
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ana María Simonet | + 1681 word(s) | 1681 | 2021-09-10 10:31:14 | | | |
| 2 | Amina Yu | Meta information modification | 1681 | 2021-10-08 05:14:15 | | |
Video Upload Options
Yucca is one of the main sources of steroidal saponins, hence different extracts are commercialized for use as surfactant additives by beverage, animal feed, cosmetics or agricultural products. For a deeper understanding of the potential of the saponins that can be found in this genus, an exhaustive review of the structural characteristics, bioactivities and analytical methods that can be used with these compounds has been carried out, since there are no recent reviews on the matter. Thus, a total of 108 saponins from eight species of the genus Yucca have been described.
1. Introduction
The Yucca genus comprises around 50 plant species of the Agavaceae family from the American continent, but mainly from the United States, Mexico and Central America [1][2]. The species that belong to the Yucca genus have been used for years in traditional medicinal practices all over the world, but especially by Native Americans [1]. Subsequently, the research on this genus has made clear that they are plants with a high content of bioactive steroidal saponins [3].
At present, some species of the Yucca genus and their saponins have been classified as Generally Recognized As Safe (GRAS) by the Food and Drug Administration of the United States (FDA) [1][4][5][6]. Therefore, several extracts and products derived from these plants have been commercialized as food supplements, parapharmaceutical complements, moisturizing agents, soil conditioners, etc. There are several producers of Y. schidigera products in the world such as Naturex, BAJA Yucca and American Extracts [7]. The saponins present in these plants have also been tested in separate studies, showing properties such as cytotoxic, phytotoxic, antifungal, molluscicidal, anti-inflammatory [8][9][10][11][12].
There are several reviews on the steroidal saponins from the Agavaceae family, including the Yucca genus, however, no work has been published to address this genus exclusively. The data that are usually collected in these studies are related to the structure and bioactivities of the saponins. An example of such work is that by Simmons-Boyce in 2007, where a total of 44 saponins found in the Yucca genus and their bioactivities were described [3].
Other papers have reviewed saponins from a specific species, such as Y. schidigera [6][13]. This species is the most widely marketed and the one with the largest number of studies. Even some very active saponins such as the so-called timosaponin A III, present in Y. macrocarpa and Y. gloriosa and their bioactivities have been the subject of a number of reviews [14].
2. Structure of Yucca Saponins
To date, 33 aglycones have been described as part of the saponins found in the Yucca genus [15][4][8][9][11][13][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35], including spirostanic and furostanic saponins ( Figure 1 ).
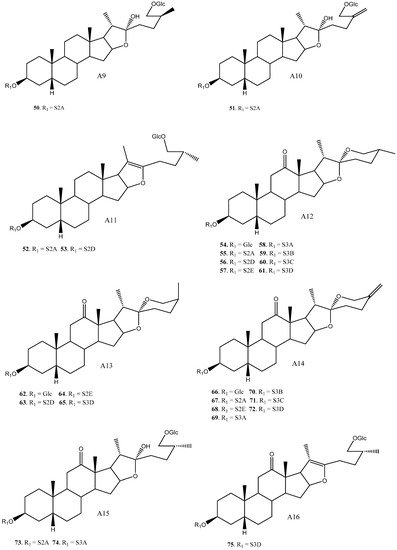
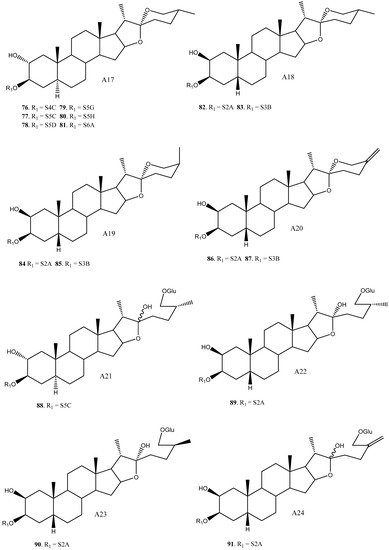
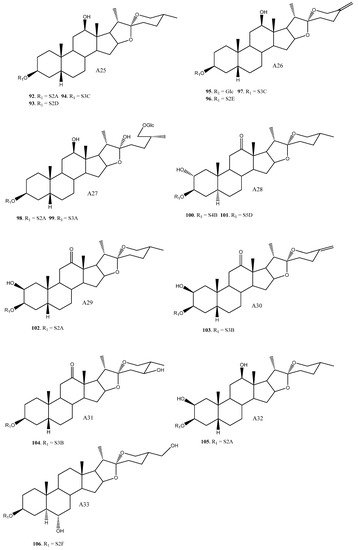
In addition to glucose, 22 different sugar chains have been described in Yucca. Disaccharides and trisaccharides exhibit a wide structural diversity. The monomer that bonds directly to the C-3 of the aglycone can be either galactopyranose or glucopyranose. These monosaccharides are linked by glycosidic bonding to glucopyranoses or xylopyranoses on the positions 2’, 3’ or 4’, in the case of galactose, and 2’ or 3’, in the case of glucose. The most common disaccharide in Yucca is glucopyranosyloxy (1→2) galactopyranoside (S2A). Sugar chains of four or more units have also been described in Yucca, although they are less abundant than the above described.
Overall, based on the data available, it is observed that some saponins such as 12, 16, 42, 55 and 82 are described in three or four different species, however, the general trend is that most of the saponins are found in only one or two species.
The saponins with a H-5β aglycone arrangement have a sugar chain of three or fewer units attached to C-3. For example, in Y. schidigera [11][13][18], the majority of the saponins are 5β-spirostanic and all of them have chains formed by two or three monosaccharides. In other species, such as Y. aloifolia [22][23][24], the isolated saponins are 5α-spirostanic with sugar chains of four and five units. The same trend is also true for Y. gloriosa [27], where the saponins with a β-arrangement at H-5 have chains formed by less than three polysaccharides, while those with an α-arrangement at H-5 present the longest chains.
3. Bioactivities by Yucca Saponins
The saponins isolated from different extracts of the genus Yucca have also been studied. Interesting cytotoxic activities against various cancer cell lines have been described for saponins from Y. glauca [8], Y. desmetiana [32] and Y. schidigera [26]. Antifungal activity has also been tested on pure saponins and epimers mixtures on the C-25 position in Y. gloriosa [29], Y. elephantipes [34] and Y. schidigera [11], showing very promising activity values. These two activities have been the most frequently tested on the saponins from Yucca , including studies on structure-activity relationships (SAR). Apart from these activities, two saponins from Y. desmetiana [9] and one from Y. aloifolia [23] presented molluscidal activity.
Sun et al. [36], demonstrated with saponins from Anemarrhena asphodeloides, which included some of the saponins contained in Yucca, that glycosylation is considered essential regarding cytotoxic activity, since when the aglycones were tested separately, they did not exhibit any activity. Yucca fraction containing furostanic saponins did not show activity, therefore, the study was focused on the spirostanic saponins [11].
The results from the tests with spirostanic saponins and their mixtures showed that the presence of oxidations on the C-2 and C-12 positions (hydroxyl and carbonyl groups) resulted in a drastic drop or even the absence of activity. On the other hand, those without such oxidations present better citotoxic activities and MIC values [11].
4. Analytical Methods for the Steroidal Saponins in Yucca
Spectrophotometric methods, such as UV, have also failed to prove accurate, since saponins do not have a strong UV chromophore that can be detected by UV analysis [15]. In order to improve results, saponins have been derivatized to form colored substances. Thus, using this method, a spectrophotometric assay claimed to have determined the deglycosylation of steroidal saponins into sapogenin within the ruminal fluid of cattle that had been fed with Y. schidigera saponins. This assay. which was a modified version of the method described by Baccou et al. [37] showed that rumen bacteria are capable of degrading saponin into sapogenin or aglycone but cannot degrade the aglycone core. This colorimetric method is based on the reactions that take place with anisaldehyde, sulphuric acid and ethyl acetate to form chromophores [38].
Other chromatographic methods have been widely used for the analysis of saponins. Thus, a low-cost method that provides a preliminary assessment and verification of the saponins found in some commercial products containing Y. schidigera has been recently developed. This method uses TLC silica gel 60 F 254, CHCl3:CH3OH: H2O (35:14:1) and 10% sulphuric acid-ethanol solution to improve plate visualization. The saponins became apparent under these chromatographic conditions when the retention factor was 0.38 or lower [39]. In another study, despite the difficulty to separate isomers, six pairs of 25 (R/S) -spirostanol saponin diastereomers from Y. schidigera were successfully separated through HPLC using a C 30 column and their structure was unequivocally confirmed by NMR analysis [26]. On the other hand, 25 (R/S)-spirostanol saponin diastereomers have been successfully separated using supercritical fluid chromatography [40].
Mass spectrometry represents an effective detection method where selectivity and specificity can be improved by tandem mass spectrometry. Thus, in the last few years, different methods using tandem mass spectrometry have been developed for a quick and reliable determination of saponins [5]. It should also be mentioned that the use of mass spectrometry as a single technique for the determination of saponins presents some drawbacks, since it does not allow to differentiate isomers, and neither the position of the sugar chain link on the aglycone, nor the connectivity between sugar residues can be determined. Mass spectrometry and chromatographic methods are not often used as a single technique for the determination of these compounds, but rather in combination or coupled to other techniques. In this way, the detection and quantification of the nineteen steroidal saponins contained in a commercial syrup and in a bark’s extract from Y. schidigera have been carried out by LC-MS. Thus, twelve saponins were confirmed by comparing their fragmentation patterns against their corresponding standards and their spectrometric data, while the rest of the saponins were structurally proposed [5]. Furthermore, Montoro et al. developed a method for the quantitative analysis of the steroidal saponins in Y. gloriosa flowers real samples using HPLC coupled to tandem mass spectrometry [4]. LC-ESI-MS analysis has also been applied to the detection of the saponins contained in different Yucca aloifolia varieties imported into Egypt [41]. HPLC/ELSD is another accurate analysis and quantification method that has been applied to determine the total steroidal saponin content in Y. schidigera extracts. This is a fast and reliable technique that allows the quantification of molecules that do not contain a chromophore group, like in the case of saponins [42]. In the same way, Sastre et al. described a method for the analysis of the four major saponins in certain commercial samples of Y. schidigera by means of HPLC coupled to an evaporative light scattering detector (ELSD) and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry [43]. Recently, Ruan and co-workers have identified 110 spirostanol saponins by multi-phase liquid chromatography (MPLC) combined with MS/MS. This approach improves chromatographic peak capacity and became the first method to provide a comprehensive characterization of spirostanol saponins from Y. schidigera [44].
Yucca has proven to be a valuable source of steroidal saponins and its commercial extracts have been classified as GRAS by the US FDA. It is, therefore, widely used as a surfactant additive for the manufacturing of beverages, animal feed, cosmetics and some agricultural products. Despite its increasing applications, a method to evaluate and control the quality of the saponins contained in this plant genus and often incorporated to commercial products is still an unresolved issue. These metabolites are usually found in complex mixtures, structurally related and their separation is still a task [45]. Thereby, the recent methods described for their identification are based in different techniques coupled between them, being the most used, LC-MS technique. Moreover, the most of the studies have been carried out with extracts of Y. schidigera , being this species one of the major commercial sources of saponins.
References
- Patel, S. Yucca: A medicinally significant genus with manifold therapeutic attributes. Nat. Prod. Bioprospect. 2012, 2, 231–234.
- Clary, K.H.; Simpson, B.B. Sistemática y evolución del género Yucca (Agavaceae): Evidencias de análisis nunfológicos y moleculares. Bot. Sci. 1995, 56, 77–88.
- Simmons-Boyce, J.L.; Tinto, W.F. Steroidal Saponins and Sapogenins from the Agavaceae Family. Nat. Prod. Commun. 2007, 2, 99–114.
- Montoro, P.; Skhirtladze, A.; Perrone, A.; Benidze, M.; Kemertelidze, E.; Piacente, S. Determination of steroidal glycosides in Yucca gloriosa flowers by LC/MS/MS. J. Pharm. Biomed. Anal. 2010, 52, 791–795.
- Kowalczyk, M.; Pecio, Ł.; Stochmal, A.; Oleszek, W. Qualitative and quantitative analysis of steroidal saponins in crude extract and bark powder of Yucca schidigera Roezl. J. Agric. Food Chem. 2011, 59, 8058–8064.
- Piacente, S.; Pizza, C.; Oleszek, W. Saponins and phenolics of Yucca schidigera Roezl: Chemistry and bioactivity. Phytochem. Rev. 2005, 4, 177–190.
- Report Insights. Yucca Schidigera Plant Extract Market 2020 by Manufacturers, Regions Analysis(Europe, Asia Pacific, North America and South America), Type and Application, Forecast to 2025; Report Insights: Maharashtra, India, 2020.
- Yokosuka, A.; Suzuki, T.; Tatsuno, S.; Mimaki, Y. Steroidal glycosides from the underground parts of Yucca glauca and their cytotoxic activities. Phytochemistry 2014, 101, 109–115.
- Diab, Y.; Ioannou, E.; Emam, A.; Vagias, C.; Roussis, V. Desmettianosides A and B, bisdesmosidic furostanol saponins with molluscicidal activity from Yucca desmettiana. Steroids 2012, 77, 686–690.
- Pérez, A.J.; Calle, J.M.; Simonet, A.M.; Guerra, J.O.; Stochmal, A.; Macías, F.A. Bioactive steroidal saponins from Agave offoyana flowers. Phytochemistry 2013, 95, 298–307.
- Miyakoshi, M.; Tamura, Y.; Masuda, H.; Mizutani, K.; Tanaka, O.; Ikeda, T.; Ohtani, K.; Kasai, R.; Yamasaki, K. Antiyeast steroidal saponins from Yucca schidigera (Mohave Yucca), a new anti-food-deteriorating agent. J. Nat. Prod. 2000, 63, 332–338.
- Xie, L.; Lee, D.Y.-W.; Shang, Y.; Cao, X.; Wang, S.; Liao, J.; Zhang, T.; Dai, R. Characterization of spirostanol glycosides and furostanol glycosides from anemarrhenae rhizoma as dual targeted inhibitors of 5-lipoxygenase and Cyclooxygenase-2 by employing a combination of affinity ultrafiltration and HPLC/MS. Phytomedicine 2020, 77, 153284.
- Oleszek, W.; Sitek, M.; Stochmal, A.; Piacente, S.; Pizza, C.; Cheeke, P. Steroidal saponins of Yucca schidigera Roezl. J. Agric. Food Chem. 2001, 49, 4392–4396.
- Han, F.Y.; Song, X.Y.; Chen, J.J.; Yao, G.D.; Song, S.J. Timosaponin AIII: A novel potential anti-tumor compound from Anemarrhena asphodeloides. Steroids 2018, 140, 125–130.
- Skhirtladze, A.; Perrone, A.; Montoro, P.; Benidze, M.; Kemertelidze, E.; Pizza, C.; Piacente, S. Steroidal saponins from Yucca gloriosa L. rhizomes: LC-MS profiling, isolation and quantitative determination. Phytochemistry 2011, 72, 126–135.
- Dragalin, I.P.; Gulya, A.P.; Krokhmalyuk, V.V.; Kintya, P.K. The structure of yuccoside E from Yucca filamentosa. Chem. Nat. Compd. 1975, 11, 772–774.
- Zhang, Y.; Yang, C.R.; Zhang, Y.J. New Steroidal Saponins from the Leaves of Yucca elephantipes. Helv. Chim. Acta 2013, 96, 1807–1813.
- Qu, L.; Wang, J.; Ruan, J.; Yao, X.; Huang, P.; Wang, Y.; Yu, H.; Han, L.; Zhang, Y.; Wang, T. Spirostane-type saponins obtained from Yucca schidigera. Molecules 2018, 23, 167.
- Skhirtladze, A.; Plaza, A.; Montoro, P.; Benidze, M.; Kemertelidze, E.; Pizza, C.; Piacente, S. Furostanol saponins from Yucca gloriosa L. rhizomes. Biochem. Syst. Ecol. 2006, 34, 809–814.
- Nakano, K.; Hara, Y.; Murakami, K.; Takaishi, Y.; Tomimatsu, T. 12-Hydroxy steroidal glycosides from the caudex of Yucca gloriosa. Phytochemistry 1991, 30, 1993–1995.
- Nakano, K.; Hara, Y.; Murakami, K.; Takaishi, Y.; Tomimatsu, T.; Midzuta, Y.; Hara, Y.; Murakami, K.; Takaishi, Y.; Tomimatsu, T. 12-Keto steroidal glycosides from the caudex of Yucca gloriosa. Phytochemistry 1991, 30, 633–636.
- Kishor, N.; Sati, P.; Sakakibarat, J.; Kaiya, T. A spirostanol glycoside from Yucca aloifolia. Phytochemistry 1992, 31, 706–707.
- Kishor, N. A new molluscicidal spirostanol glycoside of Yucca aloifolia. J. Nat. Prod. 1990, 53, 1557–1559.
- Bahuguna, S.; Kishor, N.; Sati, O.P.; Sati, S.P.; Sakakibara, J.; Kaiya, T. A new spirostanol glycoside from Yucca aloifolia. J. Nat. Prod. 1991, 54, 863–865.
- Plock, A.; Beyer, G.; Hiller, K.; Gründemann, E.; Krause, E.; Nimtz, M.; Wray, V. Application of MS and NMR to the structure elucidation of complex sugar moieties of natural products: Exemplified by the steroidal saponin from Yucca filamentosa L. Phytochemistry 2001, 57, 489–496.
- Qu, L.; Ruan, J.; Wu, S.; Huang, P.; Yan, J.; Yu, H.; Zhang, Y.; Wang, T. Separation and bioactive assay of 25R/S-spirostanol saponin diastereomers from Yucca schidigera roezl (Mojave) stems. Molecules 2018, 23, 2562.
- Kemertelidze, E.; Benidze, M.; Skhirtladze, A. Steroidal glycosides from the leaves of Yucca gloriosa L. Bull. Georg. Natl. Acad. Sci. 2011, 5, 158–163.
- Nakano, K.; Matsuda, E.; Tsurumi, K.; Yamasaki, T.; Murakami, K.; Takaishi, Y.; Tomimatsu, T. The steroidal glycosides of the flowers of Yucca gloriosa. Phytochemistry 1988, 27, 3235–3239.
- Favel, A.; Kemertelidze, E.; Benidze, M.; Fallague, K.; Regli, P. Antifungal activity of steroidal glycosides from Yucca gloriosa L. Phyther. Res. 2005, 19, 158–161.
- Kemertelidze, É.P.; Benidze, M.M.; Skhirtladze, A.V. Steroid compounds from Yucca gloriosa L. introduced into Georgia and their applications. Pharm. Chem. J. 2009, 43, 45–47.
- Jin, Y.-L.; Kuk, J.-H.; Oh, K.-T.; Kim, Y.-J.; Piao, X.-L.; Park, R.-D. A new steroidal saponin, yuccalan, from the leaves of Yucca smalliana. Arch. Pharm. Res. 2007, 30, 543–546.
- Eskander, J.; Sakka, O.K.; Harakat, D.; Lavaud, C. Steroidal saponins from the leaves of Yucca de-smetiana and their in vitro antitumor activity: Structure activity relationships through a molecular modeling approach. Med. Chem. Res. 2013, 22, 4877–4885.
- Nakano, K.; Yamasaki, T.; Imamura, Y.; Murakami, K.; Takaishi, Y.; Tomimatsu, T. The steroidal glycosides from the caudex of Yucca gloriosa. Phytochemistry 1989, 28, 1215–1217.
- Zhang, Y.; Zhang, Y.J.; Jacob, M.R.; Li, X.C.; Yang, C.R. Steroidal saponins from the stem of Yucca elephantipes. Phytochemistry 2008, 69, 264–270.
- Grishkovets, V.L.; Karpov, A.; Iksanovr, S.V.; Chirva, V.Y. A steroid glycoside from the seeds of Yucca macrocarpa. Chem. Nat. Compd. 1998, 34, 738–740.
- Sun, Y.; Wu, J.; Sun, X.; Huang, X.; Li, L.; Liu, Q.; Song, S. Steroids from the rhizome of Anemarrhena asphodeloides and their cytotoxic activities. Bioorg. Med. Chem. Lett. 2016, 26, 3081–3085.
- Baccou, J.C.; Lambert, F.; Sauvaire, Y. Spectrophotometric method for the determination of total steroidal sapogenin. Analyst 1977, 102, 458–465.
- Wang, Y.; McAllister, T.A. A modified spectrophotometric assay to estimate deglycosylation of steroidal saponin to sapogenin by mixed ruminal microbes. J. Sci. Food Agric. 2010, 90, 1811–1818.
- Sastre, F.; Ferreira, F.; Pedreschi, F. TLC fingerprint of phenolics and saponins in commercial extracts of Yucca schidigera Roezl. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 698–701.
- Zhao, Y.; McCauley, J.; Pang, X.; Kang, L.; Yu, H.; Zhang, J.; Xiong, C.; Chen, R.; Ma, B. Analytical and semipreparative separation of 25 (R/S)-spirostanol saponin diastereomers using supercritical fluid chromatography. J. Sep. Sci. 2013, 36, 3270–3276.
- El Sayed, A.M.; Basam, S.M.; El-Naggar, E.-M.B.A.; Marzouk, H.S.; El-Hawary, S. LC–MS/MS and GC–MS profiling as well as the antimicrobial effect of leaves of selected Yucca species introduced to Egypt. Sci. Rep. 2020, 10, 17778–17792.
- Tenon, M.; Feuillère, N.; Roller, M.; Birtić, S. Rapid, cost-effective and accurate quantification of Yucca schidigera Roezl. steroidal saponins using HPLC-ELSD method. Food Chem. 2017, 221, 1245–1252.
- Sastre, F.; Ferreira, F.; Pedreschi, F. MALDI-TOF mass spectrometry and reversed-phase HPLC-ELSD chromatography for structural and quantitative studies of major steroid saponins in commercial extracts of Yucca schidigera Roezl. J. Pharm. Biomed. Anal. 2016, 120, 270–282.
- Ruan, J.; Qu, L.; Zhao, W.; Gao, C.; Huang, P.; Zheng, D.; Han, L.; Yu, H.; Zhang, Z.; Zhang, Y.; et al. Identification and Structural Analysis of Spirostanol Saponin from Yucca schidigera by Integrating Silica Gel Column Chromatography and Liquid Chromatography/Mass Spectrometry Analysis. Molecules 2020, 25, 3848.
- Sastre, F.; Ferreira, F.; Pedreschi, F. A systematic approach for the chromatographic fractionation and purification of major steroid saponins in commercial extracts of Yucca schidigera Roezl. J. Chromatogr. B 2017, 1046, 235–242.




