Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Weikuan Gu | + 1163 word(s) | 1163 | 2021-09-26 05:20:11 | | | |
| 2 | Camila Xu | Meta information modification | 1163 | 2021-09-30 04:29:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gu, W. Anti-PD-1/PD-L1 to CTLA-4. Encyclopedia. Available online: https://encyclopedia.pub/entry/14750 (accessed on 12 January 2026).
Gu W. Anti-PD-1/PD-L1 to CTLA-4. Encyclopedia. Available at: https://encyclopedia.pub/entry/14750. Accessed January 12, 2026.
Gu, Weikuan. "Anti-PD-1/PD-L1 to CTLA-4" Encyclopedia, https://encyclopedia.pub/entry/14750 (accessed January 12, 2026).
Gu, W. (2021, September 29). Anti-PD-1/PD-L1 to CTLA-4. In Encyclopedia. https://encyclopedia.pub/entry/14750
Gu, Weikuan. "Anti-PD-1/PD-L1 to CTLA-4." Encyclopedia. Web. 29 September, 2021.
Copy Citation
PD-1 is an important factor in the normal immune response to prevent autoimmunity.
cancer
clinical predictor
drug
hazard ratio
lung
smoking
1. Introduction
Traditionally, it has been recognized that smoking increases the risk for enhanced disease progression and affects the efficacy of treatment in patients with non-small cell lung cancer (NSCLC). Data from the Centers for Disease Control and Prevention (CDC) show that smoking is linked to about 80% to 90% of lung cancers (https://www.cdc.gov/cancer/lung/basic_info/risk_factors.htm, accessed on 22 April 2020). In a prior analysis of patients treated for NSCLC, we found that non-smokers had a lower risk of 0·16 HR compared to those who had ever smoked, with only a single study demonstrating conflicting results [1]. However, recent results from several large clinical trials of programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1) inhibitors showed that smokers had lower HR values than non-smokers [2][3][4][5]. Wallis et al. (2019), while conducting a meta-analysis on the association between smoking and survival of patients having benefited from immunotherapy in advanced malignancies, concluded that there was no difference between smokers and non-smokers in response to immunotherapy. In the same sense, Mo et al. (2020) reported that smokers benefited from either anti-PD-1/PD-L1 monotherapy or the combined regimen compared with chemotherapy. These contrasting data raise the question of the mechanism underlying the differential response of smokers treated with PD-1/PD-L1 and potentially other immunotherapy drugs.
PD-1 is an important factor in the normal immune response to prevent autoimmunity. Currently, clinical trials have reported results from five drugs in this category: two anti-PD-1 drugs (Nivolumab and Pembrolizumab) and three anti-PD-L1 drugs (Atezolizumab, Durvalumab, and Avelumab). Nivolumab, a fully humanized monoclonal antibody against PD-1, has shown a survival benefit in a number of cancers, including malignant melanoma [6], NSCLC [7], advanced renal cell carcinoma [8], and other types of cancers [9]. Pembrolizumab was the first anti-PD-1 antibody to be approved by the US Food and Drug Administration (FDA) for the treatment of patients with unresectable or metastatic melanoma with disease progression following Ipilimumab. It has also been used for the treatment of NSCLC [10] and other cancers. Atezolizumab is a PD-L1 inhibitor used in cancer therapy with a focus on bladder and NSCLC [11]. Durvalumab is reported as a selective, high-affinity, human IgG1 monoclonal antibody that blocks PD-L1. It has been used for the treatment of NSCLC [12] and urothelial carcinoma [13]. Avelumab is a promising new therapeutic agent for patients with metastatic Merkel cell carcinoma that has also been used in the treatment of NSCLC [14].
CTLA-4 is the second molecule of the immune checkpoint that has been targeted by monoclonal drugs [15]. Data on the status of smokers in response to the anti-CTLA-4 drug treatment are available. The anti-CTLA drug, Ipilimumab was efficient intreating NSCLC [16][17]. Both anti-PD-1/PD-L1 and CTLA-4 are also cell surface molecules [16][17][18][19][20][21]. The question is whether smokers respond better to both checkpoint drugs and their combination.
2. Smoking as a Favorite Predictor for Drugs of Anti PD-1/PD-L1, MUC1, CTLA-4
The effects of smoking on treatment outcome data were analyzed with a meta-analysis for two major categories of drugs, immune checkpoint inhibitors (anti-PD-1/PD-L1 and CTLA-4 drugs) and other drugs including MUC1, EGFR, and angiogenesis inhibitors. Diseases treated with anti-PD-1 and anti-PD-L1 drugs included NSCLC and other types of cancers. Data analyzed for other drugs were only from NSCLC patients. To examine the effect of only anti-PD-1 and anti-PD-L1, within the data of anti-PD-1 and anti-PD-L1 drugs, a sub-group analysis was conducted based on the treatment matrix, including monotherapy versus combination treatment and maintenance therapy. To examine the effect of combination between anti-PD-1/PD-L1 and CTLA-4 drugs, patients’ responses to the combination treatment between drugs from these two types of checkpoint drugs were analyzed.
Our review is based on published results from clinical trials from PubMed, PMC/MEDLINE, and Scopus. Inclusion criteria were reported in clinical trials with different smoking statuses. Table 1 shows data from a set of 36 clinical trials including 13 on NSCLC and 6 on other cancers treated with PD-1/PD-L1 drugs, 5 on cancer patients treated with anti-CTLA-4 drugs, 2 treated with anti-MUC1 drugs, and 10 with anti-VEGF drugs.
Table 1. Additional data collected from clinical trials.
| Drugs/First Author | Drug Comparison | Subgroup Analysis | Current/# Patients |
Former/# Patients | Never/# Patients |
Overall/# Patients |
Note |
|---|---|---|---|---|---|---|---|
| Pembrolizumab/Shaverdian (50) | KEYNOTE-001 phase 1 trial Former smokers vs non-smokers |
Progression-free survival (PFS) | 0.60 (0.38–0.95) vs. 1 * | ||||
| Any previous radiotherapy and PFS | 0.78 (0.47–1.31) vs. 1 * | ||||||
| Previous extracranial radiotherapy and PFS | 0.82 (0.49–1.37) vs. 1 * | ||||||
| Pembrolizumab/Reck (51) | Update on KEYNOTE-024 vs. Chemotherapy |
Subgroup analysis of overall survival (OS) | 0.81 (0.41–1.60)/65 | 0.59 (0.41–0.85)/216 | 0.90 (0.11–7.59)/24 | 0.63 (0.47–0.86)/305 | All are PD-L1 expression on at least 50% of tumor cells |
| Nivolumab/Lee (52) | To patients failed prior platinum-based chemotherapy | Objective response rates | 15/78 (19.2) | 5/22 (22.7) | PD-L1 status not known. | ||
| Nivoluma/Dumenil (37) | Prospectively and treated by nivolumab in two French academic hospitals | PFS | 1 (1.00–1.00) * | 0.85(0.45–1.58) | 1.93 (0.79–4.68) | Previously not included because of patients less than 100 | |
| OS | 1 (1.00–1.00) * | 1.09 (0.58–2.05) | 2.15 (0.89–5.22) | ||||
| Durvalumab/ Sridhar (Sridhar et al., 2019) | Study 1108/ durvalumab monotherapy | Adjusted HR for OS | Ever versus Never: 0.85 by Cox Proportional Hazards Model (Cox Model) | - | - | ||
| HR for | Ever versus Never: 0.85 (OS by Cox Model, Including PD-L1/LM Subgroups) | - | - | ||||
| HR for PFS by | Ever versus Never: 0.66 (Cox Model, Including PD-L1/LM Subgroups) | - | - | ||||
| ATLANTIC /Durvalumab as third-line or later treatment (53) | Adjusted HR for OS | Ever versus Never: 1.67 (by Cox Model) | - | - | |||
| HR for OS | Ever versus Never: 1.67 (by Cox Model, Including PD-L1/LM Subgroups) | ||||||
| HR for PFS | Ever versus Never: 1.08 (by Cox Model, Including PD-L1/LM Subgroups) | - | - | ||||
* Assumed values based on information from the study.
For anti-PD-1/PD-L1 drugs, the mean HR value from the smoking subgroups in these trials was 0.702; non-smoking subgroups was 0.848. When we combined the anti-PD-1/PD-L1 and anti-CTLA-4 drugs, the mean HR values of smokers and non-smokers were 0.735 and 0.882, respectively. When we combined the anti-PD-1/PD-L1, anti-CTLA-4 drugs, and anti-MUC1 drugs, the mean HR values of smokers and non-smokers were 0.751 and 1.016, respectively. The p-values for comparison between smokers and non-smokers for data of three types of drugs, two types of drugs, and anti-PD-1/PD-L1 drugs only are 0.0325, 0.0299, and 0.0685, respectively (Figure 1A). The meta-analysis indicated that the smokers have an HR value of 0.023 lower than that of the non-smokers (Figure 1B) with heterogeneity of I2 = 0% and p < 0.001. In contrast, for anti-VEGF drugs, the mean HR value from the smoking subgroups in these trials was 0.868; the mean HR value from the non-smoking subgroup subgroups was 0.654, with a p-value of 0.0013 (Figure 1C). The meta-analysis indicated that the smokers have an HR value of 0.194 higher that of the non-smokers (Figure 1D), again with heterogeneity of I2 = 0% and p < 0.001. These data suggest a significant difference in the response to treatment between smokers and no-smokers when different drugs were used. Accordingly, a detailed description and subgroup and stratified meta-analysis were conducted to further analyze the difference between smokers and non-smokers for these drugs.
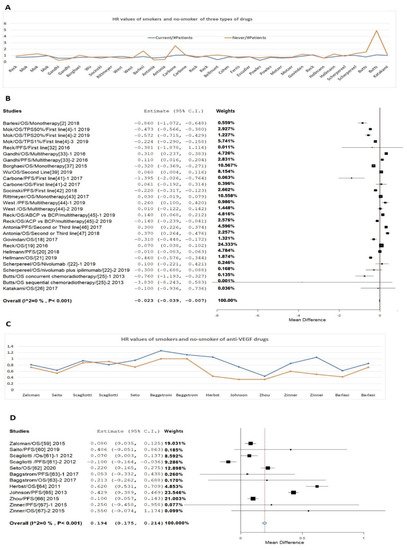
Figure 1. HR values of patients treated with different drugs. (A) HR data from three types of drugs (anti-PD-1/PD-L1, anti-CTLA-4 drug, and anti-MUC1 drug). (B) Meta-analysis of HR for these three types of drugs. (C) HR data from Bevacizumab on NSCLC between smokers and non-smokers. (D) Meta-analysis of HR Bevacizumab on NSCLC between smokers and non-smokers.
References
- Wang, L.; Cao, Y.; Ren, M.; Chen, A.; Cui, J.; Sun, D.; Gu, W. Sex Differences in Hazard Ratio during Drug Treatment of Non-small-cell Lung Cancer in Major Clinical Trials: A Focused Data Review and Meta-analysis. Clin. Ther. 2017, 39, 34–54.
- Barlesi, F.; Vansteenkiste, J.; Spigel, D.; Ishii, H.; Garassino, M.; de Marinis, F.; Ozguroglu, M.; Szczesna, A.; Polychronis, A.; Uslu, R.; et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): An open-label, randomised, phase 3 study. Lancet Oncol. 2018, 19, 1468–1479.
- Ng, T.L.; Liu, Y.; Dimou, A.; Patil, T.; Aisner, D.L.; Dong, Z.; Jiang, T.; Su, C.; Wu, C.; Ren, S.; et al. Predictive value of oncogenic driver subtype, programmed death-1 ligand (PD-L1) score, and smoking status on the efficacy of PD-1/PD-L1 inhibitors in patients with oncogene-driven non-small cell lung cancer. Cancer 2019, 125, 1038–1049.
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830.
- Calles, A.; Liao, X.; Sholl, L.M.; Rodig, S.J.; Freeman, G.J.; Butaney, M.; Lydon, C.; Dahlberg, S.E.; Hodi, F.S.; Oxnard, G.R.; et al. Expression of PD-1 and Its Ligands, PD-L1 and PD-L2, in Smokers and Never Smokers with KRAS-Mutant Lung Cancer. J. Thorac. Oncol. 2015, 10, 1726–1735.
- Sundar, R.; Cho, B.C.; Brahmer, J.R.; Soo, R.A. Nivolumab in NSCLC: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2015, 7, 85–96.
- Zarrabi, K.; Wu, S. An evaluation of nivolumab for the treatment of metastatic renal cell carcinoma. Expert Opin. Biol. Ther. 2018, 18, 695–705.
- Amraee, A.; Evazi, M.R.; Shakeri, M.; Roozbeh, N.; Ghazanfarpour, M.; Ghorbani, M.; Ansari, J.; Darvish, L. Efficacy of nivolumab as checkpoint inhibitor drug on survival rate of patients with relapsed/refractory classical Hodgkin lymphoma: A meta-analysis of prospective clinical study. Clin. Transl. Oncol. 2019, 21, 1093–1103.
- Li, J.; Guo, S.; Wang, L.; Zhuang, T.Z.; Ren, M.; Cho, W.; Gu, W.; Wu, S. Attention on Smoking Status to PD-1/PD-L1 Drugs on Non-Small Lung Cancer Patients. J. Cancer Sci. Clin. Ther. 2019, 3, 178–185.
- Dang, T.O.; Ogunniyi, A.; Barbee, M.S.; Drilon, A. Pembrolizumab for the treatment of PD-L1 positive advanced or metastatic non-small cell lung cancer. Expert Rev. Anticancer Ther. 2016, 16, 13–20.
- Krishnamurthy, A.; Jimeno, A. Atezolizumab: A novel PD-L1 inhibitor in cancer therapy with a focus in bladder and non-small cell lung cancers. Drugs Today 2017, 53, 217–237.
- Mezquita, L.; Planchard, D. Durvalumab in non-small-cell lung cancer patients: Current developments. Future Oncol. 2018, 14, 205–222.
- Faiena, I.; Cummings, A.L.; Crosetti, A.M.; Pantuck, A.J.; Chamie, K.; Drakaki, A. Durvalumab: An investigational anti-PD-L1 monoclonal antibody for the treatment of urothelial carcinoma. Drug Des. Dev. Ther. 2018, 12, 209–215.
- Cordes, L.M.; Gulley, J.L. Avelumab for the treatment of metastatic Merkel cell carcinoma. Drugs Today 2017, 53, 377–383.
- Jie, H.B.; Schuler, P.J.; Lee, S.C.; Srivastava, R.M.; Argiris, A.; Ferrone, S.; Whiteside, T.L.; Ferris, R.L. CTLA-4(+) Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer Res. 2015, 75, 2200–2210.
- Govindan, R.; Szczesna, A.; Ahn, M.J.; Schneider, C.P.; Gonzalez Mella, P.F.; Barlesi, F.; Han, B.; Ganea, D.E.; Von Pawel, J.; Vladimirov, V.; et al. Phase III Trial of Ipilimumab Combined With Paclitaxel and Carboplatin in Advanced Squamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2017, 35, 3449–3457.
- Reck, M.; Luft, A.; Szczesna, A.; Havel, L.; Kim, S.W.; Akerley, W.; Pietanza, M.C.; Wu, Y.L.; Zielinski, C.; Thomas, M.; et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 3740–3748.
- Hellmann, M.D.; Ciuleanu, T.E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018, 378, 2093–2104.
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031.
- Scherpereel, A.; Mazieres, J.; Greillier, L.; Lantuejoul, S.; Do, P.; Bylicki, O.; Monnet, I.; Corre, R.; Audigier-Valette, C.; Locatelli-Sanchez, M.; et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): A multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019, 20, 239–253.
- Schmidt, C. The benefits of immunotherapy combinations. Nature 2017, 552, S67–S69.
More
Information
Subjects:
Medicine, Research & Experimental
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
929
Revisions:
2 times
(View History)
Update Date:
30 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




