Han, Y.; Fong, G.H.; Pandolfi, P.P.; Lee, L.Y.; Li, S. PTEN induces apoptosis and cavitation via HIF-2-dependent Bnip3 upregulation during epithelial lumen formation. Cell Death Differ. 2015, 22, 875–884.
- Coucouvanis, E.; Martin, G.R. Signals for death and survival: A two-step mechanism for cavitation in the vertebrate embryo. Cell 1995, 83, 279–287.
- Murray, P.; Edgar, D. Regulation of programmed cell death by basement membranes in embryonic development. J. Cell Biol 2000, 150, 1215–1221.
- Mishina, Y.; Suzuki, A.; Ueno, N.; Behringer, R.R. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995, 9, 3027–3037.
- Itskovitz-Eldor, J.; Schuldiner, M.; Karsenti, D.; Eden, A.; Yanuka, O.; Amit, M.; Soreq, H.; Benvenisty, N. Differentiation of Human Embryonic Stem Cells into Embryoid Bodies Comprising the Three Embryonic Germ Layers. Mol. Med. 2000, 6, 88–95.
- Bauwens, C.L.; Peerani, R.; Niebruegge, S.; Woodhouse, K.A.; Kumacheva, E.; Husain, M.; Zandstra, P.W. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells 2008, 26, 2300–2310.
- Dias, A.D.; Unser, A.M.; Xie, Y.; Chrisey, D.B.; Corr, D.T. Generating size-controlled embryoid bodies using laser direct-write. Biofabrication 2014, 6, 025007.
- Valamehr, B.; Jonas, S.J.; Polleux, J.; Qiao, R.; Guo, S.; Gschweng, E.H.; Stiles, B.; Kam, K.; Luo, T.J.; Witte, O.N.; et al. Hydrophobic surfaces for enhanced differentiation of embryonic stem cell-derived embryoid bodies. Proc. Natl. Acad. Sci. USA 2008, 105, 14459–14464.
- Park, J.; Cho, C.H.; Parashurama, N.; Li, Y.; Berthiaume, F.; Toner, M.; Tilles, A.W.; Yarmush, M.L. Microfabrication-based modulation of embryonic stem cell differentiation. Lab Chip 2007, 7, 1018–1028.
- Ji, R.P.; Phoon, C.K.; Aristizabal, O.; McGrath, K.E.; Palis, J.; Turnbull, D.H. Onset of cardiac function during early mouse embryogenesis coincides with entry of primitive erythroblasts into the embryo proper. Circ. Res. 2003, 92, 133–135.
- Yamamoto, K.; Sokabe, T.; Watabe, T.; Miyazono, K.; Yamashita, J.K.; Obi, S.; Ohura, N.; Matsushita, A.; Kamiya, A.; Ando, J. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am. J. Physiol Heart Circ. Physiol. 2005, 288, H1915–H1924.
- Carpenedo, R.L.; Sargent, C.Y.; McDevitt, T.C. Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cells 2007, 25, 2224–2234.
- Guven, S.; Lindsey, J.S.; Poudel, I.; Chinthala, S.; Nickerson, M.D.; Gerami-Naini, B.; Gurkan, U.A.; Anchan, R.M.; Demirci, U. Functional maintenance of differentiated embryoid bodies in microfluidic systems: A platform for personalized medicine. Stem Cells Transl. Med. 2015, 4, 261–268.
- Fung, W.T.; Beyzavi, A.; Abgrall, P.; Nguyen, N.T.; Li, H.Y. Microfluidic platform for controlling the differentiation of embryoid bodies. Lab Chip 2009, 9, 2591–2595.
- Nsiah, B.A.; Ahsan, T.; Griffiths, S.; Cooke, M.; Nerem, R.M.; McDevitt, T.C. Fluid shear stress pre-conditioning promotes endothelial morphogenesis of embryonic stem cells within embryoid bodies. Tissue Eng. Part A 2014, 20, 954–965.
- Sargent, C.Y.; Berguig, G.Y.; Kinney, M.A.; Hiatt, L.A.; Carpenedo, R.L.; Berson, R.E.; McDevitt, T.C. Hydrodynamic modulation of embryonic stem cell differentiation by rotary orbital suspension culture. Biotechnol. Bioeng. 2010, 105, 611–626.
- Goetzke, R.; Sechi, A.; De Laporte, L.; Neuss, S.; Wagner, W. Why the impact of mechanical stimuli on stem cells remains a challenge. Cell Mol. Life Sci 2018, 75, 3297–3312.
- Geuss, L.R.; Wu, D.C.; Ramamoorthy, D.; Alford, C.D.; Suggs, L.J. Paramagnetic beads and magnetically mediated strain enhance cardiomyogenesis in mouse embryoid bodies. PLoS ONE 2014, 9, e113982.
- Sharifpanah, F.; Behr, S.; Wartenberg, M.; Sauer, H. Mechanical strain stimulates vasculogenesis and expression of angiogenesis guidance molecules of embryonic stem cells through elevation of intracellular calcium, reactive oxygen species and nitric oxide generation. Biochim. Biophys. Acta 2016, 1863, 3096–3105.
- Schmelter, M.; Ateghang, B.; Helmig, S.; Wartenberg, M.; Sauer, H. Embryonic stem cells utilize reactive oxygen species as transducers of mechanical strain-induced cardiovascular differentiation. FASEB J. 2006, 20, 1182–1184.
- Wang, B.; Tu, X.; Wei, J.; Wang, L.; Chen, Y. Substrate elasticity dependent colony formation and cardiac differentiation of human induced pluripotent stem cells. Biofabrication 2018, 11, 015005.
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689.
- Gerardo, H.; Lima, A.; Carvalho, J.; Ramos, J.R.D.; Couceiro, S.; Travasso, R.D.M.; Pires das Neves, R.; Graos, M. Soft culture substrates favor stem-like cellular phenotype and facilitate reprogramming of human mesenchymal stem/stromal cells (hMSCs) through mechanotransduction. Sci. Rep. 2019, 9, 9086.
- Ferreira, L.S.; Gerecht, S.; Fuller, J.; Shieh, H.F.; Vunjak-Novakovic, G.; Langer, R. Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials 2007, 28, 2706–2717.
- Levenberg, S.; Huang, N.F.; Lavik, E.; Rogers, A.B.; Itskovitz-Eldor, J.; Langer, R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc. Natl. Acad. Sci. USA 2003, 100, 12741–12746.
- Hribar, K.C.; Finlay, D.; Ma, X.; Qu, X.; Ondeck, M.G.; Chung, P.H.; Zanella, F.; Engler, A.J.; Sheikh, F.; Vuori, K.; et al. Nonlinear 3D projection printing of concave hydrogel microstructures for long-term multicellular spheroid and embryoid body culture. Lab Chip 2015, 15, 2412–2418.
- Qi, H.; Du, Y.; Wang, L.; Kaji, H.; Bae, H.; Khademhosseini, A. Patterned differentiation of individual embryoid bodies in spatially organized 3D hybrid microgels. Adv. Mater. 2010, 22, 5276–5281.
- Schukur, L.; Zorlutuna, P.; Cha, J.M.; Bae, H.; Khademhosseini, A. Directed differentiation of size-controlled embryoid bodies towards endothelial and cardiac lineages in RGD-modified poly(ethylene glycol) hydrogels. Adv. Healthc. Mater. 2013, 2, 195–205.
- Sun, Y.; Villa-Diaz, L.G.; Lam, R.H.; Chen, W.; Krebsbach, P.H.; Fu, J. Mechanics regulates fate decisions of human embryonic stem cells. PLoS ONE 2012, 7, e37178.
- Abagnale, G.; Sechi, A.; Steger, M.; Zhou, Q.; Kuo, C.C.; Aydin, G.; Schalla, C.; Muller-Newen, G.; Zenke, M.; Costa, I.G.; et al. Surface Topography Guides Morphology and Spatial Patterning of Induced Pluripotent Stem Cell Colonies. Stem Cell Rep. 2017, 9, 654–666.
- Stenberg, J.; Elovsson, M.; Strehl, R.; Kilmare, E.; Hyllner, J.; Lindahl, A. Sustained embryoid body formation and culture in a non-laborious three dimensional culture system for human embryonic stem cells. Cytotechnology 2011, 63, 227–237.
- Correction for Dixon et al., Combined hydrogels that switch human pluripotent stem cells from self-renewal to differentiation. Proc. Natl. Acad. Sci. USA 2017, 114, E8940–E8942.




