
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mário Bezerra | + 5477 word(s) | 5477 | 2021-08-18 11:28:50 | | | |
| 2 | Lily Guo | Meta information modification | 5477 | 2021-09-30 03:13:09 | | |
Video Upload Options
Tree nuts are considered an important food in healthy diets. However, for part of the world’s population, they are one of the most common sources of food allergens causing acute allergic reactions that can become life-threatening. They are part of the Big Eight food groups which are responsible for more than 90% of food allergy cases in the United States, and within this group, almond allergies are persistent and normally severe and life-threatening. Almond is generally consumed raw, toasted or as an integral part of other foods. Its dietary consumption is generally associated with a reduced risk of cardiovascular diseases. Several almond proteins have been recognized as allergens. Six of them, namely Pru du 3, Pru du 4, Pru du 5, Pru du 6, Pru du 8 and Pru du 10, have been included in the WHO-IUIS list of allergens. Nevertheless, further studies are needed in relation to the accurate characterization of the already known almond allergens or putative ones and in relation to the IgE-binding properties of these allergens to avoid misidentifications.
1. Introduction
2. Almond
2.1. Almond Allergy
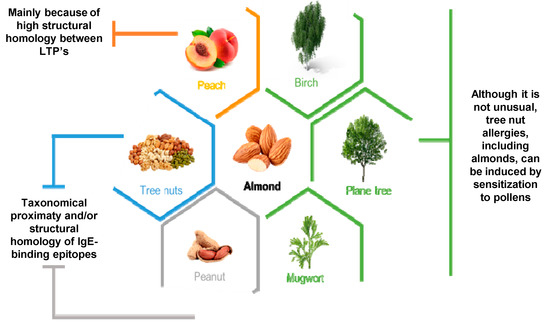
2.2. Almond Allergens
| Allergen | Biochemical Name | WHO-IUIS | Isoallergen and Variants | GenBank Nucleotide | UniProt | Biological Function | MW (kDa) | Processing | Clinical Relevance | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Pru du 3 | non-specific Lipid Transfer Protein 1 nsLTP1 | Yes (2009) | Pru du 3.0101 | FJ652103 | C0L0I5 | Non-specific lipid transfer protein (nslTP1) and plant defense proteins against pathogens | 9 | Very resistant to pH, thermal and enzyme treatments | Systemic and life-threatening symptoms; cross reactivity among Rosaceae fruit | [58] |
| Pru du 4 | Profilin | Yes (2006) | Pru du 4.0101 Pru du 4.0102 |
AY081850 AY081852 |
Q8GSL5 Q8GSL5 |
Actin-binding protein for cellular function | 14 | Unstable during heat processing | Mild symptoms and mainly in oral cavity | [48] |
| Pru du 5 | 60S acidic ribosomal protein P2 | Yes (2007) | Pru du 5.0101 | DQ836316 | Q8H2B9 | Protein synthesis | 10 | Unknown | Unknown | [59] |
| Pru du 6 | Amandin, 11S globulin legumin-like protein | Yes (2010) | Pru du 6.0101 Pru du 6.0201 |
GU059260 GU059261 |
E3SH28 E3SH29 |
Major storage protein | 360 | Stable to dry heat but can be denatured by boiling | Severe IgE allergic reactions | [60] |
| Pru du 8 | Antimicrobial seed storage protein | Yes (2018) | Pru du 8.0101 | MH922028 | A0A516F3L2 | Antimicrobial and seed storage function | 31 | Unknown | Unknown | [61] |
| Pru du 10 | Mandelonitrile lyase 2 | Yes (2019) | Pru du 10.0101 | AF412329.1 | Q945K2 | Highly efficient catalytical enzyme | 60 | Resistant to enzyme digestion | Unknown | [62][63] |
| Pru du γ-conglutin | Cupin superfamily | No | _______ | _______ | _______ | 7S vicilin storage protein | 45 for each subunit | Unknown | Unknown | [64] |
| Pru du 1 | PR-10 protein | No | _______ | _______ | _______ | Plant pathogenic and stress response | 17 | Wet heat processing reduces IgE reactivity | Unknown | [65] |
| Pru du 2 | PR-5/thaumatin-like protein | No | _______ | _______ | _______ | Pathogenic response | 23–27 | Resistant to protease, pH or heat treatment | Unknown | [66] |
| Pru 2S albumin | Prolamin super family | No | _______ | _______ | _______ | Seed storage protein | 12 | Stable to heat treatment | Unknown | [64] |
2.2.1. WHO/IUIS Designated Almond Allergens
Pru du 6 (Amandin)
Pru du 5 (60S Acidic Ribossomal Protein P2)
Pru du 3 (nsLTP)
Pru du 4 (Profilins)
Pru du 8
Pru du 10
2.2.2. Allergens Not Included in the WHO/IUIS Allergen List
Pru du γ-Conglutin
Pru du 1-PR-10 Protein (Pathogenesis Related-10 Protein)
Pru du 2 (PR-5/Thaumatin-Like Protein)
Pru du 2S Albumin
2.3. Methods for Almond Allergens Detection
| Kit 1 | Assay Time | Assay Type | LOD (ppm) | LOQ (ppm) |
Company |
|---|---|---|---|---|---|
| ELISA-based | |||||
| MonoTrace ELISA kit | 40 min | Monoclonal antibody-based ELISA | 0.15 | 1 | BioFront Technologies, Tallahassee, FL, USA |
| SENSISpec ELISA almond | 75 min | Sandwich enzyme immunoassay | 0.2 | 0.4 | Eurofins Technologies, Budapest, Hungary |
| RIDASCREEN FAST Mandel/Almond |
50 min | Polyclonal antibody specifically for almond protein detection, sandwich ELISA |
0.1 | 2.5 | R-Biopharm AG, Madrid, Spain |
| AgraQuant® Plus Almond | 30 min | Sandwich enzyme-linked immunosorbent assay | 0.5 | 1 | Romer Labs®, Getzersdorf, Austria |
| LFD-based | |||||
| AgraStrip® Almond | 11 min | Lateral flow device | 2 | __________ | Romer Labs®, Getzersdorf, Austria |
| Reveal 3-D Almond Test | 10 min | Lateral flow device | 5 | __________ | Neogen Corp., Lansing, MI, USA |
| Lateral Flow Almond incl. Hook Line 2 | 10 min | Lateral flow device | 1 | __________ | R-Biopharm AG, Madrid, Spain |
References
- Gupta, R.S.; Springston, E.E.; Warrier, M.R.; Smith, B.; Kumar, R.; Pongracic, J.; Holl, J.L. The Prevalence, Severity, and Distribution of Childhood Food Allergy in the United States. Pediatrics 2011, 128, e9–e17.
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prev-alence and severity of food allergies among US adults. JAMA Netw. Open 2019, 2, e185630.
- Kumfer, A.M.; Commins, S.P. Primary prevention of food allergy. Curr. Allergy Asthma Rep. 2019, 19, 7.
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58.
- Moore, L.E.; Stewart, P.H.; Deshazo, R.D. Food Allergy: What We Know Now. Am. J. Med. Sci. 2017, 353, 353–366.
- Sánchez-García, S.; Del Río, P.R.; Escudero, C.; Martínez-Gómez, M.J.; Ibáñez, M.D. Possible eosin-ophilic esophagitis induced by milk oral immunotherapy. J. Allergy Clin. Immunol. 2012, 129, 1155–1157.
- Varshney, P.; Steele, P.H.; Vickery, B.P.; Bird, J.A.; Thyagarajan, A.; Scurlock, A.M.; Perry, T.T.; Jones, S.M.; Burks, A.W. Adverse reactions during peanut oral immunotherapy home dosing. J. Allergy Clin. Immunol. 2009, 124, 1351–1352.
- Zhang, Y.; Jin, T. Almond allergens: Update and perspective on identification and characterization. J. Sci. Food Agric. 2020, 100, 4657–4663.
- Costa, J.; Mafra, I.; Carrapatoso, I.; Oliveira, B. Almond Allergens: Molecular Characterization, Detection, and Clinical Relevance. J. Agric. Food Chem. 2012, 60, 1337–1349.
- Mandalari, G.; Mackie, A.R. Almond Allergy: An Overview on Prevalence, Thresholds, Regulations and Allergen Detection. Nutrients 2018, 10, 1706.
- Rehm, C.D.; Drewnowski, A. Replacing American snacks with tree nuts increases consumption of key nutrients among US children and adults: Results of an NHANES modeling study. Nutr. J. 2017, 16, 17.
- Bottone, A.; Montoro, P.; Masullo, M.; Pizza, C.; Piacente, S. Metabolomics and antioxidant activity of the leaves of Prunus dulcis Mill. (Italian cvs. Toritto and Avola). J. Pharm. Biomed. Anal. 2018, 158, 54–65.
- Nanos, G.D.; Kazantzis, I.; Kefalas, P.; Petrakis, C.; Stavroulakis, G.G. Irrigation and harvest time affect almond kernel quality and composition. Sci. Hortic. 2002, 96, 249–256.
- Piscopo, A.; Romeo, F.; Petrovicova, B.; Poiana, M. Effect of the harvest time on kernel quality of several almond varieties (Prunus dulcis (Mill.) D.A. Webb). Sci. Hortic. 2010, 125, 41–46.
- de Giorgio, D.; Leo, L.; Zacheo, G.; Lamascese, N. Evaluation of 52 almond (Prunus amygdalusBatsch) cultivars from the Apulia region in Southern Italy. J. Hortic. Sci. Biotechnol. 2007, 82, 541–546.
- FAOSTAT. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#home (accessed on 10 October 2020).
- Griel, A.E.; Kris-Etherton, P.M. Tree nuts and the lipid profile: A review of clinical studies. Br. J. Nutr. 2006, 96, S68–S78.
- Jenkins, D.J.A.; Hu, F.B.; Tapsell, L.C.; Josse, A.; Kendall, C.W.C. Possible Benefit of Nuts in Type 2 Diabetes. J. Nutr. 2008, 138, 1752S–1756S.
- Mandalari, G.; Tomaino, A.; Arcoraci, T.; Martorana, M.; Turco, V.L.; Cacciola, F.; Rich, G.; Bisignano, G.; Saija, A.; Dugo, P.; et al. Characterization of polyphenols, lipids and dietary fibre from almond skins (Amygdalus communis L.). J. Food Compos. Anal. 2010, 23, 166–174.
- Richardson, D.P.; Astrup, A.; Cocaul, A.; Ellis, P. The nutritional and health benefits of almonds: A healthy food choice. Food Sci. Technol. Bull. Funct. Foods 2009, 6, 41–50.
- E Berryman, C.; Preston, A.G.; Karmally, W.; Deckelbaum, R.J.; Kris-Etherton, P. Effects of almond consumption on the reduction of LDL-cholesterol: A discussion of potential mechanisms and future research directions. Nutr. Rev. 2011, 69, 171–185.
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids--food sources and health benefits. Roczniki Państwowego Zakładu Higieny 2014, 65, 65.
- Mandalari, G.; Genovese, T.; Bisignano, C.; Mazzon, E.; Wickham, M.; Di Paola, R.; Bisignano, G.; Cuzzocrea, S. Neuroprotective effects of almond skins in experimental spinal cord injury. Clin. Nutr. 2011, 30, 221–233.
- Seo, K.; Lee, D.; Kang, H.; Kim, H.; Kim, Y.; Baek, N.; Lee, D. Hepatoprotective and neuroprotective tocopherol analogues isolated from the peels of Citrus unshiuMarcovich. Nat. Prod. Res. 2014, 29, 571–573.
- Tian, H.; Zhang, H.; Zhan, P.; Tian, F. Composition and antioxidant and antimicrobial activities of white apricot almond (Amygdalus communis L.) oil. Eur. J. Lipid Sci. Technol. 2011, 113, 1138–1144.
- Cabrita, L.; Apostolova, E.; Neves, A.; Marreiros, A.; Leitão, J. Genetic diversity assessment of the almond (Prunus dulcis (Mill.) D.A. Webb) traditional germplasm of Algarve, Portugal, using molecular markers. Plant Genet. Resour. 2014, 12, S164–S167.
- Monastra, F.; Raparelli, E. Inventory of almond research, germplasm and references. In REUR Technical Series; FAO: Rome, Italy, 1997.
- Bargman, T.R.J.; Rupnow, J.O.H.; Taylor, S.T.L. IgE-binding proteins in almonds (Prunus amygdalus); identi-fication by immunoblotting with sera from almond-allergic adults. J. Food Sci. 1992, 57, 717–720.
- Bolling, B.W.; Dolnikowski, G.; Blumberg, J.B.; Chen, C.-Y.O. Polyphenol content and antioxidant activity of California almonds depend on cultivar and harvest year. Food Chem. 2010, 122, 819–825.
- Summo, C.; Palasciano, M.; De Angelis, D.; Paradiso, V.M.; Caponio, F.; Pasqualone, A. Evaluation of the chemical and nu-tritional characteristics of almonds (Prunus dulcis (Mill). DA Webb) as influenced by harvest time and cultivar. J. Sci. Food Agric. 2018, 98, 5647–5655.
- Ewan, P.W. Clinical study of peanut and nut allergy in 62 consecutive patients: New features and associations. BMJ 1996, 312, 1074–1078.
- Sicherer, S.H.; Muñoz-Furlong, A.; A Sampson, H. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: A 5-year follow-up study. J. Allergy Clin. Immunol. 2003, 112, 1203–1207.
- Geiselhart, S.; Hoffmann-Sommergruber, K.; Bublin, M. Tree nut allergens. Mol. Immunol. 2018, 100, 71–81.
- Weinberger, T.; Sicherer, S. Current perspectives on tree nut allergy: A review. J. Asthma Allergy 2018, 11, 41.
- Kim, S.R.; Park, H.J.; Park, K.H.; Lee, J.-H.; Park, J.-W. IgE Sensitization Patterns to Commonly Consumed Foods Determined by Skin Prick Test in Korean Adults. J. Korean Med. Sci. 2016, 31, 1197–1201.
- McWilliam, V.; Koplin, J.; Lodge, C.; Tang, M.; Dharmage, S.; Allen, K.J. The Prevalence of Tree Nut Allergy: A Systematic Review. Curr. Allergy Asthma Rep. 2015, 15, 1–13.
- Segura, L.R.; Pérez, E.F.; Nowak-Wegrzyn, A.; Siepmann, T.; Larenas-Linnemann, D. Food allergen sensitization pat-terns in a large allergic population in Mexico. Allergol. Immunopathol. 2020, 48, 553–559.
- Rentzos, G.; Johanson, L.; Goksör, E.; Telemo, E.; Lundbäck, B.; Ekerljung, L. Prevalence of food hypersensitivity in relation to IgE sensitisation to common food allergens among the general adult population in West Sweden. Clin. Transl. Allergy 2019, 9, 22.
- Stiefel, G.; Anagnostou, K.; Boyle, R.; Brathwaite, N.; Ewan, P.; Fox, A.T.; Huber, P.; Luyt, D.; Till, S.J.; Venter, C.; et al. BSACI guideline for the diagnosis and management of peanut and tree nut allergy. Clin. Exp. Allergy 2017, 47, 719–739.
- Cheng, C.-W.; Lin, Y.-C.; Nong, B.-R.; Liu, P.-Y.; Huang, Y.-F.; Lu, L.-Y.; Lee, H.-S. Nut sensitization profile in Southern Taiwan. Immunol. Infect. 2020, 53, 791–796.
- Ben Kayale, L.; Ling, J.; Henderson, E.; Carter, N. The influence of cultural attitudes to nut exposure on reported nut allergy: A pilot cross sectional study. PLoS ONE 2020, 15, e0234846.
- Byrne, A.M.; Malka-Rais, J.; Burks, A.W.; Fleischer, D.M. How do we know when peanut and tree nut allergy have resolved, and how do we keep it resolved? Clin. Exp. Allergy 2010, 40, 1303–1311.
- Albin, S.; Nowak-Węgrzyn, A.J.I.; Clinics, A. Potential treatments for food allergy. Immunol. Allergy Clin. 2015, 35, 77–100.
- Kulis, M.; Vickery, B.; Burks, A.W. Pioneering immunotherapy for food allergy: Clinical outcomes and modulation of the immune response. Immunol. Res. 2011, 49, 216–226.
- Egger, M.; Hauser, M.; Mari, A.; Ferreira, F.; Gadermaier, G. The Role of Lipid Transfer Proteins in Allergic Diseases. Curr. Allergy Asthma Rep. 2010, 10, 326–335.
- Noble, K.A.; Liu, C.; Sathe, S.K.; Roux, K.H. A Cherry Seed-Derived Spice, Mahleb, is Recognized by Anti-Almond Antibodies Including Almond-Allergic Patient IgE. J. Food Sci. 2017, 82, 1786–1791.
- Lee, S.-H.; Benmoussa, M.; Sathe, S.K.; Roux, K.H.; Teuber, S.S.; Hamaker, B.R. A 50 kDa Maize γ-Zein Has Marked Cross-Reactivity with the Almond Major Protein. J. Agric. Food Chem. 2005, 53, 7965–7970.
- Tawde, P.; Venkatesh, Y.P.; Wang, F.; Teuber, S.S.; Sathe, S.K.; Roux, K.H. Cloning and characterization of profilin (Pru du 4), a cross-reactive almond (Prunus dulcis) allergen. J. Allergy Clin. Immunol. 2006, 118, 915–922.
- de Leon, M.; Drew, A.; Glaspole, I.; Suphioglu, C.; O’Hehir, R.; Rolland, J. IgE cross-reactivity between the major peanut allergen Ara h 2 and tree nut allergens. Mol. Immunol. 2007, 44, 463–471.
- Maleki, S.J.; Teuber, S.S.; Cheng, H.; Chen, D.; Comstock, S.S.; Ruan, S.; Schein, C.H. Computationally predicted IgE epitopes of walnut allergens contribute to cross-reactivity with peanuts. Allergy 2011, 66, 1522–1529.
- Wallowitz, M.; Teuber, S.; Beyer, K.; Sampson, H.; Roux, K.; Sathe, S.; Wang, F.; Robotham, J. Cross-reactivity of walnut, cashew, and hazelnut legumin proteins in tree nut allergic patients. J. Allergy Clin. Immunol. 2004, 113, S156.
- Flinterman, A.E.; Hoekstra, M.O.; Meijer, Y.; Van Ree, R.; Akkerdaas, J.H.; Bruijnzeel-Koomen, C.A.; Knulst, A.C.; Pasmans, S.G. Clinical reactivity to hazelnut in children: Association with sensitization to birch pollen or nuts? J. Allergy Clin. Immunol. 2006, 118, 1186–1189.
- Vieths, S.; Scheurer, S.; BALLMER-WEBER, B. Current understanding of cross-reactivity of food allergens and pollen. Ann. N. Y. Acad. Sci. 2002, 964, 47–68.
- Hasegawa, M.; Inomata, N.; Yamazaki, H.; Morita, A.; Kirino, M.; Ikezawa, Z. Clinical Features of Four Cases with Cashew Nut Allergy and Cross-Reactivity between Cashew Nut and Pistachio. Allergol. Int. 2009, 58, 209–215.
- Noorbakhsh, R.; Mortazavi, S.A.; Sankian, M.; Shahidi, F.; Tehrani, M.; Azad, F.J.; Behmanesh, F.; Varasteh, A. Pistachio Allergy-Prevalence and In vitro Cross-Reactivity with Other Nuts. Allergol. Int. 2011, 60, 425–432.
- Asero, R. 7 Lipid Transfer Protein Cross-reactivity Assessed In Vivo and In Vitro in the Office: Pros and Cons. J. Investig. Allergol. Clin. Immunol. 2011, 21, 129.
- KewalRamani, A.; Maleki, S.; Cheng, H.; Teuber, S. Cross-Reactivity Among Almond, Peanut and Other Tree Nuts in Almond Allergic Patients. J. Allergy Clin. Immunol. 2006, 117, S32.
- Buhler, S.; Tedeschi, T.; Faccini, A.; Garino, C.; Arlorio, M.; Dossena, A.; Sforza, S. Isolation and full characterisation of a potentially allergenic lipid transfer protein (LTP) in almond. Food Addit. Contam. Part A 2015, 32, 648–656.
- Abou Alhasani, M.; Roux, K.H. cDNA Cloning, expression and characterization of an allergenic 60s ribosomal protein of almond (Prunus dulcis). Iran. J. Allergy Asthma Immunol. 2009, 8, 77–84.
- Willison, L.N.; Tripathi, P.; Sharma, G.; Teuber, S.S.; Sathe, S.K.; Roux, K.H. Cloning, Expression and Patient IgE Reactivity of Recombinant Pru du 6, an 11S Globulin from Almond. Int. Arch. Allergy Immunol. 2011, 156, 267–281.
- Che, H.; Zhang, Y.; Jiang, S.; Jin, T.; Lyu, S.-C.; Nadeau, K.C.; McHugh, T. Almond (Prunus dulcis) Allergen Pru du 8, the First Member of a New Family of Food Allergens. J. Agric. Food Chem. 2019, 67, 8626–8631.
- Yao, L.; Li, H.; Yang, J.; Li, C.; Shen, Y. Purification and characterization of a hydroxynitrile lyase from Amygdalus pedunculata Pall. Int. J. Biol. Macromol. 2018, 118, 189–194.
- De Angelis, E.; Bavaro, S.L.; Forte, G.; Pilolli, R.; Monaci, L. Heat and Pressure Treatments on Almond Protein Stability and Change in Immunoreactivity after Simulated Human Digestion. Nutrients 2018, 10, 1679.
- Poltronieri, P.; Cappello, M.; Dohmae, N.; Conti, A.; Fortunato, D.; Pastorello, E.; Ortolani, C.; Zacheo, G. Identification and characterisation of the IgE-binding proteins 2S albumin and conglutin γ in almond (Prunus dulcis) seeds. Int. Arch. Allergy Immunol. 2002, 128, 97–104.
- Fernandes, H.; Michalska, K.; Sikorski, M.; Jaskolski, M. Structural and functional aspects of PR-10 proteins. FEBS J. 2013, 280, 1169–1199.
- Liu, J.-J.; Sturrock, R.; Ekramoddoullah, A.K.M. The superfamily of thaumatin-like proteins: Its origin, evolution, and expression towards biological function. Plant Cell Rep. 2010, 29, 419–436.
- Albillos, S.M.; Jin, T.; Howard, A.; Zhang, Y.; Kothary, M.H.; Fu, T.-J. Purification, Crystallization and Preliminary X-ray Characterization of Prunin-1, a Major Component of the Almond (Prunus dulcis) Allergen Amandin. J. Agric. Food Chem. 2008, 56, 5352–5358.
- Albillos, S.M.; Menhart, N.; Fu, T.-J. Structural Stability of Amandin, a Major Allergen from Almond (Prunus dulcis), and Its Acidic and Basic Polypeptides. J. Agric. Food Chem. 2009, 57, 4698–4705.
- Roux, K.H.; Teuber, S.S.; Robotham, J.M.; Sathe, S.K. Detection and Stability of the Major Almond Allergen in Foods. J. Agric. Food Chem. 2001, 49, 2131–2136.
- Sathe, S.K.; Wolf, W.J.; Roux, K.H.; Teuber, S.S.; Venkatachalam, M.; Sze-Tao, K.W.C. Biochemical Characterization of Amandin, the Major Storage Protein in Almond (Prunus dulcis L.). J. Agric. Food Chem. 2002, 50, 4333–4341.
- Venkatachalam, M.; Teuber, S.S.; Roux, K.H.; Sathe, S.K. Effects of Roasting, Blanching, Autoclaving, and Microwave Heating on Antigenicity of Almond (Prunus dulcis L.) Proteins. J. Agric. Food Chem. 2002, 50, 3544–3548.
- Mandalari, G.; Rigby, N.M.; Bisignano, C.; Curto, R.B.L.; Mulholland, F.; Su, M.; Venkatachalam, M.; Robotham, J.M.; Willison, L.N.; Lapsley, K.; et al. Effect of food matrix and processing on release of almond protein during simulated digestion. LWT 2014, 59, 439–447.
- Holden, L.; Sletten, G.B.; Lindvik, H.; Fæste, C.K.; Dooper, M.M. Characterization of IgE binding to lupin, peanut and al-mond with sera from lupin-allergic patients. Int. Arch. Allergy Immunol. 2008, 146, 267–276.
- Mills, E.C.; Sancho, A.; Moreno, J.; Kostyra, H. The effects of food processing on allergens. In Managing Allergens in Food; Elsevier: Amsterdam, The Netherlands, 2007; pp. 117–133.
- Marcus, J.P.; Green, J.L.; Goulter, K.C.; Manners, J.M. A family of antimicrobial peptides is produced by processing of a 7S globulin protein in Macadamia integrifolia kernels. Plant J. 1999, 19, 699–710.
- Garino, C.; De Paolis, A.; Coïsson, J.D.; Arlorio, M. Pru du 2S albumin or Pru du vicilin? Comput. Biol. Chem. 2015, 56, 30–32.
- Kolivas, S.; Gayler, K.R. Structure of the cDNA coding for conglutin γ, a sulphur-rich protein from Lupinus angusti-folius. Plant Mol. Biol. 1993, 21, 397–401.
- Burks, A.W.; Williams, L.W.; Helm, R.M.; Connaughton, C.; Cockrell, G.; O’Brien, T. Identification of a major peanut allergen, Ara h I, in patients with atopic dermatitis and positive peanut challenges. J. Allergy Clin. Immunol. 1991, 88, 172–179.
- Burks, A.W., Jr.; Brooks, J.R.; Sampson, H.A. Allergenicity of major component proteins of soybean determined by enzyme-linked immunosorbent assay (ELISA) and immunoblotting in children with atopic dermatitis and positive soy challenges. J. Allergy Clin. Immunol. 1988, 81, 1135–1142.
- Wang, F.; Robotham, J.M.; Teuber, S.S.; Tawde, P.; Sathe, S.K.; Roux, K.H. Ana o 1, a cashew (Ana-cardium occidental) allergen of the vicilin seed storage protein family. J. Allergy Clin. Immunol. 2002, 110, 160–166.
- Scarafoni, A.; Consonni, A.; Pessina, S.; Balzaretti, S.; Capraro, J.; Galanti, E.; Duranti, M. Structural basis of the lack of endoglucanase inhibitory activity of Lupinus albus γ-conglutin. Plant Physiol. Biochem. 2016, 99, 79–85.
- Mittag, D.; Akkerdaas, J.; Ballmer-Weber, B.K.; Vogel, L.; Wensing, M.; Becker, W.-M.; Koppelman, S.J.; Knulst, A.C.; Helbling, A.; Hefle, S.L.; et al. Ara h 8, a Bet v 1–homologous allergen from peanut, is a major allergen in patients with combined birch pollen and peanut allergy. J. Allergy Clin. Immunol. 2004, 114, 1410–1417.
- Scala, E.; Alessandri, C.; Palazzo, P.; Pomponi, D.; Liso, M.; Bernardi, M.L.; Ferrara, R.; Zennaro, D.; Santoro, M.; Rasi, C. IgE recognition patterns of profilin, PR-10, and tropomyosin panallergens tested in 3,113 allergic patients by allergen microar-ray-based technology. PLoS ONE 2011, 6, e24912.
- Chen, L.; Zhang, S.; Illa, E.; Song, L.; Wu, S.; Howad, W.; Arús, P.; Van de Weg, E.; Chen, K.; Gao, Z. Genomic characterization of putative allergen genes in peach/almond and their synteny with apple. BMC Genom. 2008, 9, 543.
- Palacin, A.; Tordesillas, L.; Gamboa, P.; Sanchez-Monge, R.; Cuesta-Herranz, J.; Sanz, M.; Barber, D.; Salcedo, G.; Díaz-Perales, A. Characterization of peach thaumatin-like proteins and their identification as major peach allergens. Clin. Exp. Allergy 2010, 40, 1422–1430.
- Roux, K.H.; Teuber, S.S.; Sathe, S.K. Tree Nut Allergens. Int. Arch. Allergy Immunol. 2003, 131, 234–244.
- Shewry, P.R.; Napier, J.A.; Tatham, A.S. Seed storage proteins: Structures and biosynthesis. Plant Cell 1995, 7, 945.
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958.
- Moreno, F.J.; Clemente, A. 2S Albumin Storage Proteins: What Makes them Food Allergens? Open Biochem. J. 2008, 2, 16–28.
- Clemente, A.; Chambers, S.J.; Lodi, F.; Nicoletti, C.; Brett, G.M. Use of the indirect competitive ELISA for the detection of Brazil nut in food products. Food Control. 2004, 15, 65–69.
- Koppelman, S.J.; Wensing, M.; Ertmann, M.; Knulst, A.C.; Knol, E. Relevance of Ara h1, Ara h2 and Ara h3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h2 is the most important peanut allergen. Clin. Exp. Allergy 2004, 34, 583–590.
- Nicolaou, N.; Poorafshar, M.; Murray, C.; Simpson, A.; Winell, H.; Kerry, G.; Härlin, A.; Woodcock, A.; Ahlstedt, S.; Custovic, A. Allergy or tolerance in children sensitized to peanut: Prevalence and differentiation using component-resolved diagnostics. J. Allergy Clin. Immunol. 2010, 125, 191–197.e113.
- Palmer, G.W.; Dibbern, D.A., Jr.; Burks, A.W.; Bannon, G.A.; Bock, S.A.; Porterfield, H.S.; McDermott, R.A.; Dreskin, S.C. Comparative potency of Ara h 1 and Ara h 2 in immunochemical and functional assays of allergenicity. Clin. Immunol. 2005, 115, 302–312.
- Jackson, L.S.; Al-Taher, F.M.; Moorman, M.; DeVRIES, J.W.; Tippett, R.; Swanson, K.M.J.; Fu, T.-J.; Salter, R.; Dunaif, G.; Estes, S.; et al. Cleaning and Other Control and Validation Strategies To Prevent Allergen Cross-Contact in Food-Processing Operations. J. Food Prot. 2008, 71, 445–458.
- Wang, X.; Young, O.; Karl, D. Evaluation of Cleaning Procedures for Allergen Control in a Food Industry Environment. J. Food Sci. 2010, 75, T149–T155.
- Pafundo, S.; Gulli, M.; Marmiroli, N. SYBR® GreenER™ Real-Time PCR to detect almond in traces in processed food. Food Chem. 2009, 116, 811–815.
- Van Hengel, A.J. Food allergen detection methods and the challenge to protect food-allergic consumers. Anal. Bioanal. Chem. 2007, 389, 111–118.
- Hoffmann-Sommergruber, K. Proteomics and its impact on food allergy diagnosis. EuPA Open Proteom. 2016, 12, 10–12.
- Piras, C.; Roncada, P.; Rodrigues, P.; Bonizzi, L.; Soggiu, A. Proteomics in food: Quality, safety, microbes, and allergens. Proteomics 2015, 16, 799–815.
- Johnson, P.E.; Baumgartner, S.; Aldick, T.; Bessant, C.; Giosafatto, V.; Heick, J.; Mamone, G.; O’connor, G.; Poms, R.; Pop-ping, B. Current perspectives and recommendations for the development of mass spectrometry methods for the determination of allergens in foods. J. AOAC Int. 2011, 94, 1026–1033.
- Monaci, L.; De Angelis, E.; Montemurro, N.; Pilolli, R. Comprehensive overview and recent advances in proteomics MS based methods for food allergens analysis. TrAC Trends Anal. Chem. 2018, 106, 21–36.
- Bignardi, C.; Elviri, L.; Penna, A.; Careri, M.; Mangia, A. Particle-packed column versus silica-based monolithic column for liquid chromatography–electrospray-linear ion trap-tandem mass spectrometry multiallergen trace analysis in foods. J. Chromatogr. A 2010, 1217, 7579–7585.
- Lupinek, C.; Wollmann, E.; Baar, A.; Banerjee, S.; Breiteneder, H.; Broecker, B.M.; Bublin, M.; Curin, M.; Flicker, S.; Garmatiuk, T.; et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: The MeDALL allergen-chip. Methods 2014, 66, 106–119.
- Santos, A.F.; Lack, G. Basophil activation test: Food challenge in a test tube or specialist research tool? Clin. Transl. Allergy 2016, 6, 1–9.
- Ebo, D.; Bridts, C.H.; Hagendorens, M.; Aerts, N.E.; De Clerck, L.S.; Stevens, W.J. Basophil activation test by flow cytometry: Present and future applications in allergology. Cytom. Part B Clin. Cytom. 2008, 74, 201–210.
- Duan, L.; Celik, A.; Hoang, J.A.; Schmidthaler, K.; So, D.; Yin, X.; Ditlof, C.M.; Ponce, M.; Upton, J.E.; Lee, J.; et al. Basophil activation test shows high accuracy in the diagnosis of peanut and tree nut allergy: The Markers of Nut Allergy Study. Allergy 2021, 76, 1800–1812.





