1. Introduction
Pain, particularly severe and chronic pain, constitutes a major public health problem with an enormous impact on both the individual and society. Successful pain management can be viewed as a combination of adequate analgesia and minimal unwanted side effects. However, currently available analgesics are either ineffective in a large proportion of patients or the benefit/risk ratio is suboptimal due to multiple and severe adverse effects
[1][2]. Pain medicine is currently one of the most rapidly developing medical specialties, with effective pain control being a therapeutic priority
[3][4]. Furthermore, comorbidity of chronic pain with mood (e.g., depression and anxiety) and addictive disorders (e.g., alcohol and drug abuse) in pain patients is well-documented
[4][5][6].
Strong opioids are the mainstay for the treatment of moderate to severe nociceptive pain, such as post-operative pain and pain due to major trauma. They are only third-line treatments for neuropathic pain
[1][7]. Over the past decades, there has been an increased focus on pain management, which has resulted in a societal escalation in opioid use and misuse, with opioids being presently the most prescribed medications in the USA
[2]. As a consequence, a dramatic growth in the number of opioid-related overdose deaths and diagnoses of opioid-use disorder associated with prescription and over-the-counter opioids was experienced in recent years
[8][9]. Therefore, pain medicine and analgesic drug discovery are intensively dedicated to the discovery of effective, safer and nonaddictive analgesic drugs
[9][10]. Diverse strategies are being evaluated to mitigate the deleterious side effects of opioids, ranging from abuse-deterrent formulations of existing opioids to peripherally restricted opioids, and from multifunctional drugs to biased agonists. A comprehensive review on such developments is beyond the scope of this mini-review, and we recommend extended and topical reviews and books
[11][12][13][14][15][16][17][18][19][20][21].
The therapeutic analgesia of opioid drugs is mediated by the opioid receptors
[22][23]. To date, four opioid receptors have been cloned, i.e., the mu (MOP), delta (DOP), kappa (KOP) and nociceptin (NOP) receptors. All opioid receptor types are G protein-coupled receptors (GPCRs) with seven transmembrane domains, which bind endogenous opioid ligands
[22][23]. A key milestone in the field was realized with the high-resolution crystal structures of all opioid receptors attained in active and inactive conformations
[24][25][26][27][28][29][30]. Furthermore, the present understanding of the opioid system’s function and signaling, together with significant information on the structural basis for the pharmacological profile of endogenous and exogenous ligands, is constantly growing with the crystal structures of the receptors being nowadays available
[31][32].
Historically, the MOP receptor has been of most clinical interest because it mediates the desired analgesic action of opioids, but also their detrimental effects, which include respiratory depression, constipation, sedation and, with prolonged treatment, tolerance, dependence and abuse liability
[7][22][23][33]. The MOP receptor is the endogenous target of naturally occurring peptides, while it is also responsible for the analgesic effect of morphine, structurally derived compounds and other opioid drugs. Morphine, the natural alkaloid from the poppy plant,
Papaver somniferum, has been used for decades for pain relief, and its addictive and other side effects are well-known
[34]. Since its structure elucidation in 1925 by Gulland and Robinson
[35] and first synthesis of morphine in 1956 by Gates and Tschudi
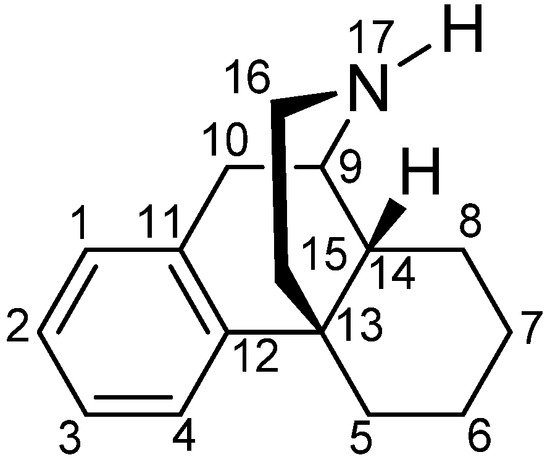
[36], research has been constantly focused on finding a non-narcotic opioid drug. The skeleton of morphine is a nitrogen-containing phenanthrene derivative belonging to the class of morphinans (
Figure 1). One of the first ‘nonaddictive morphine substitutes’ to be introduced was the 3,6-diacetylated derivative, diacetylmorphine (heroin), but the claims for reduced respiratory depression and dependence liability were soon shown to be ill-founded
[37]. Numerous chemical efforts have contributed to the design and synthesis of various derivatives of morphine that are used as therapeutics and/or important research tools (
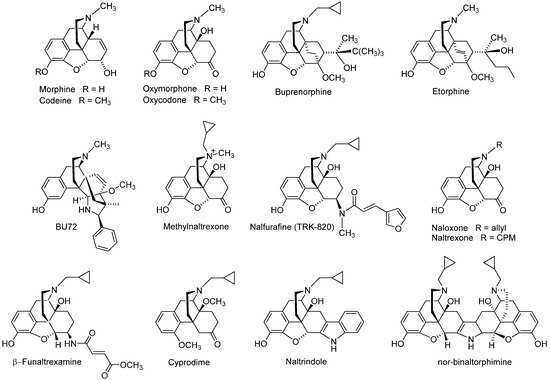
Figure 2), with references to comprehensive literature reviewed over the years
[38][39][40][41][42][43][44][45][46][47][48][49][50].
Figure 1. The (−)-morphinan skeleton.
Figure 2. Examples of opioid morphinans used in the clinics or as research tools. CPM, cyclopropylmethyl.
Clinically used opioid analgesics from the class of morphinans comprise naturally occurring alkaloids (e.g., morphine, codeine), semi-synthetic derivatives (e.g., oxycodone, oxymorphone, buprenorphine) and synthetic analogues (e.g., levorphanol, butorphanol, etorphine) (
Figure 2). Such drugs proved to be of the utmost significance for the treatment of moderate to severe nociceptive pain as a result of their binding and agonist action at the MOR, while they all share the same general pharmacological profile, including undesired side effects
[51][52]. A KOP receptor agonist from the class of morphinans, nalfurafine (
Figure 2)
[53], was originally developed as an analgesic drug for post-operative pain, but the safety margin was found to be insufficient for analgesic use
[54]. Because of nalfurafine’s anti-scratching/anti-itching effects in animals and humans, it is clinically used in Japan for the treatment of uremic pruritus in individuals with chronic kidney disease undergoing hemodialysis
[55][56].
Development of opioid antagonists with a morphinan structure was also reported, exemplified by the non-selective antagonists naloxone and naltrexone, the MOP receptor selective antagonists β-funaltrexamine and cyprodime, the DOP receptor selective antagonists naltrindole and the KOP receptor selective antagonist nor-binaltophimine (
Figure 2)
[41][48][57][58]. Whereas antagonists were initially employed as pharmacological tools in opioid research, there has been attention in generating selective antagonists as potential pharmacotherapies for the treatment of human disorders where the opioid system (opioid receptors and their ligands) plays an important role. For example, naloxone and naltrexone are in clinical practice as treatments for narcotic overdose and alcoholism or opioid abuse/dependence
[48][57][59]. The quaternary analogue of naltrexone, methylnaltrexone (
Figure 2), a peripheral MOP antagonist, is used as a medication for the treatment of opioid-induced constipation
[60]. The MOP selective antagonist β-funaltrexamine and agonist BU-72 (
Figure 2) were used to elucidate the X-ray crystal structures of the MOP receptor in inactive
[25] and active conformations
[28], respectively, whereas the crystal inactive structure of the DOP receptor bound to the antagonist, naltrindole (
Figure 2) was reported
[24].
This review summarizes recent chemical and pharmacological developments in the area of opioid analgesics through exploring the morphinan scaffold in the search of safer pain therapeutics. We particularly focus on the structure–activity (SAR) relationships of differently substituted 14-oxygenated morphinans, including design strategies, synthetical procedures and pharmacology.
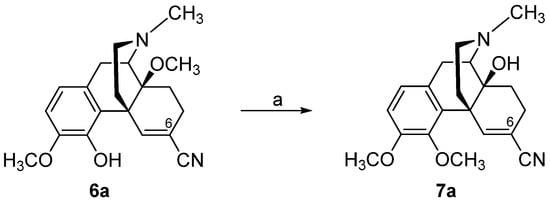
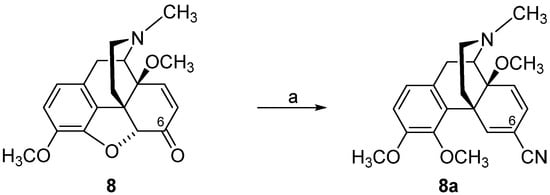
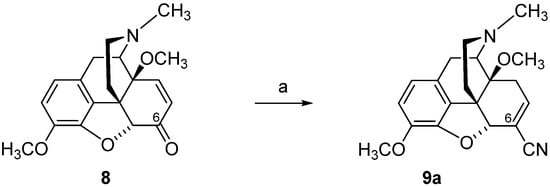
3. Modifications in Position 14 of 14-Oxygenated-N-methylmorphinan-6-ones: Design, Synthesis and SAR Studies
The 14-position of natural opioids (e.g., morphine and codeine, see
Figure 2) are unsubstituted; however, synthetic approaches have uncovered that functionalizing position 14 gives rise to a broad array of activities. Diverse modifications in position 14 of the morphinan skeleton were targeted by us and others with the prospect of designing novel effective opioid analgesics, with fewer unwanted adverse effects
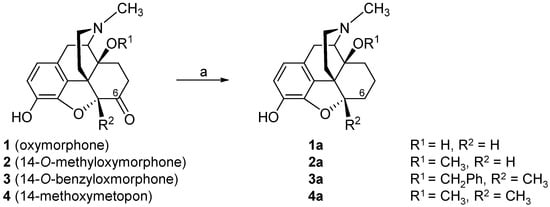
[43][44][45][46][47][48][85][86][87]. We have reported on the substitution of the hydroxyl group in position 14 in the clinically used MOP agonist oxymorphone (
1) with a methoxy group leading to 14-
O-methyloxymorphone (
2,
Scheme 1)
[88]. Introduction of a 14-methoxy group in oxymorphone (
1) not only increased binding affinity and agonist potency at the MOP receptor, but also caused a significant improvement in the antinociceptive activity of 14-
O-methyloxymorphone (
2) in different pain models in rats and mice (for reviews, see
[47][48]). It was reported to exhibit about 40-fold higher antinociceptive potency than oxymorphone and was up to 400-fold more effective that the “gold standard” morphine. Unfortunately, 14-
O-methyloxymorphone (
2) induced the typical MOP-mediated side effects, including respiratory depression, physical dependence, constipation and motor dysfunction
[88][89][90][91].
Using 14-
O-methyloxymorphone (
2) as a lead compound, numerous series of differently substituted 14-alkoxymorphinan-6-ones were designed and their pharmacology was reported (for reviews, see
[44][46][47][48]). A notable example is 14-
O-benzyloxymorphone (
3), where the 14-methoxy group in
2 was replaced by a benzyloxy group
[89]. 14-
O-Benzyloxymorphone (
3) retained the high affinity in the subnanomolar at the MOP receptor of
2, while having increased MOP agonist potency in vitro and in vivo (
Table 1 and
Table 4). Furthermore, antinociceptive potency of derivative
3 was reported as 5-fold higher compared to
2 (
Table 1 and
Table 5), in addition to its limited inhibition of gastrointestinal motility in mice at antinociceptive doses. It exhibited 2.5-fold less constipation than morphine and was 7-fold less effective than
2 in this respect
[89].
Table 4. Binding affinities and agonist potencies of N-methylmorphinan-6-ones 2, 3 and 13.
| |
Binding Affinity (Ki, nM) a |
Agonist Activity (EC50, nM; %stim) b |
| MOP |
DOP |
KOP |
Ki ratios MOP/DOP/KOP |
MOP |
DOP |
KOP |
| 2 |
0.10 |
4.80 |
10.2 |
1/48/102 |
1.62; 97 |
43.8; 106 |
144; 65 |
| 3 |
0.12 |
2.14 |
1.18 |
1/18/10 |
0.32; 101 |
- c |
- |
| 13 |
0.073 |
0.13 |
0.30 |
1/1.8/4.1 |
0.082; 100 |
0.28; 91 |
0.38; 39 |
Table 5. Antinociceptive activities and gastrointestinal transit inhibition by N-methylmorphinan-6-ones 2, 3 and 13 and morphine.
| |
Antinociception a
(AD50 nmol/kg, s.c.) |
Gastrointestinal Transit b
(EC50 nmol/kg, s.c.) |
| 2 |
53 |
37 |
| 3 |
9.6 |
27 c |
| 13 |
0.70 |
1.70 |
| Morphine |
6690 |
3800
7622 c |
Using the crystal structure of the MOP receptor in the active conformation (PDB code: 5C1M)
[28], the first molecular modeling study aided by docking and molecular dynamics (MD) simulations reported on the binding modes and interaction pattern differences related to specific structural features of oxymorphone (
1) and 14-
O-methyl and 14-
O-benzyl substituted analogues
2 and
3, respectively
[92]. Similar to oxymorphone (
1), derivatives
2 and
3 bind at the MOP receptor and form a charged interaction with Asp
147 and a hydrogen bond to His
297 via a water network
[92]. The later interaction is recognized as a conserved interaction between morphinan ligands and the binding pocket of the MOP receptor
[25][28][66][93][94]. The presence of the 14-methoxy group in
2 does not change the ligand–receptor interaction profile compared to
1, as the polar contacts of
2 were comparable to those of
1 except for the hydrogen bond formed by the 14-hydroxyl group of
1 to the Asp
147 residue. Interesting were the docking results on the 14-benzyloxy substituted derivative
3, where the intermolecular hydrogen bonds were maintained, established by the comparison of the binding mode of this analogue to the docking solution of 14-methoxy analogue
2. An additional hydrophobic interaction to Ile
144 residue was described for
3 [92].
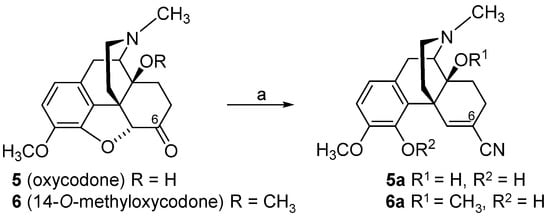
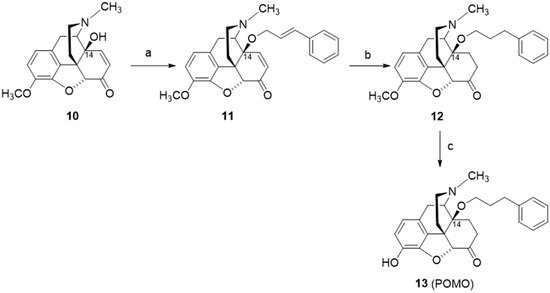
The design and synthesis of the 14-
O-phenylpropyl substituted analog of 14-
O-methyloxymorphone (
2), namely 14-
O-phenylpropyloxymorphone (POMO,
13) (
Scheme 6), as an interesting representative of the series of
N-methyl-14-arylalkoxy-morphinan-6-ones, was described
[91][95]. Starting from 14-hydroxycodeinone (
10), POMO was prepared in three synthetic steps (
Scheme 6)
[95].
Scheme 6. Synthetic route to POMO (13). Reagents and conditions: (a) cinnamyl bromide, NaH, DMF, 0 °C; (b) hydrogenation over Pd/C, glacial acetic acid, r.t., 40 psi; (c) 3-O-ether cleavage, refluxing 48% HBr solution.
Recent pharmacological investigations reported POMO (
13) as a potent mixed MOP/DOP/KOP receptor agonist with reduced propensity for constipation in mice after s.c. administration
[91]. An interesting SAR observation was revealed where replacing the 14-
O-methyl in
2 by a 14-
O-phenylpropyl moiety in
13 converted an MOP receptor selective ligand into a nonselective agonist (
Table 4). In vivo, POMO (
13) was highly effective in acute thermal nociception (hot-plate test) in mice after s.c. administration, with over 70- and 9000-fold increased potency than 14-
O-methyloxymorphone (
2) and morphine, respectively, while producing a four-fold lower inhibition of the gastrointestinal transit than
2 and morphine (
Table 5)
[91]. The pharmacological profile established for POMO (
13), as a ligand that can simultaneously bind and activate multiple opioid receptors, is of major relevance nowadays, with the design of bi- and multifunctional opioids becoming increasingly attractive as novel strategy for an effective and safer pain management
[16][18][85][87][96][97][98][99].
Other SAR observations were made in 4,5α-epoxypyridomorphinans, where the addition of a 3-phenylpropoxy substituent at C-14 on the framework containing a cyclopropylmethyl group on the morphinan nitrogen led to ligands with an MOP agonist/DOP antagonist profile, whereas
N-methyl-14-
O-phenylpropyl substituted analogues were reported as dual MOP/DOP agonists
[85]. Recently, the MOP/DOP agonist SRI-22141 was described to have efficacy with reduced tolerance and dependence in mouse models of neuropathic pain
[98].
4. Modifications in Position 5 of 14-Oxygenated-N-methylmorphinan-6-ones: Design, Synthesis and SAR Studies
Further chemical and synthetical efforts focused on structural modifications in position 5 of 14-methoxy-
N-methylmorphinan-6-ones with evolving compounds showing an interesting pharmacological profile, as potent antinociceptives with an improved side effect profile
[47][100]. Chemical derivatization of 14-
O-methyloxymorphone (
2) by introducing a methyl group in position 5 created 14-methoxymetopon (
4)
[101]. This new opioid morphinan retained the high subnanomolar affinity at the MOP receptor, shown by its 5-unsubstituted analogue
2, while DOP and KOP receptor affinities were reduced by two- to three-fold, causing an increase in the MOP receptor selectivity (
Table 6). It was also established that methylation in position 5 of
2 did not substantially alter MOP receptor agonist activity in vitro and in vivo. In the [
35S]GTPγS binding assays with CHO cells expressing human opioid receptors (
Table 6)
[94] and in the rat brain
[90], 14-methoxymetopon (
4) showed comparable MOP receptor agonist potency and equal efficacy to its analogue
2 (
Table 6). In vivo, the addition of a 5-methyl substituent in 14-
O-methyloxymorphone (
2) was well-tolerated, leading to a highly potent MOP analgesic
4, which was very effective in reducing pain response in various thermal, chemical and inflammatory pain models in mice, rats and dogs, after different routes of administration (
Table 7) (for a review, see
[100]). Numerous behavioral studies reported on the significantly reduced propensity of 14-methoxymotopon (
4) to induce MOP-mediated unwanted liabilities (i.e., respiratory depression, hypotension, bradycardia, constipation, analgesic tolerance, physical dependence, addiction) in animals (rodents and dogs) in comparison to conventional MOP analgesics, such as morphine and sufentanil (for a review, see
[100]).
Table 6. Binding affinities and agonist potencies of differently 5-substituted N-methylmorphinan-6-ones 2, 4, 17 and 21.
| |
Binding Affinity (Ki, nM) a |
Agonist Activity (EC50, nM; %stim) b |
| MOP |
DOP |
KOP |
Ki Ratios MOP/DOP/KOP |
MOP |
DOP |
KOP |
| 2 |
0.10 |
4.8 |
10.2 |
1/48/102 |
1.21; 95
23.7; 103 d |
38.5; 102 |
135; 65.6 |
| 4 |
0.15 |
13.3 |
25.2 |
1/89/168 |
2.66; 99
63.0; 99 d |
36.8; 100 |
181; 68.9 |
| 17 |
0.20 |
0.14 |
0.40 |
1/0.7/2 |
- c |
- |
- |
| 21 |
0.31 |
13.1 |
22.8 |
1/42/73 |
1.86; 95 e
13.8; 85 d |
31.7; 126 e |
116; 59.0 e |
Table 7. Antinociceptive potencies of differently 5-substituted N-methylmorphinan-6-ones 2, 4, 17 and 21.
| |
ED50 (µg/kg, s.c.) a |
| Hot-Plate Test |
Tail-Flick Test |
PPQ Abdominal Stretching Test |
| 2 |
17 |
14 |
- b |
| 4 |
30 |
30 |
9.0 |
| 17 |
0.10 |
0.08 |
0.16 |
| 21 |
53 |
43 |
- |
| Morphine |
850 |
1920 |
400 |
In view of the remarkable in vitro and in vivo functional profile of 14-methoxymetopon (
4), our interest in the field of opioid analgesics from the class of 14-alkoxymorphinan-6-ones was directed towards the design of analogues of
4. Two representative derivatives, including the 14-phenylpropoxy, 5-methyl substituted PPOM (
17) (
Scheme 7)
[102] and the 14-methoxy-5-benzyl substituted
21 (
Scheme 8)
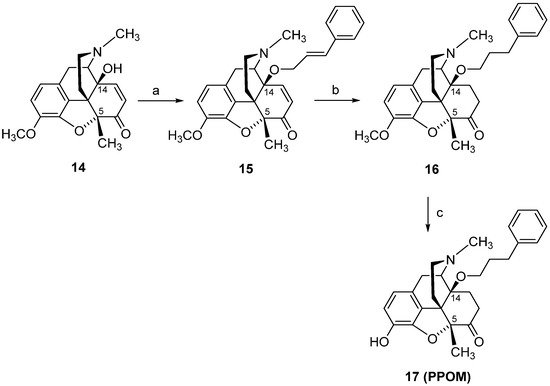
[90], are described herein. Starting from 14-hydroxy-5-methylcodeinone (
14)
[103], PPOM (
17) was prepared in three synthetic steps (
Scheme 7). The 5-benzyl substituted analogue
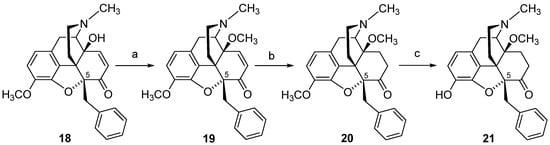
21 was also prepared in three synthetic steps from 5ß-benzyl-14-methoxycodeinone (
19), which is readily available from 5β-benzylthebaine (
18) (
Scheme 8)
[104].
Scheme 7. Synthetic route to PPOM (17). Reagents and conditions: (a) a mixture of 14, cinnamyl bromide, DMF, NaH, 0 °C; (b) a mixture of 15, EtOH, Pd/C, was hydrogenated at 50 °C for 2 h; (c) a mixture of 16 and 48% HBr was refluxed for 20 min, and after cooling to r.t., the mixture was treated with MeOH and evaporated to dryness.
Scheme 8. Synthetic route to 14-methoxy, 5-benzyl substituted 21. Reaction scheme for the synthesis of 5-benzyl substituted morphinans. Reagents and conditions: (a) NaH, (CH3O)2SO2, DMF, 0 °C; (b) 10% Pd/C, H2, MeOH, 30 psi, r.t.; (c) 48% HBr, reflux.
The phenylpropoxy substitution in position 14 of 14-methoxymetopon (
4) led to a new structure
17, with significant enhanced binding affinities at both DOP and KOP receptors, in the subnanomolar range, while the high MOP receptor affinity remained unchanged compared to
4, with the result in a complete loss of MOP receptor selectivity for PPOM (
17) (
Table 6)
[102]. Noteworthy are the behavioral findings in different pain models (hot-plate, tail-flick and writhing tests) in mice with PPOM (
17), evolving as an extremely potent agonist in vivo with a considerably improved antinociceptive potency compared to its 14-methoxy substituted
4 (up to 400-fold) and morphine (up to 24,000-fold) (
Table 7). PPOM (
17) also showed a superior antinociceptive activity compared to etorphine (
Figure 2) (up to 25-fold)
[102], an MOP agonist used for anesthesia in veterinary medicine of large animals and wildlife species
[105]. Such SAR observation on conversion of a selective MOP ligand into a multifunctional ligand, which displays activity at multiple opioid receptor subtypes, is very exciting in the light of the current research on analgesic drug discovery
[16][18][96][97].
The SAR outcome on the substitution of the 5-methyl group in 14-methoxymetopon (
4) by a benzyl group resulting in analogue
21 was investigated in order to understand the role of the substitution pattern in position 5 in
N-methylmorphinan-6-ones on the opioid activity in vitro and in vivo
[90]. In vitro, 5-benzyl substituted
21 showed a similar binding profile to the opioid receptors in the rodent brain to its 5-methyl analogue
4. Replacing the 5-methyl group in
4 by a benzyl group in
21 kept the high affinity in the subnanomolar range at the MOP receptor and the MOP selectivity (
Table 6). In the [
35S]GTPγS binding assays with membranes from the rat brain and CHO cells expressing human opioid receptors, the presence of a 5-benzyl group in
21 yielded a potent MOP full agonist with a 5-fold increased potency than its 5-methyl analogue
4 at the rat MOP receptor and similar potency at the human MOP receptor (
Table 6). A comparable in vitro MOP agonism was observed between the 5-benzyl analogue
21 and the 5-unsubstituted derivative 14-
O-methyloxymorphone (
2) (
Table 6). Pharmacological in vivo studies in mice also demonstrated that the substitution of the 5-methyl group in
4 by a benzyl group was well-tolerated, leading to a highly potent and efficacious MOP antinociceptive agent
21 [90]. Antinociceptive potency of
21 in mouse models of acute thermal nociception (hot-plate and tail-flick tests) after s.c. administration was comparable to that of 14-methoxymetopon (
4), whereas a significantly increased potency to morphine was reported (
Table 7)
[90]. Additional behavioral studies evaluated the consequences of the replacement of the 5-methyl group in
4 by the 5-benzyl group in
21 on motor coordination in mice using the rotarod assay. Whereas 14-methoxymetopon (
4), as well as its 5-unsubstituted analogue
2 and morphine, caused a significant motor deficit, the 5-benzyl substituted
21 did not affect the evoked locomotor activity of mice at doses producing a full antinociceptive efficacy
[90].
The importance of the substitution pattern in position 5 to the binding mode of differently 5-substituted
N-methylmorphinan-6 ones (
2,
4,
17 and
21) to the active conformation of the human MOP receptor (PDB code: 5C1M)
[28] was examined by molecular docking and MD stimulations
[92]. A 5-methyl group introduced during targeted derivatization resulted in enhanced hydrophobic ligand-receptor interactions, as the orientation of these derivatives relative to the receptor was influenced by this structural modification. It was also proposed that this might influence the orientation of further groups (i.e., at position 14), predicted to interact favorably with the MOP receptor. Notable were the in silico findings on the 14-phenylpropoxy-5-methyl substituted analogue POMO (
17). The in vitro and in vivo activity profiles of
17, as a high affinity MOP agonist and one of the most potent opioid analgesic drug (
Table 6 and
Table 7), were supported by docking of the ligand to the active MOP receptor. Compared to other derivatives, an additional hydrophobic region was seen for
17, embedding the phenyl group in this region formed by hydrophobic or aromatic residues (i.e., Trp
133, Val
143 and Ile
144)
[92]. In the SAR exploration on how the nature of the substituent at position 5 affects the ligand–MOP receptor interaction profile, the 5-H (
2), 5-methyl (
4) and 5-benzyl (
21)-substituted derivatives were directly compared. Although all ligands exhibit similar MOP binding affinities (
Table 6) and polar interactions with Asp
147 and with His
297 residues via a water network, other important ligand–receptor contacts were different or unexpected for
21 [92]. It was reported that
21, as a potent MOP agonist, interacts with Asp
147, albeit with reduced proneness, while the polar contact to the nearby Tyr
326 residue was formed rather frequently, which is a unique characteristic of
21. In MD stimulations, the 5-benzyl substituted
21 shows a remarkable tendency to form a hydrogen bond to Tyr
148 residue, by either the 3-phenol group, the 4,5α-epoxy group or both. It was also observed that the basic nitrogen reorients in an unexpected manner, moving toward Tyr
326 and away from the conserved Asp
147, the counterpart in the charge-enhanced hydrogen bond, which is (more or less) common among the other morphinans
[92].
5. Modifications in Position 17 of N-Methylmorphinan-6-ones: Design, Synthesis and SAR Studies
Over many years of research on morphine and structurally related derivatives, SAR analyses have assigned an important role to the N-substituent on the morphinan skeleton in defining the functional activity profile, extending from agonism to mixed agonism–antagonism to antagonism
[38][44]. Historically, exchanging the
N-methyl group in morphine by an allyl group generated the first compound to be reported as an opioid antagonist, nalorphine
[38]. Nalorphine, also known as
N-allylmorphine, was synthesized from morphine in 1942
[106]. Clinical studies showed that nalorphine reversed the analgesic and respiratory depressant effects of morphine and other narcotic analgesics, and precipitated the abstinence syndrome in morphine-dependent subjects
[42][43][47]. Nalorphine acts as an antagonist at the MOP receptor and a partial agonist to full agonist at the KOP receptor, being the first drug used for narcotic overdose treatment. However, it exhibits undesirable psychotomimetic side effects, such as dysphoria and visual hallucinations due to its KOP activity; therefore, it is no longer used medically
[43]. Further substitutions at the N-17 position were targeted with numerous compounds synthesized and pharmacologically characterized. In the 1960s, synthetical work on the replacement of the
N-methyl group in oxymorphone by
N-cycloproplymethyl or
N-allyl groups led to the discovery of naloxone and naltrexone (
Figure 2)
[39][107]. They are well-known, non-selective opioid antagonists used as therapeutic agents (for the treatment of opioid overdose and drug and alcohol dependence) and important research tools in opioid receptors pharmacology
[47][59][108].
Replacement of the
N-methyl group with a different substituent, the
N-phenethyl moiety, was also shown to influence the in vitro and in vivo functional profile of the opioid ligand. Among the first synthesized
N-phenethyl substituted compounds were
N-phenethylnormorphine
[109][110],
N-phenethylmorphine (
22b,
Figure 3)
[111],
N-phenethylnoroxymorphone
[112] and
N-phenethyloxymorphone (
1b,
Figure 3)
[113], which were found to be potent MOP agonists and effective antinociceptive agents with increased potency compared to their
N-methyl analogues
[94][114][115][116]. Furthermore, exchanging the
N-methyl group by an
N-phenethyl substituent in other opioid ligands, such as 5-phenylmorphans, led to MOP antagonists
[117][118]. A recent study reported on (-)-
N-phenethyl analogs of
N-norhydromorphone, with the
N-
p-chlorophenethylnorhydromorphone as a bifunctional MOP/DOP ligand with partial agonism at the MOP receptor and a full agonism at the DOP receptor, and antinociceptive efficacy without respiratory depression in squirrel monkeys after s.c. administration
[119].
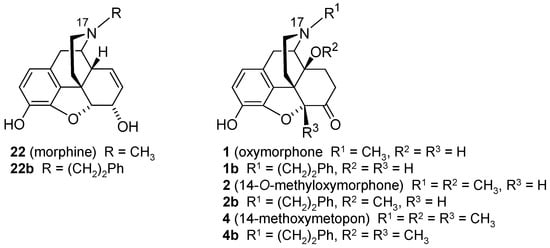
Figure 3. Structures of N-methylmorphinans morphine (22), oxymorphone (1), 14-O-methyoxymorphone (2) and 14-methoxymetopon (4), and their N-phenethyl counterparts 22b, 1b, 2b and 4b, respectively. Ph, phenyl.
The design of two
N-phenethyl substituted 14-methoxymorphinan-6-ones was recently reported
[94]. Based on the interesting pharmacological profile shown by 14-
O-methyloxymorphone (
2) and 14-methoxymetopon (
4), it was aimed at investigating the effect of the replacement of the
N-methyl group in
2 and
4, by an
N-phenethyl group in
2b and
4b, respectively, on binding and agonist in vitro activity at the opioid receptors and in vivo behavioral pharmacology
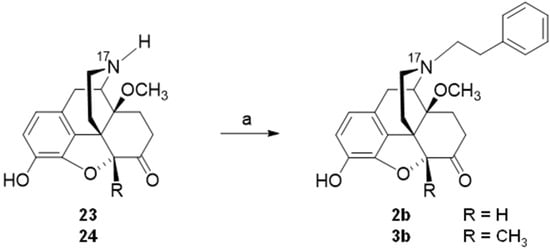
[94]. Starting from
23 (4,5α-epoxy-3-hydroxy-14-methoxy-
N-phenethylmorphinan-6-one) or
24 (4,5α-epoxy-3-hydroxy-14-methoxy-5-methyl-
N-phenethylmorphinan-6-one), compounds
2b and
3b, respectively, were prepared in one synthetic step (
Scheme 9)
[94].
Scheme 9. Synthetic route to N-phenethyl, 14-methoxy substituted 2b and 3b. Reagents and conditions: (a) phenethyl bromide, NaHCO3, DMF, 80 °C, 48 h.
Remarkable SAR observations were made for
N-methyl substituted
2 and
4 and their
N-phenethyl substituted counterparts
2b and
4b, and also extended to the
N-phenethyl analogues of morphine and oxymorphone,
22b and
1b, respectively (
Figure 3)
[94]. Whereas substitution of the
N-methyl group in morphine (
22b) and oxymorphone (
1) by an
N-phenethyl group enhances binding affinity, selectivity and agonist potency at the MOP receptor of
22b and
1b, the
N-phenethyl substitution in 14-methoxy-
N-methylmorphinan-6-ones (
2 and
4) converts selective MOP ligands into dual MOP/DOP agonists (
2b and
4b) (
Table 8 and
Table 9). On the other hand, the high antinociceptive potency of
N-methyl-14-methoxy substituted
2 and
4 in mice after s.c. administration was not affected by the presence of an
N-phenethyl substituent in
2b and
4b, with both compounds being very effective antinociceptive agents against acute thermal nociception (
Table 9)
[94]. In contrast, the replacement of the
N-methyl group in morphine (
22) and oxymorphone (
1) by an
N-phenethyl moiety produced a significant increase in antinociceptive potencies of analogues
22b and
1b (
Table 9)
[94][114][115][116]. Behavioral studies also demonstrated that the
N-phenethyl substituted
2b and
4b, with a dual MOP/DOP agonism, did not impair motor function of mice in the rotarod test at doses equivalent to the 4-fold the antinociceptive ED
50 dose
[94]. As noted earlier in this review, currently, there is a large interest in the discovery of multifunctional ligands, including bifunctional MOP/DOP ligands, expected to produce fewer undesirable side effects caused by selective MOP agonists
[16][18][96][97][98].
Table 8. Binding affinities at the opioid receptors of N-methylmorphinans 22, 1, 2 and 4, and their N-phenethyl substituted analogues 22b, 1b, 2b and 4b.
| |
Binding Affiniy Ki (nM) a |
| MOP |
DOP |
KOP |
Ki Ratios MOP/DOP/KOP |
| Morphine (22) |
3.35 |
195 |
96.4 |
1/58/29 |
| 22b |
0.25 |
24.5 |
93.5 |
1/98/374 |
| Oxymorphone (1) |
1.41 |
79.1 |
32.6 |
1/56/23 |
| 1b |
0.12 |
10.7 |
42.2 |
1/89/352 |
| 2 |
0.27 |
9.08 |
10.3 |
1/34/38 |
| 2b |
0.19 |
1.81 |
15.8 |
1/9.5/83 |
| 4 |
0.25 |
18.6 |
12.8 |
1/74/51 |
| 4b |
0.24 |
1.45 |
35.3 |
1/6.0/147 |
Table 9. In vitro agonist activities and antinociceptive of N-methylmorphinans 22, 1, 2 and 4, and their N-phenethyl substituted analogues 22b, 1b, 2b and 4b.
| |
In Vitro Agonist Activity a |
Antinocieption b |
| MOP |
DOP |
KOP |
ED50 mg/kg, s.c. |
| EC50 (nM) |
% stim. |
EC50 (nM) |
% stim. |
EC50 (nM) |
% stim. |
| >Morphine (22) |
34.4 |
89 |
668 |
109 |
710 |
76 |
3.06 |
| 22b |
10.3 |
113 |
712 |
138 |
1049 |
19 |
0.11 |
| Oxymorphone (1) |
7.80 |
92 |
259 |
87 |
463 |
48 |
0.35 |
| 1b |
2.67 |
97 |
131 |
101 |
225 |
7.5 |
0.15 |
| 2 |
1.21 |
95 |
38.5 |
102 |
135 |
65.9 |
0.014 |
| 2b |
1.26 |
98 |
9.34 |
107 |
144 |
35.4 |
0.014 |
| 4 |
2.66 |
99 |
36.8 |
100 |
181 |
68.9 |
0.024 |
| 4b |
1.86 |
102 |
9.54 |
103 |
334 |
51.3 |
0.024 |
Pharmacological findings in the series of
N-phenethyl substituted
22b,
1b,
2b and
4b and their
N-methyl analogues morphine (
22), oxymorphone (
1), 14-
O-methyoxymorphone (
2) and 14-methoxymetopon (
4) were supported by docking and MD simulations analysis using the active conformation of the MOP receptor (PDB code: 5C1M)
[28]. The recent report
[94] established that 14-methoxy substituted
N-methylmorphinan-6-one and their
N-phenethyl counterparts share several essential receptor–ligand interactions, but also interaction pattern differences related to specific structural features. The
N-methyl substituted morphine (
22) and oxymorphone (
1) showed a slightly different orientation in the binding pocket of the MOP receptor compared to their related
N-phenethyl analogues,
22b and
1b, respectively. This was not observed for pairs
2 vs.
2b and
4 vs.
4b, indicating that an increased MOP receptor affinity can be achieved by either a 14-methoxy or by an
N-phenethyl substitution, as key sites to be targeted in modulating the binding affinity and efficacy of morphinans at the MOP receptor
[94].