Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wei, J. Tumor Microenvironment with Nanoparticle-Based Therapies. Encyclopedia. Available online: https://encyclopedia.pub/entry/14802 (accessed on 11 January 2026).
Wei J. Tumor Microenvironment with Nanoparticle-Based Therapies. Encyclopedia. Available at: https://encyclopedia.pub/entry/14802. Accessed January 11, 2026.
Wei, Jia. "Tumor Microenvironment with Nanoparticle-Based Therapies" Encyclopedia, https://encyclopedia.pub/entry/14802 (accessed January 11, 2026).
Wei, J. (2021, October 01). Tumor Microenvironment with Nanoparticle-Based Therapies. In Encyclopedia. https://encyclopedia.pub/entry/14802
Wei, Jia. "Tumor Microenvironment with Nanoparticle-Based Therapies." Encyclopedia. Web. 01 October, 2021.
Copy Citation
Therapies mobilizing host immunity against cancer cells have profoundly improved prognosis of cancer patients. However, efficacy of immunotherapies depends on local immune conditions. The “cold” tumor, which is characterized by lacking inflamed T cells, is insensitive to immunotherapy. Current strategies of improving the “cold” tumor microenvironment are far from satisfying. Nanoparticle-based therapies provide novel inspiration in firing up the tumor microenvironment.
immunotherapy
tumor microenvironment
cancer vaccine
adoptive cell therapy
1. Introduction
Immunotherapies have already switched the pattern of cancer treatment and incredibly extended patient survival in melanoma, non-small-cell lung carcinoma (NSCLC), and gastric/gastro-esophageal junction cancer [1]. Immune checkpoint inhibitors (ICI), adoptive cell therapy (ACT), and cancer vaccines are major strategies of cancer immunotherapies. Several ICIs and ACT have been approved by Food and Drug Administration (FDA) and recommended by National Comprehensive Cancer Network (NCCN) guidelines as standard therapies for specific solid tumors and hematology neoplasms [2]. However, the overall response rate of immunotherapies is inferior, which indicates that it is necessary to screen potential beneficial patients [3]. Meanwhile, resistance to immunotherapies seems inevitable. Suppressive tumor microenvironment plays an important role in primary or secondary resistance to immunotherapy [4]. Tumor microenvironment (TME) remodeling exhibits synergism with immunotherapies [5]. According to immune cell infiltration and reactivity to immunotherapies, malignancies can be divided into “hot”, “cold”, and transitional types [6]. Tumors with “cold” immune landscapes are considered as refractory cases and resistant to immune agents. Although vast efforts have been made to improve immune cell infiltration and reverse immune suppressive TME, clinical outcomes are far from satisfying [7][8]. There is an urgent need to develop new methods to heat up TME.
The rapid development of nanotechnology in recent years brings a novel choice of immune agent carrier [9]. Nanoparticles are defined as materials, structures, devices, and systems with their size and shape in the nanoscale range (1 to 100 nm) [10]. Due to the similarity to biologic molecules in scale, nanoparticles are designed to execute different functions as medical agents. According to material, nanoparticles can be divided into lipid based nanoparticles, polymeric nanoparticles and inorganic nanoparticles [11].Nanoparticles have been envisioned as an attractive adjunctive approach to enhance immunotherapies [12][13]. Nanoparticles can deliver immunogens accurately and activate both antigen presenting cells (APC) and effector cells to enhance every step of anti-tumor immune cycle [14]. Besides, multiple types of drugs have been proved to display synergistic effect with immunotherapies [15]. Nanoparticles construct a platform which facilitates the combination of different therapies. In this review, we discuss the barriers of immunotherapy and focus on the application of nanoparticles to heat up the “cold” TME from different aspects.
2. Features of “Cold” Tumor and Barriers for Immunotherapy
Recent advances of technology, analysis methods, and mechanisms in immunology enable more specific classification of immune landscape of tumor. Due to the relationship between immune contexture and prognosis, scientists constructed immunoscore to quantitatively evaluate immune cell infiltration in both tumor center and invasive margin [16]. The cut point between a cold tumor and hot tumor is not explicit. Thus, transitional types may be more common. According to immunoscore, Galon et al. suggested a more comprehensive main four-category classification of tumors—hot, altered-excluded, altered-immunosuppressed, and cold [17]. Beyond hot, the remaining three types of tumors exhibit cold immune features in various degrees. Cold tumors feature in barrier molecules in extracellular matrix (ECM), low immunogenicity, and low antigen presentation. Cold tumors possess intrinsic insensitivity to immunotherapy. Excluded type displays notable hypoxia and angiogenesis in the center of tumor bulk thus blocking T cell trafficking. The immunosuppressed type has relatively better T cell infiltration and striking immunosuppressive factors or cells.
Taken together, the obstacles of cold tumor immunotherapy include local immune barriers and systematic immune dysfunction. In the tumor area, molecules such as collagen and hyaluronic acid (HA) construct the ECM barrier and block immune cell infiltration [18]. Aberrant tumor vessels attenuate T cell adhesion and penetration [19]. Subsequently, tumor angiogenesis-caused hypoxia restrains the priming of immune system. Moreover, the local immunogenicity of tumor is not enough for APCs [20]. Meanwhile, cancer patients, especially in late stages, may have malnutrition and T cell exhaustion, which are systematic immune disadvantages (Figure 1).
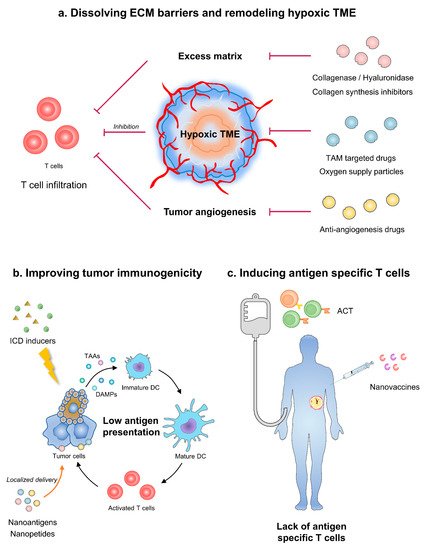
Figure 1. Strategies of nanoparticles in improving cancer immunotherapy. (a) Nanoparticles dissolving ECM barriers and remodeling angiogenesis-caused hypoxia could improve T cell infiltration. (b) Nanomodified ICD inducers or vaccines can augment tumor immunogenicity. (c) ACT equipped with nanoparticles induce antigen specific T cells and relieve systematic immune dysfunction. ECM, extracellular matrix; TME, tumor microenvironment; TAM, tumor-associated macrophage; ICD, immunogenetic cell death; TAAs, tumor-associated antigens; DC, dendritic cells; ACT, adoptive cell therapy.
Multifunctional nanoparticles can augment the effect of immunotherapy by resolving critical immune barriers. Nanoparticles mainly act as carriers for immunotherapy. According to distinctive design, nanoparticles are divided as tumor targeting and lymph organ targeting. Tumor-targeting nanoparticles mainly rely on the enhanced permeability and retention effect (EPR) of solid tumors [21]. Therefore, less kidney, liver, and spleen clearance also contribute to the concentration of nanoparticles in tumor bulk [22]. Tumor targeting nanoparticles provide drugs with the targetability towards TME, including ECM components, aberrant angiogenesis, hypoxia, and immune cells. Meanwhile, lymph organ targeting nanoparticles are usually administered through subcutaneous, intradermal, intramuscular, or intraperitoneal injection, which facilitates nanoparticles entering lymph circulation [23]. When reaching lymph nodes, nanoparticles with suitable diameter are absorbed by macrophages or dendritic cells and initiate antigen processing and presentation [24]. Apart from carriers, nanoparticles with multiple modification facilitate the combination of immunotherapy and other therapies. For example, nanomodification endows T cells with enhanced cytokine secretion which augments systematic antitumor immune.
3. Dissolving ECM Barriers
A large amount of ECM is one of the features of cold tumors [25]. Ingredients of tumor ECM, such as collagen and HA, compose physical barriers for lymphocyte infiltration or immune agents entering TME [26]. Researchers have already developed several ways to break through the ECM barriers. It is an effective strategy to break through ECM barrier by directly decomposing matrix components through nanomaterials. In addition, nanomaterials can also improve matrix properties and enhance immune infiltration by regulating stromal cells. Conventional preparation caused relatively short half-life and less-than-effective local concentration when systematically injected. Nanoparticles as carriers for those drugs avoid inappropriate activation in peripheral blood and release drugs accurately in TME.
3.1. Enzymolysis of Collagen and HA
Enzymes that can dissolve collagen or HA have been under trial [27]. Methods to decompose collagen could modulate tumor ECM, which could potentially increase immune cell infiltration and tumor cell evasion simultaneously [28]. Therefore, collagenase is usually applied as combination instead of monotherapy. Collagenase modification on the surface of pegylated gold nanoparticles could increase tumor penetration by 35% in the NSCLC xenograft murine model [29]. In the breast cancer murine model, collagenase conjugated with gold-nanoparticles can also improve the penetration of metformin gold-nanoparticles’ conjugation, thus reforming tumor suppression [30].
When combined with chemotherapy, collagenase could raise drug penetration and immunogenetic cell death (ICD). Fibrosis in ECM is typical of ductal adenocarcinoma of pancreas (PDAC), which has little inflamed T cells [31]. Zinger et al. developed nano-liposome encapsulated collagenase type-I called “Collagozome” [32]. Pretreatment with Collagozome followed by paclitaxel micelles decreased tumor volume by 60% in orthotopic PDAC murine model, comparing with unmodified collagenase pretreatment. Masson’s trichrome histological staining confirmed that the collagen level was 37% less in the Collagozome-treated group than free collagenase. Besides, the application of Collagozome did not increase the amount of circulating tumor cells or metastasis. Improved tumor suppression of paclitaxel micelles was in line with a higher level of ICD. It has been proven that paclitaxel-induced ICD could promote antigen presentation by dendritic cells and activate antitumor immunity [33]. Instead of utilizing collagenase as pretreatment, Huang et al. designed a novel collagenase IV and clusterin modified polycaprolactone-polyethylene glycol (PCL-PEG) nanoparticles that load doxorubicin, which exhibited impressive ECM penetration and anti-tumor effects both in vitro and in vivo [34].
HA is another druggable target of tumor ECM. The most frequently applied hyaluronidase is pegylated recombinant human hyaluronidase (PEGPH20). PEGPH20 monotherapy could increase NK cell infiltration in the high-HA tumor model [35]. Besides, PEGPH20 has been combined with chemotherapies and immunotherapies in various trials. However, the effect of nanoparticle as carriers for hyaluronidase was more controversial in clinical trials, especially combined with chemotherapies. In SWOG S1313, a phase I b /II trial, PEGPH20 plus FOLFIRINOX caused more adverse events, reduced treatment duration, and seemed to be deleterious in unselected metastasis pancreas cancer (mPC) patients [36]. While in the HALO-202 trial, retrospective analysis showed that the PEGPH20 plus nab-paclitaxel/gemcitabine group had a better objective response rate (ORR, 45% vs. 31%) and medium overall survival (OS, 11.5 vs. 8.5 months) in HA-high untreated PDAC patients [37]. Thus, the following phase III clinical trial HALO-301 was limited to high-HA PDAC patients. Considering the similar clinical background of SWOG S1313 and HALO-202, the divergence of outcomes lay in the type of chemotherapy. Patients in the PEGPH20-treated group tended to have more adverse events, although not reaching statistical significance in HALO-202 trial [38]. When combined with intense chemotherapy, enhanced adverse events may be intolerable and result in relatively more drop-outs. Taken together, caution should be paid when combing nanoparticle modified hyaluronidase with chemotherapy.
Immunotherapy is a better choice to combine with hyaluronidase, which could directly increase T cell infiltration or improve the concentration of immune agents in TME. Blair et al. applied an irradiated whole-cell PDAC vaccine along with PEGPH20 in metastasis PDAC murine model, resulting in increased effector memory T cell infiltration, IFNγ secretion, and improved survival [39]. Apart from the vaccine, hyaluronidase coordinated with the immune checkpoint inhibitor as well. PEGPH20 sensitized HA accumulating cancer to PD-L1 blockade in the breast cancer murine model [40].
Overall, nanoparticle-modified enzymolysis of collagen and HA itself enhanced immune infiltration and exhibited a synergistic effect with other immunotherapy. Enzymolysis of ECM ingredients may be potential adjuvant of immunotherapy.
3.2. Reprogramming ECM Producing Cells
Aside from directly dissolving ECM components, the strategy of restraining fibroblasts from secreting excessive stroma is another way of remodeling tumor ECM for better immune infiltration. Angiotensin receptor II blockers, namely losartan, inhibit collagen I synthesis in cancer-associated fibroblasts and facilitate the distribution and efficacy of pegylated liposomal doxorubicin in murine breast, pancreas, and skin cancer models [41]. A phase II clinical trial further confirmed that in neoadjuvant setting, FOLFIRINOX along with losartan brought about survival benefits to PDAC patients with an R0 resection rate of 61% [42]. Considering the regulation of TGFβ signaling of losartan, the drug may have undefine influence on anticancer immune [43]. Hou et al. developed transformable nano assemblies of carbon dots containing doxorubicin and Fe ions on the surface and losartan encapsuled within the mesopores, which exhibited about 2.40-fold higher CD8+ and CD4+ T cell infiltration than those of control [44]. Another angiotensin II type 1 receptor blocker telmisartan inhibits the development of transient hypoxia and sensitizes tumor to radiation, causing higher level of ICD [45].
Taken together, nanoparticles as carriers for ECM targeting therapies have exhibited impressive improvement of pharmacokinetics, either in prevention from premature emission or systematic toxicities. Nanoparticles when combined with chemotherapies increase T cell infiltration by inducing higher levels of ICD. Immune agents modified with nanoparticles could remodel TME more directly through better penetration and raised local concentration.
4. Improving Tumor Immunogenicity
Efficient antitumor strategies must fully activate endogenous tumor immunity. However, one of the major obstacles to tumor clearance is loss of tumor antigen expression and low adjuvancity [46]. To solve this problem, increasing efforts have been made to improve tumor immunogenicity by enhancing local antigen presentation or adjuvanticity and inducing ICD, in order to form the cycle of immune priming, tumor cell death, antigen release, and immune reactivating, to maximize the anti-tumor effect. Nanomaterials play an important role in antigen delivery and ICD induction.
4.1. Assisting in Exogenous Antigen Delivery
Effector T cells recognize and bind the peptide-MHC complex on target cells to start the killing process. Lacking of endogenously presented antigen-derived peptides on tumor cells is one of the important reasons for forming “cold” TME. Therefore, the innovative approach to modify tumor cells via immunogenic antigen or peptide delivery can be an option to induce the cytotoxic T lymphocyte (CTL)-mediated antitumor activity.
Delivering alloantigens to “foreignize” tumor cells is a feasible way to enhance the immunogenicity of tumor. A nanoplatform of hyaluronic acid, which modified CD44+ tumor-targeting ligand and loaded with foreign antigen ovalbumin (OVA), realized preferential aggregation on tumor surface, phagocytosis by tumor cells, degradation by hyaluromycin, and release of OVA intracellularly. The OVA was degraded by proteasome into recognized peptide to boost T cell response [47]. In addition, based on the characteristics of acid pH, hypoxia, high levels of MMP, and other specific enzymes of TME, the stimulation sensitive nanodelivery system can improve the safety of the antigen delivery therapy. For example, a conjugated polymer nanoplatform modified by matrix metalloproteinase 9 (MMP9) cleavable linker allowed foreign antigen to be delivered and conditionally released into the local tumor site [48]. This kind of exogenous antigen-loading therapy theoretically overcomes the tumor heterogeneity and has a certain clinical application potential. However, the current preclinical studies are mostly the introduction of heterologous proteins, and its safety needs to be further evaluated.
Delivering viral peptides can also subtly enhance the immunogenicity of tumor cells. Memory T cells specific to previous virus infections can produce immediate and effective response to secondary infection. Injecting non-replicating viral peptides into TME effectively reactivated these antiviral T cells by mimicking a viral reinfection to poorly immunogenic tumor cells [49]. Encouragingly, antibody-peptide epitope conjugates (APECs), a nanoscale antibody and viral peptide conjugates, successfully loaded CMV antigens to the tumor surface, mobilized pre-stored virus specific memory T cells to attack tumor cells, effectively increasing the immunogenicity of tumor cells and avoiding the potential biosafety problem of oncolytic virus infection [50]. Redirection of virus-specific T cells to tumors may yield new therapeutic opportunities for cancer patients. However, previous reports mainly focused on intratumoral injection. It is only suitable for superficial and puncture accessible lesions, rather than microsatellite lesions and metastatic lesions. Therefore, it is necessary to develop effective tumor targeting and penetrable materials for systemic administration in order to eliminate occult lesions.
4.2. Promoting the Release of Endogenous Antigen
Apoptotic tumor cells induced by subtherapeutic doses of chemotherapeutics, radiotherapy, or photodynamic therapy could release tumor associated antigens (TAA), damage-associated molecular patterns (DAMPs) and pro-inflammatory cytokines to trigger antitumor immune response, which called ICD [51]. ICD-inducing modalities can effectively provoke specific T cell responses while killing tumors, and eventually transform a “cold” TME to an immunogenic, “hot” TME [52].
The application of the nanomaterials endows ICD inducers with superior antitumor activity. Apart from being a synergist of ICD to improve penetration and hypoxia microenvironment as above-mentioned, nanoparticles have several unique advantages in inducing ICD. First, aggregation in tumor site is necessary for ICD inducers. Integrated mesoporous silica nanoparticles armed with classical ICD inductors doxorubicin (DOX), named DOX@HIMSNs, initiated an anti-tumor immune response characterized by DC maturation and antitumor cytokines release [53]. Second, nanotechnology allows ICD inducers to release in a predictable and designable manner. DOX@HIMSNs has been confirmed to mostly accumulate in tumor tissue and controllably release DOX in acidic microenvironment with high concentration of GSH with the help of integrating a pH and GSH dual stimulated rotaxane [53]. Third, nanoparticles could effectively induce ICD while reducing their side effect. NPs can selectively deliver photosensitizers to tumors with minimize damage to normal tissues by spatially controlled light irradiation [52]. Fourth, co-loading multi-components on nanoparticles could significantly improve ICD and anti-tumor effect. Sen et al. engineered a redox-active Au(I) bis-N-heterocyclic carbine (Au(I) bis-NHC) that realized the double effect combining TrxR2 inhibition (damaging biological antioxidants) with increased oxidative stress [54]. The combination of photodynamic therapy with oxygen therapy based on C@HPOC showed enhanced specific CD8+ T cell response and abscopal effect [55]. Fifth, the development of nanoparticles broadens the selection range of ICD inducers. Classical metallic ICD inducer oxaliplatin failed to induce ICD in non-small cell lung cancer (NSCLC) [56]. An ER-targeting iridium(III) complex, armed with an N,N-bis(2-chloroethyl)-azane derivate, significantly triggered endoplasmic reticulum stress and increased reactive oxygen species by targeting endoplasmic reticulum, resulting in antitumor CD8+ T cell response and Foxp3+ T cell depletion, successfully suppling the selection of ICD inducers for NSCLC [57]. At last, nanoparticles can be used as a synergist of ICD inducers. Min et al. engineered Antigen-capturing NPs (AC-NPs) could play a good synergy with radiotherapy by capturing TAAs released after radiation with different surface chemistry and transport them to APCs [58].
References
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668.
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249.
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35.
- Horn, L.A.; Fousek, K.; Palena, C. Tumor Plasticity and Resistance to Immunotherapy. Trends Cancer 2020, 6, 432–441.
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840.
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618.
- Ding, N.; Zou, Z.; Sha, H.; Su, S.; Qian, H.; Meng, F.; Chen, F.; Du, S.; Zhou, S.; Chen, H.; et al. iRGD synergizes with PD-1 knockout immunotherapy by enhancing lymphocyte infiltration in gastric cancer. Nat. Commun. 2019, 10, 1336.
- Zhou, S.; Meng, F.; Du, S.; Qian, H.; Ding, N.; Sha, H.; Zhu, M.; Yu, X.; Wang, L.; Liu, B.; et al. Bifunctional iRGD-anti-CD3 enhances antitumor potency of T cells by facilitating tumor infiltration and T-cell activation. J. Immunother. Cancer 2021, 9, e001925.
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196.
- Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124.
- Wan, M.M.; Chen, H.; Da Wang, Z.; Liu, Z.Y.; Yu, Y.Q.; Li, L.; Miao, Z.Y.; Wang, X.W.; Wang, Q.; Mao, C.; et al. Nitric Oxide-Driven Nanomotor for Deep Tissue Penetration and Multidrug Resistance Reversal in Cancer Therapy. Adv. Sci. 2021, 8, 2002525.
- Chen, H.; Shi, T.; Wang, Y.; Liu, Z.; Liu, F.; Zhang, H.; Wang, X.; Miao, Z.; Liu, B.; Wan, M.; et al. Deep Penetration of Nanolevel Drugs and Micrometer-Level T Cells Promoted by Nanomotors for Cancer Immunochemotherapy. J. Am. Chem. Soc. 2021, 143, 12025–12037.
- Wan, M.; Wang, Q.; Li, X.; Xu, B.; Fang, D.; Li, T.; Yu, Y.; Fang, L.; Wang, Y.; Wang, M.; et al. Systematic Research and Evaluation Models of Nanomotors for Cancer Combined Therapy. Angew. Chem. Int. Ed. Engl. 2020, 59, 14458–14465.
- Meric-Bernstam, F.; Larkin, J.; Tabernero, J.; Bonini, C. Enhancing anti-tumour efficacy with immunotherapy combinations. Lancet 2021, 397, 1010–1022.
- Angell, H.K.; Bruni, D.; Barrett, J.C.; Herbst, R.; Galon, J. The Immunoscore: Colon Cancer and Beyond. Clin. Cancer Res. 2020, 26, 332–339.
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218.
- Hessmann, E.; Buchholz, S.M.; Demir, I.E.; Singh, S.K.; Gress, T.M.; Ellenrieder, V.; Neesse, A. Microenvironmental Determinants of Pancreatic Cancer. Physiol. Rev. 2020, 100, 1707–1751.
- Huang, Y.; Kim, B.Y.S.; Chan, C.K.; Hahn, S.M.; Weissman, I.L.; Jiang, W. Improving immune-vascular crosstalk for cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 195–203.
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168.
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1252–1276.
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 28–51.
- Chen, Y.; De Koker, S.; De Geest, B.G. Engineering Strategies for Lymph Node Targeted Immune Activation. Acc. Chem. Res. 2020, 53, 2055–2067.
- Zeng, Q.; Li, H.; Jiang, H.; Yu, J.; Wang, Y.; Ke, H.; Gong, T.; Zhang, Z.; Sun, X. Tailoring polymeric hybrid micelles with lymph node targeting ability to improve the potency of cancer vaccines. Biomaterials 2017, 122, 105–113.
- Neesse, A.; Bauer, C.A.; Öhlund, D.; Lauth, M.; Buchholz, M.; Michl, P.; Tuveson, D.A.; Gress, T.M. Stromal biology and therapy in pancreatic cancer: Ready for clinical translation? Gut 2019, 68, 159–171.
- Piersma, B.; Hayward, M.K.; Weaver, V.M. Fibrosis and cancer: A strained relationship. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188356.
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309.
- Alaseem, A.; Alhazzani, K.; Dondapati, P.; Alobid, S.; Bishayee, A.; Rathinavelu, A. Matrix Metalloproteinases: A challenging paradigm of cancer management. Semin. Cancer Biol. 2019, 56, 100–115.
- Murty, S.; Gilliland, T.; Qiao, P.; Tabtieng, T.; Higbee, E.; Al Zaki, A.; Puré, E.; Tsourkas, A. Nanoparticles functionalized with collagenase exhibit improved tumor accumulation in a murine xenograft model. Part. Part. Syst. Charact. 2014, 31, 1307–1312.
- Abdolahinia, E.D.; Nadri, S.; Rahbarghazi, R.; Barar, J.; Aghanejad, A.; Omidi, Y. Enhanced penetration and cytotoxicity of metformin and collagenase conjugated gold nanoparticles in breast cancer spheroids. Life Sci. 2019, 231, 116545.
- Hosein, A.N.; Brekken, R.A.; Maitra, A. Pancreatic cancer stroma: An update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 487–505.
- Zinger, A.; Koren, L.; Adir, O.; Poley, M.; Alyan, M.; Yaari, Z.; Noor, N.; Krinsky, N.; Simon, A.; Gibori, H.; et al. Collagenase Nanoparticles Enhance the Penetration of Drugs into Pancreatic Tumors. ACS Nano 2019, 13, 11008–11021.
- Yang, Q.; Shi, G.; Chen, X.; Lin, Y.; Cheng, L.; Jiang, Q.; Yan, X.; Jiang, M.; Li, Y.; Zhang, H.; et al. Nanomicelle protects the immune activation effects of Paclitaxel and sensitizes tumors to anti-PD-1 Immunotherapy. Theranostics 2020, 10, 8382–8399.
- Huang, H.-Y.; Chen, L.-Q.; Sun, W.; Du, H.-H.; Dong, S.; Ahmed, A.M.Q.; Cao, D.; Cui, J.-H.; Zhang, Y.; Cao, Q.-R. Collagenase IV and clusterin-modified polycaprolactone-polyethylene glycol nanoparticles for penetrating dense tumor tissues. Theranostics 2021, 11, 906–924.
- Singha, N.C.; Nekoroski, T.; Zhao, C.; Symons, R.; Jiang, P.; Frost, G.I.; Huang, Z.; Shepard, H.M. Tumor-associated hyaluronan limits efficacy of monoclonal antibody therapy. Mol. Cancer Ther. 2015, 14, 523–532.
- Ramanathan, R.K.; McDonough, S.L.; Philip, P.A.; Hingorani, S.R.; Lacy, J.; Kortmansky, J.S.; Thumar, J.; Chiorean, E.G.; Shields, A.F.; Behl, D.; et al. Phase IB/II Randomized Study of FOLFIRINOX Plus Pegylated Recombinant Human Hyaluronidase Versus FOLFIRINOX Alone in Patients With Metastatic Pancreatic Adenocarcinoma: SWOG S1313. J. Clin. Oncol. 2019, 37, 1062–1069.
- Hingorani, S.R.; Zheng, L.; Bullock, A.J.; Seery, T.E.; Harris, W.P.; Sigal, D.S.; Braiteh, F.; Ritch, P.S.; Zalupski, M.M.; Bahary, N.; et al. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J. Clin. Oncol. 2018, 36, 359–366.
- Wang-Gillam, A. Targeting Stroma: A Tale of Caution. J. Clin. Oncol. 2019, 37, 1041–1043.
- Blair, A.B.; Kim, V.M.; Muth, S.T.; Saung, M.T.; Lokker, N.; Blouw, B.; Armstrong, T.D.; Jaffee, E.M.; Tsujikawa, T.; Coussens, L.M.; et al. Dissecting the Stromal Signaling and Regulation of Myeloid Cells and Memory Effector T Cells in Pancreatic Cancer. Clin. Cancer Res. 2019, 25, 5351–5363.
- Clift, R.; Souratha, J.; Garrovillo, S.A.; Zimmerman, S.; Blouw, B. Remodeling the Tumor Microenvironment Sensitizes Breast Tumors to Anti-Programmed Death-Ligand 1 Immunotherapy. Cancer Res. 2019, 79, 4149–4159.
- Diop-Frimpong, B.; Chauhan, V.P.; Krane, S.; Boucher, Y.; Jain, R.K. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl. Acad. Sci. USA 2011, 108, 2909–2914.
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Clark, J.W.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Ly, L.; Baglini, C.V.; Blaszkowsky, L.S.; et al. Total Neoadjuvant Therapy With FOLFIRINOX in Combination With Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 1020–1027.
- Hauge, A.; Rofstad, E.K. Antifibrotic therapy to normalize the tumor microenvironment. J. Transl. Med. 2020, 18, 207.
- Hou, L.; Chen, D.; Wang, R.; Wang, R.; Zhang, H.; Zhang, Z.; Nie, Z.; Lu, S. Transformable Honeycomb-Like Nanoassemblies of Carbon Dots for Regulated Multisite Delivery and Enhanced Antitumor Chemoimmunotherapy. Angew. Chem. Int. Ed. Engl. 2021, 60, 6581–6592.
- Wadsworth, B.J.; Cederberg, R.A.; Lee, C.-M.; Firmino, N.S.; Franks, S.E.; Pan, J.; Colpo, N.; Lin, K.-S.; Benard, F.; Bennewith, K.L. Angiotensin II type 1 receptor blocker telmisartan inhibits the development of transient hypoxia and improves tumour response to radiation. Cancer Lett. 2020, 493, 31–40.
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167.
- Lee, Y.H.; Yoon, H.Y.; Shin, J.M.; Saravanakumar, G.; Noh, K.H.; Song, K.H.; Jeon, J.H.; Kim, D.W.; Lee, K.M.; Kim, K.; et al. A polymeric conjugate foreignizing tumor cells for targeted immunotherapy in vivo. J. Control. Release 2015, 199, 98–105.
- Shin, J.M.; Oh, S.J.; Kwon, S.; Deepagan, V.G.; Lee, M.; Song, S.H.; Lee, H.J.; Kim, S.; Song, K.H.; Kim, T.W.; et al. A PEGylated hyaluronic acid conjugate for targeted cancer immunotherapy. J. Control. Release 2017, 267, 181–190.
- Rosato, P.C.; Wijeyesinghe, S.; Stolley, J.M.; Nelson, C.E.; Davis, R.L.; Manlove, L.S.; Pennell, C.A.; Blazar, B.R.; Chen, C.C.; Geller, M.A.; et al. Virus-specific memory T cells populate tumors and can be repurposed for tumor immunotherapy. Nat. Commun. 2019, 10, 567.
- Millar, D.G.; Ramjiawan, R.R.; Kawaguchi, K.; Gupta, N.; Chen, J.; Zhang, S.; Nojiri, T.; Ho, W.W.; Aoki, S.; Jung, K.; et al. Antibody-mediated delivery of viral epitopes to tumors harnesses CMV-specific T cells for cancer therapy. Nat. Biotechnol. 2020, 38, 420–425.
- Musetti, S.; Huang, L. Nanoparticle-Mediated Remodeling of the Tumor Microenvironment to Enhance Immunotherapy. ACS Nano 2018, 12, 11740–11755.
- Duan, X.; Chan, C.; Lin, W. Nanoparticle-Mediated Immunogenic Cell Death Enables and Potentiates Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2019, 58, 670–680.
- Zheng, D.W.; Chen, J.L.; Zhu, J.Y.; Rong, L.; Li, B.; Lei, Q.; Fan, J.X.; Zou, M.Z.; Li, C.; Cheng, S.X.; et al. Highly Integrated Nano-Platform for Breaking the Barrier between Chemotherapy and Immunotherapy. Nano Lett. 2016, 16, 4341–4347.
- Sen, S.; Hufnagel, S.; Maier, E.Y.; Aguilar, I.; Selvakumar, J.; DeVore, J.E.; Lynch, V.M.; Arumugam, K.; Cui, Z.; Sessler, J.L.; et al. Rationally Designed Redox-Active Au(I) N-Heterocyclic Carbene: An Immunogenic Cell Death Inducer. J. Am. Chem. Soc. 2020, 142, 20536–20541.
- Chen, Z.; Liu, L.; Liang, R.; Luo, Z.; He, H.; Wu, Z.; Tian, H.; Zheng, M.; Ma, Y.; Cai, L. Bioinspired Hybrid Protein Oxygen Nanocarrier Amplified Photodynamic Therapy for Eliciting Anti-tumor Immunity and Abscopal Effect. ACS Nano 2018, 12, 8633–8645.
- Flieswasser, T.; Van Loenhout, J.; Freire Boullosa, L.; Van den Eynde, A.; De Waele, J.; Van Audenaerde, J.; Lardon, F.; Smits, E.; Pauwels, P.; Jacobs, J. Clinically Relevant Chemotherapeutics Have the Ability to Induce Immunogenic Cell Death in Non-Small Cell Lung Cancer. Cells 2020, 9, 1474.
- Wang, L.; Guan, R.; Xie, L.; Liao, X.; Xiong, K.; Rees, T.W.; Chen, Y.; Ji, L.; Chao, H. An ER-Targeting Iridium(III) Complex That Induces Immunogenic Cell Death in Non-Small-Cell Lung Cancer. Angew. Chem. Int. Ed. Engl. 2021, 60, 4657–4665.
- Min, Y.; Roche, K.C.; Tian, S.; Eblan, M.J.; McKinnon, K.P.; Caster, J.M.; Chai, S.; Herring, L.E.; Zhang, L.; Zhang, T.; et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol. 2017, 12, 877–882.
More
Information
Subjects:
Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
763
Revisions:
2 times
(View History)
Update Date:
08 Oct 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




