Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Modinat Tolani Lambo | + 1569 word(s) | 1569 | 2021-09-28 09:47:35 | | | |
| 2 | Lindsay Dong | + 696 word(s) | 2265 | 2021-09-29 05:30:38 | | | | |
| 3 | Lindsay Dong | + 696 word(s) | 2265 | 2021-09-29 05:31:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lambo, M. Multistrain Probiotics in Livestock Production. Encyclopedia. Available online: https://encyclopedia.pub/entry/14674 (accessed on 07 February 2026).
Lambo M. Multistrain Probiotics in Livestock Production. Encyclopedia. Available at: https://encyclopedia.pub/entry/14674. Accessed February 07, 2026.
Lambo, Modinat. "Multistrain Probiotics in Livestock Production" Encyclopedia, https://encyclopedia.pub/entry/14674 (accessed February 07, 2026).
Lambo, M. (2021, September 28). Multistrain Probiotics in Livestock Production. In Encyclopedia. https://encyclopedia.pub/entry/14674
Lambo, Modinat. "Multistrain Probiotics in Livestock Production." Encyclopedia. Web. 28 September, 2021.
Copy Citation
Multistrain probiotics comprise two or more species or strains of important microorganisms as a consortium beneficial to the administered animal. Several studies are being carried out to explore their potency or efficiency. They have proven to be a promising alternative to antibiotics growth promoters and were responsible for enhancing gut health, growth performance, maintaining a balance in gut microbiota, stimulating immunity against pathogenic organisms, improving digestion, and overall production efficiency in ruminants, poultry, and swine production.
gut microbes
feed additives
growth performance
cattle
chicken
pigs
1. Introduction
Probiotics preparations come in various forms, and their efficacy sometimes varies depending on whether they are mono- or multistrain. The new approach in probiotics utilization has been to use a combination of probiotics strains. This strategy is presumed to have highly influenced animal nutrition, exerted increased health benefits, and created an even more favorable balance of intestinal metabolism, animal welfare [1], and performance than single-strain cultures [2]. They can be administered via several routes (Figure 1), but the oral method is most common in animal husbandry.
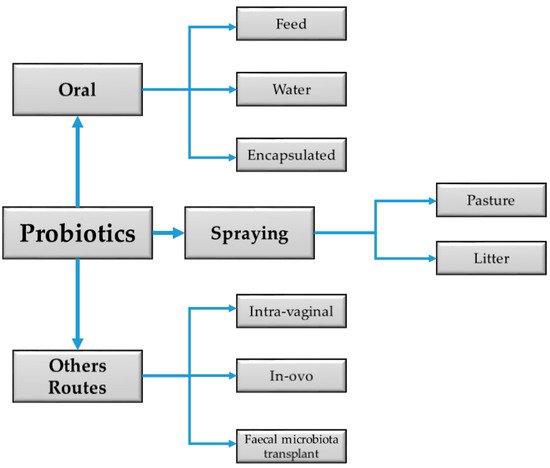
Figure 1. Diagram summarizing the common routes of administering probiotics in livestock.
2. Common Probiotic Strains and Their Mode of Action
Bacteria, bacteriophages, microalgae, and yeasts are all examples of probiotics [3]. Although numerous microorganisms have probiotic potential, Lactobacillus, Streptococcus, Enterococcus, Lactococcus, and Bifidobacteria remain the most commonly used probiotic agents in livestock to date [2][3][4]. Saccharomyces (S. cerevisiae and S. bourlardii), Candida pintolopesii, and Aspergillus oryzae are typical non-bacterial probiotics [4][5][6]. There are currently numerous commercially available mono- and multistrain probiotics [7].
Even though probiotics are considered a possible replacement for antibiotic growth promoters, their mode of action appears to be distinct [8]. Probiotics impacts are species-specific [9] and may also rely on the physiological and immunological condition of the administered animal. Different probiotics exert their benefits via mechanisms yet to be fully understood but are presumed to be related to their gastrointestinal lumen or wall activities. Their primary function results from the production of a range of antibacterial and bacteriostatic substances, such as organic acids, bacteriocins, diacetyl, antibiotics, and hydrogen peroxide [10], which exert beneficial effects through three primary pathways [11]:
(1) Competitive exclusion,
(2) Bacterial antagonism, and
(3) Immune system stimulation.
(2) Bacterial antagonism, and
(3) Immune system stimulation.
Probiotics also impact the health of the administered host via competition between beneficial bacteria and pathogens, replacement of pathogens by probiotic bacteria, and regulation of innate and adaptive immunity [12]. Due to their antagonistic effect, probiotics can hinder the growth of noxious bacteria by altering the gut microbiome, reduce the spread of pathogens and their emission during infection, decrease gut permeability, ameliorate clinical symptoms in livestock, boost immunity, and improve disease resistance and health [13][14][15]. In addition, they appear to be effective in foodborne pathogen reduction, for example, Salmonella, Escherichia coli, Campylobacter, Clostridium, Staphylococcus aureus, and perfringens [16][17], hence improving intestinal digestion and nutrient absorption and supporting a healthy micro ecological state. They can even aid pollution reduction by preventing the accumulation of harmful chemicals and lowering ammonia emissions in animal manure [18][19].
3. Multistrain Probiotic Use in Ruminants
Several studies have shown that probiotics can help increase milk quality, improve growth performance, increase average daily weight gain, improve feed efficiency, and reduce diarrhea in ruminants [20][21][22][23][24][25].
At the onset of diarrhea in dairy calves, a multispecies probiotic containing five bacteria strains (Bifidobacterium bifidum, Pediococcus acidilactici, Lactobacillus acidophilus, Lactobacillus casei, Enterococcus faecium), peptide extract, dead yeast extract, dried whey, an enzyme blend, and natural flavor rapidly resolved the condition by reducing the duration of symptoms. The calves’ daily weight gain improved with the combination as well [26]. Buffaloes supplemented with a multistrain probiotic-containing six bacterial strains (Streptococcus faecium, Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus reuteri, Lactobacillus lactis) and two yeast strains (Aspergillus oryzae, Saccharomyces cerevisiae) had no improvement with respect to body condition score and dry matter intake but had a higher average daily milk yield, and reduced feed conversion ratio [27].
Furthermore, Kembabazi et al. [28] discovered that a mixture of Lactobacilli plantarum and Saccharomyces cerevisiae could operate as a probiotic. According to the findings, the mechanism by which they exert their probiotic function involves producing a low and stable lactate concentration in the rumen, resulting in a low pH medium suitable for the activity of S. cerevisiae, which usually amplifies the rumen bacteria population and competes against starch-utilizing bacteria. Owing to the potentiality of yeast to regulate pH and scavenge oxygen, they limit lactate build-up, creating a more conducive habitat for the cellulolytic activity of bacteria. Therefore, resulting in enhanced fodder consumption [29] as indicated by improved dry matter intake in nursing dairy cows.
In another study, Olchowy et al. [30] top-dressed pasture with a liquid commercial probiotic product containing a mixture of multispecies constituting four bacteria strains (Lactobacillus rapi, Lactobacillus parafarraginis, Lactobacillus zeae, and Lactobacillus buchneri with a minimum concentration of each strain, 106 CFU/mL), Acetobacter fabarum (minimum concentration of 105 CFU/mL) and yeast from the environment (Candida ethanolica; minimum concentration of 106 CFU/mL). Based on the result, cows that grazed pasture treated with the product produced a significantly higher volume of milk and a higher quantity of milk protein with tendencies towards producing more milk fat. Similarly, when dairy cows were directly fed the pasture from paddocks treated with the same probiotic mixture, the treatment group still produced more milk and higher milk protein content than the control group. In addition, Deng et al. [31] used an intravaginal infusion to give transition dairy cows a lactic acid bacteria cocktail containing Lactobacillus sakei, P. acidilactici FUA3138, and P. acidilactici FUA3140 combinations around parturition. The result revealed lower non-esterified fatty acids, higher cholesterol, and higher lactate levels, indicating that the concentrations of specified metabolites in the blood serum of transition dairy cows had been altered. A summary of several other combinations used in cattle, sheep, and goat of different physiological status and age are presented in Table 1.
Table 1. Various combinations of multistrain probiotics and their effect on ruminant production.
| Multistrain | Cell Count | Mode of Administration/Dose | Host | Duration | Effect | No Effect | Ref. |
|---|---|---|---|---|---|---|---|
| Bacillus foraminis, B. firmus B. licheniformis, Staphylococcus saprophyticus bovis |
107 CFU/g | Oral inoculant using a syringe (1 mL/day at 1–2 weeks, 2 mL/day at 3–9 weeks) |
Neonate lamb | 9 weeks |
|
No effect on BWG and wool quality | [32] |
| P. acidilactici 3G3 L. plantarum BS S. cerevisiae 2030 |
5 × 109 CFU/mL | Orally using a syringe (6 mL) | Dairy goats | 9 weeks |
|
No effect on total milk yield, glucose, hemoglobin, and RBC count | [33] |
| E. faecalis L. rhamnous |
2 × 109 CFU/mL | Orally using dosing gun (5 mL) | South African goats | 30 days |
|
No effect on feed intake | [34] |
| L. acidophilus L. casei B. thermophilum E. faecium |
107 CFU/g | (Orally) mixed with concentrate | Lactating Ewes | 8 weeks |
|
Rumen conversion pathway of Fatty acid was not altered | [35] |
| L. acidophilus L. plantarum B. bifidum, B. subtilis, A. oryzae |
1 × 108 9.8 × 107 2 × 106 CFU/g |
Orally (3 g or 20 g/cow/day mixed with diet) |
Pre-partum dairy cow | 6 months |
|
No effect on BW, birth weight of calves, blood biochemical concentrations | [36] |
| (Locally produced probiotic bacteria) containing: L. farraginis L. reuteri L. rhamnosus |
108 CFU/g DM | Orally (mixed with diet) |
Pre-partum dairy cows | 3 months |
|
No effect on milk lactose, solid non-fat, and ash | [37] |
| L. casei Zhang L. plantarum P-8 |
1.3 × 109 (50 g/head/day) |
Orally (mixed with basal diet) |
Lactating primiparous dairy cows | 4 weeks |
|
No effect on milk fat, protein, and lactose | [38] |
| L. acidophilus S. cerevisiae E. faecium A. oryza B. subtilis |
50 mL/day | Orally (mixed with endotoxin-free water) |
Dairy cows | 60 days |
|
No effect on BW, PCV, and total protein concentration in plasma | [39] |
| L. fermentum L. plantarum M. elsdenii S. cerevisiae |
4.5 × 108 4.5 × 108 4.5 × 108 1.4 × 1010 |
Orally (dosing of 50 mL microbial suspension) |
Fattening lamb | 63 days |
|
No effect on feed intake and blood metabolite | [40] |
BWG, Body weight gain; PCV, packed cell volume; DMI, Dry matter intake; RBC, Red blood cell; PUN, Plasma urea nitrogen; n. s, not stated by the author.
4. Multistrain Probiotic Use in Poultry
Pathogenic bacteria including E. coli, Clostridium, and Salmonella appear to be a severe concern in chicken production, causing mortality, lowered growth rate, and low output. Antibiotics had previously played an important role in combating or regulating this problem; however, their prohibition has resulted in the use of probiotics to fill the void. Generally, because of their high fermentation utilization activity, probiotics promote protein and lipid digestion and interacts with enzymes to break down dietary molecules into simpler forms for digestion and absorption. They stimulate the production of digestive enzymes for carbohydrate metabolism, lower cholesterol, help in the synthesis of nutrients such as vitamins, influence the pH level in the poultry gut, and improve the productive performance, intestinal flora, and histomorphometry in heat-stressed chickens [18][41][42][43].
When broiler chickens were experimentally challenged with Pasteurella multocida, a highly contagious poultry disease that causes fowl cholera [44][45], supplementing dietary multistrain containing Saccharomyces cerevisiae, Lactobacillus fermentum, Pediococcus acidilactici, Lactobacillus plantarum, and Enterococcus faecium improved feed efficiency, growth performance, and intestinal health. It mitigated clinical signs, inflammatory reactions, and mortality-related symptoms [46]. In previous studies, successes have been recorded on probiotics’ potency in attenuating the colonization of avian pathogens in the chicken gut [47][48][49][50][51]. These antimicrobial effects are traceable to bacteriocins, organic acids, hydrogen peroxide, and short-chain fatty acids secreted by probiotic bacteria [52]. Besides, the transcriptional profiles of anti-inflammatory genes in the intestinal mucosa of probiotic-fed birds were elevated, haemato-biochemical markers such as packed cell volume, total cholesterol, glucose, proteins, white blood cells, and lymphocytes were also improved. There is a possibility that perhaps the synergy between lactic acid bacteria and yeast strains resulted in higher antimicrobial activity against P. multocida and enterobacteria in the guts of supplemented birds, as well as the ability of the combination to out-compete pathogens, thereby preventing them from attaching to the intestinal walls and as a result improve intestinal microbial balance [53].
5. Multistrain Probiotic Use in Swine
Feed prices contribute to almost two-thirds of overall swine production expenses; hence, to ensure profitability in the pig industry, efficiency in converting feed into pig body mass is essential [54]. Moreover, improved metabolic utilization of dietary nutrients is dependent primarily on a healthy gut, which can lead to improved feed digestion and nutrient absorption [55]. Research has shown that multistrain probiotics could enhance growth performance, feed efficiency, and nutrient digestibility [56][57][58]. It has also been effective in maintaining a balance in the intestinal microbial flora [59][60], stimulating immunity [32][61], increasing litter size, vitality, and weight, and reducing fecal noxious gas emission in pigs [57][58]. A summary of the effects of some multistrain probiotics on pigs of different physiological statuses is presented in Table 2.
Table 2. Various combinations of multistrain probiotics and their effect on swine production.
| Multistrain | Cell Count | Mode of Administration/Dose | Host | Duration | Effect | No Effect | Ref. |
|---|---|---|---|---|---|---|---|
| L. acidophilus B. subtilis S. cerevisiae |
1 × 107 1 × 107 1 × 107 CFU/g |
Orally (0.1% and 0.2% mixed with basal diet) |
Finishing pigs | 10 weeks |
|
No effect on meat quality parameters | [62] |
| Product A: L. plantarum L21 L. plantarum L80 L. paraplantarum L103 Product B: B. subtilis L. acidophilus S. cerevisiae |
1 × 109 1 × 109 1 × 109 1 × 1012 1.5 × 107 1 × 109 CFU/mL |
Oral gavage (0.25 g/day) |
Weaned pigs | 28 days |
|
n.s | [63] |
| B.coagulans B. licheniformis B. subtilis C. butyricum |
1 × 109 5 × 108 1 × 109 1 × 108 CFU/g |
Orally (0.1 or 0.2 g/kg mixed with basal diet) |
Growing-finishing pigs | 16 weeks |
|
No effect on average daily feed intake and meat color | [64] |
| L. amylovorus L. reuteri LAB 26 L. reuteri LAB 49 L. johnsonii L. salivarius L. mucosae |
1.7 × 1019 CFU/mL | Orally (1 mL mixed with PBS and 13% glycerol, aliquots added to feed) |
Piglets | 3 weeks |
|
No effect on the population of lactobacilli and bacteria in the large intestine digesta and growth enhancement | [65] |
| B. subtilitis B. licheniformis |
1 × 109 CFU/g | Orally (0.1 and 0.2% inoculated into limestone and maltodextrin as carriers) | Lactating sow and their suckling piglets | 28 days |
|
No effect on reproductive performance, H2S concentration, and fecal score in sows | [66] |
BWG, Body weight gain; ADWG, Average daily weight gain; n.s, not stated by the author.
6. Conclusions
In ruminants, poultry, and swine, multistrain probiotics have proven to be a viable alternative to antibiotics, and their usage in animal husbandry continues to grow. The effect on and responses of host animals, however, differs among literature. The variability in results might be due to the microorganism type or strains combined, as different species could possess distinct metabolic effects. The survivability of all the strains until delivery to the gut may also be difficult to ascertain. Probiotic dosage, the number of viable organisms in each dose, host animal physiological status and age, environment, diet composition, production procedures, and the mode of administering to the animal could all have a role. There were also limited reports on the greater benefits of multistrain probiotics over single strains in livestock. As a result, further research is needed to understand the interaction mechanisms among the combined microbes and the host’s gut microbiota and the unique role played by the individual microbe. In addition, comparison among the investigated animals and direct comparisons between the mono- and multispecies probiotics should be considered. Finally, stringent recommendations for optimal benefits should be provided.
References
- Yirga, H. The Use of Probiotics in Animal Nutrition. J. Probiotics Health 2015, 03.
- Collado, M.C.; Gueimonde, M.; Hernández, M.; Sanz, Y.; Salminen, S. Adhesion of Selected Bifidobacterium Strains to Human Intestinal Mucus and the Role of Adhesion in Enteropathogen Exclusion. J. Food Prot. 2005, 68, 2672–2678.
- Llewellyn, M.S.; Boutin, S.; Hoseinifar, S.H.; Derome, N. Teleost microbiomes: The state of the art in their characterization, manipulation and importance in aquaculture and fisheries. Front. Microbiol. 2014, 5, 207.
- Abdelqader, A.; Irshaid, R.; Al-Fataftah, A.-R. Effects of dietary probiotic inclusion on performance, eggshell quality, cecal microflora composition, and tibia traits of laying hens in the late phase of production. Trop. Anim. Health Prod. 2013, 45, 1017–1024.
- Mookiah, S.; Sieo, C.C.; Ramasamy, K.; Abdullah, N.; Ho, Y.W. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens: Effects of dietary prebiotics, probiotic and synbiotics on performance. J. Sci. Food Agric. 2014, 94, 341–348.
- Pedroso, A.A.; Hurley-Bacon, A.L.; Zedek, A.S.; Kwan, T.W.; Jordan, A.P.; Avellaneda, G.; Hofacre, C.L.; Oakley, B.B.; Collett, S.R.; Maurer, J.J.; et al. Can Probiotics Improve the Environmental Microbiome and Resistome of Commercial Poultry Production? Int. J. Environ. Res. Public. Health 2013, 10, 4534–4559.
- van Doan, H.; Hoseinifar, S.H.; Dawood, M.A.O.; Chitmanat, C.; Tayyamath, K. Effects of Cordyceps militaris spent mushroom substrate and Lactobacillus plantarum on mucosal, serum immunology and growth performance of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2017, 70, 87–94.
- Fajardo, P.; Pastrana, L.; Méndez, J.; Rodríguez, I.; Fuciños, C.; Guerra, N.P. Effects of Feeding of Two Potentially Probiotic Preparations from Lactic Acid Bacteria on the Performance and Faecal Microflora of Broiler Chickens. Sci. World J. 2012, 2012, 1–9.
- Hoseinifar, S.H.; Sun, Y.-Z.; Wang, A.; Zhou, Z. Probiotics as Means of Diseases Control in Aquaculture, a Review of Current Knowledge and Future Perspectives. Front. Microbiol. 2018, 9, 2429.
- Cholewińska, P.; Czyż, K.; Nowakowski, P.; Wyrostek, A. The microbiome of the digestive system of ruminants—A review. Anim. Health Res. Rev. 2020, 21, 3–14.
- Ohinaim, E.I.; Ofongo, R.T.S. The Effect of Probiotic and Prebiotic Feed Supplementationon Chicken Health and Gut Microflora: A Review. Int. J. Anim. Vet. Adv. 2012, 4, 135–143.
- Zeng, W.; Shen, J.; Bo, T.; Peng, L.; Xu, H.; Nasser, M.I.; Zhuang, Q.; Zhao, M. Cutting Edge: Probiotics and Fecal Microbiota Transplantation in Immunomodulation. J. Immunol. Res. 2019, 2019, 1–17.
- Cao, L.; Yang, X.J.; Li, Z.J.; Sun, F.F.; Wu, X.H.; Yao, J.H. Reduced lesions in chickens with Clostridium perfringens-induced necrotic enteritis by Lactobacillus fermentum 1.2029. Poult. Sci. 2012, 91, 3065–3071.
- Chaves, B.D.; Brashears, M.M.; Nightingale, K.K. Applications and safety considerations of Lactobacillus salivarius as a probiotic in animal and human health. J. Appl. Microbiol. 2017, 123, 18–28.
- Safari, R.; Adel, M.; Lazado, C.C.; Caipang, C.M.A.; Dadar, M. Host-derived probiotics Enterococcus casseliflavus improves resistance against Streptococcus iniae infection in rainbow trout (Oncorhynchus mykiss) via immunomodulation. Fish Shellfish Immunol. 2016, 52, 198–205.
- Arsène, M.M.; Davares, A.K.; Andreevna, S.L.; Vladimirovich, E.A.; Carime, B.Z.; Marouf, R.; Khelifi, I. The use of probiotics in animal feeding for safe production and as potential alternatives to antibiotics. Vet. World 2021, 14, 319–328.
- Jungersen, M.; Wind, A.; Johansen, E.; Christensen, J.; Stuer-Lauridsen, B.; Eskesen, D. The Science behind the Probiotic Strain Bifidobacterium animalis subsp. lactis BB-12®. Microorganisms 2014, 2, 92–110.
- Liao, S.F.; Nyachoti, M. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 2017, 3, 331–343.
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 31.
- Liu, K.; Zhang, Y.; Xu, Q.; Zheng, N.; Zhao, S.; Huang, G.; Wang, J. Ruminal microbiota–host interaction and its effect on nutrient metabolism. Anim. Nutr. 2021, 7, 49–55.
- Kritas, S.K.; Govaris, A.; Christodoulopoulos, G.; Burriel, A.R. Effect of Bacillus licheniformis and Bacillus subtilis Supplementation of Ewe’s Feed on Sheep Milk Production and Young Lamb Mortality. J. Vet. Med. Ser. A 2006, 53, 170–173.
- Adjei-Fremah, S.; Ekwemalor, K.; Asiamah, E.K.; Ismail, H.; Ibrahim, S.; Worku, M. Effect of probiotic supplementation on growth and global gene expression in dairy cows. J. Appl. Anim. Res. 2018, 46, 257–263.
- Whitley, N.C.; Cazac, D.; Rude, B.J.; Jackson-O’Brien, D.; Parveen, S. Use of a commercial probiotic supplement in meat goats1. J. Anim. Sci. 2009, 87, 723–728.
- Hasunuma, T.; Kawashima, K.; Nakayama, H.; Murakami, T.; Kanagawa, H.; Ishii, T.; Akiyama, K.; Yasuda, K.; Terada, F.; Kushibiki, S. Effect of cellooligosaccharide or synbiotic feeding on growth performance, fecal condition and hormone concentrations in Holstein calves: Cellobiose or Synbiotic Feeding in Calves. Anim. Sci. J. 2011, 82, 543–548.
- Vibhute, V.; Shelke, R.; Chavan, S.; Nage, S. Effect of Probiotics Supplementation on the Performance of Lactating Crossbred Cows. Vet. World 2011, 4, 557.
- Renaud, D.L.; Kelton, D.F.; Weese, J.S.; Noble, C.; Duffield, T.F. Evaluation of a multispecies probiotic as a supportive treatment for diarrhea in dairy calves: A randomized clinical trial. J. Dairy Sci. 2019, 102, 4498–4505.
- Rao, Y.Y.N.K.A.; Kumar, C.V.S.D.S.; Lekha, M.S. Effect of Feeding Multi-Strain Probiotic on Feed Intake and Milk Production Performance in Murrah Buffaloes. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 409–417.
- Kembabazi, B.; Ondiek, J.O.; Migwi, P.K. Effect of single or mixed strain probiotics on milk yield of dairy cows. Livest. Res. Rural Dev. 2021, 33. Available online: http://www.lrrd.org/lrrd33/1/brend3307.html (accessed on 4 August 2021).
- Thomas, A.D. Supplementation of Two Novel Probiotics in the Dietof Lactating Dairy Cows. Iowa State University. 2017. Available online: http://lib.dr.iastate.edu/etd/16110 (accessed on 4 August 2021).
- Olchowy, T.W.J.; Soust, M.; Alawneh, J. The effect of a commercial probiotic product on the milk quality of dairy cows. J. Dairy Sci. 2019, 102, 2188–2195.
- Deng, Q.; Odhiambo, J.F.; Farooq, U.; Lam, T.; Dunn, S.M.; Ametaj, B.N. Intravaginal probiotics modulated metabolic status and improved milk production and composition of transition dairy cows1. J. Anim. Sci. 2016, 94, 760–770.
- Yang, C.M.; Cao, G.T.; Ferket, P.R.; Liu, T.T.; Zhou, L.; Zhang, L.; Xiao, Y.P.; Chen, A.G. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult. Sci. 2012, 91, 2121–2129.
- Dlamini, Z.C.; Langa, R.L.S.; Aiyegoro, O.A.; Okoh, A.I. Effects of probiotics on growth performance, blood parameters, and antibody stimulation in piglets. S. Afr. J. Anim. Sci. 2017, 47, 765.
- Lan, R.X.; Lee, S.I.; Kim, I.H. Effects of multistrain probiotics on growth performance, nutrient digestibility, blood profiles, faecal microbial shedding, faecal score and noxious gas emission in weaning pigs. J. Anim. Physiol. Anim. Nutr. 2016, 100, 1130–1138.
- Hu, J.; Kim, Y.H.; Kim, I.H. Effects of two bacillus strains probiotic supplement on reproduction performance, nutrient digestibility, blood profile, fecal score, excreta odor contents and fecal microflora in lactation sows, and growth performance in sucking piglets. Livest. Sci. 2021, 244, 104293.
- Ishaq, S.L.; Kim, C.J.; Reis, D.; Wright, A.-D.G. Fibrolytic Bacteria Isolated from the Rumen of North American Moose (Alces alces) and Their Use as a Probiotic in Neonatal Lambs. PLoS ONE 2015, 10, e0144804.
- Salvedia, C.; Supangco, E.; Vega, R.; Elegado, F.; Rayos, A. Effect of Probiotic Feeding on Milk Yield and Components of Crossbred Dairy Goats. Philipp. J. Vet. Anim. Sci. 2015, 41, 21–30.
- Maake, T.W.; Adeleke, M.; Aiyegoro, O.A. Effect of lactic acid bacteria administered as feed supplement on the weight gain and ruminal pH in two South African goat breeds. Trans. R. Soc. S. Afr. 2021, 76, 35–40.
- Aalaei, M.; Khatibjoo, A.; Zaghari, M.; Taherpour, K.; Gharaei, M.A.; Soltani, M. Comparison of single- and multistrain probiotics effects on broiler breeder performance, egg production, egg quality and hatchability. Br. Poult. Sci. 2018, 59, 531–538.
- Shreedhar, J.N.; Patil, M.; Kumar, P. Effect of Probiotics Supplementation on Milk Yield and Its Composition in Lactating Holstein Fresien and Deoni Cross Bred Cows. J. Med. Bioeng. 2016, 5, 19–23.
- Yoo, J.; Kim, S. Probiotics and Prebiotics: Present Status and Future Perspectives on Metabolic Disorders. Nutrients 2016, 8, 173.
- Ding, S.; Yan, W.; Ma, Y.; Fang, J. The impact of probiotics on gut health via alternation of immune status of monogastric animals. Anim. Nutr. 2021, 7, 24–30.
- Elbaz, A.M.; Ibrahim, N.S.; Shehata, A.M.; Mohamed, N.G.; Abdel-Moneim, A.-M.E. Impact of multistrain probiotic, citric acid, garlic powder or their combinations on performance, ileal histomorphometry, microbial enumeration and humoral immunity of broiler chickens. Trop. Anim. Health Prod. 2021, 53, 115.
- Fenton, H.; McManamon, R.; Howerth, E.W. Anseriformes, Ciconiiformes, Charadriiformes, and Gruiformes. In Pathology of Wildlife and Zoo Animals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 697–721.
- Mbuthia, P.G.; Njagi, L.W.; Nyaga, P.N.; Bebora, L.C.; Minga, U.; Kamundia, J.; Olsen, J.E. Pasteurella multocida in scavenging family chickens and ducks: Carrier status, age susceptibility and transmission between species. Avian Pathol. 2008, 37, 51–57.
- Monteverde, V.; Congiu, F.; Vazzana, I.; Dara, S.; di Pietro, S.; Piccione, G. Serum lipid profile modification related to polyunsaturated fatty acid supplementation in thoroughbred horses. J. Appl. Anim. Res. 2017, 45, 615–618.
- Giannenas, I.; Papadopoulos, E.; Tsalie, E.; Triantafillou, E.L.; Henikl, S.; Teichmann, K.; Tontis, D. Assessment of dietary supplementation with probiotics on performance, intestinal morphology and microflora of chickens infected with Eimeria tenella. Vet. Parasitol. 2012, 188, 31–40.
- Rajabi, S.; Darban, D.; Tabatabaei, R.R.; Hosseini, F. Antimicrobial effect of spore-forming probiotics Bacillus laterosporus and Bacillus megaterium against Listeria monocytogenes. Arch. Microbiol. 2020, 202, 2791–2797.
- Rahimi, S.; Kathariou, S.; Fletcher, O.; Grimes, J.L. Effect of a direct-fed microbial and prebiotic on performance and intestinal histomorophology of turkey poults challenged with Salmonella and Campylobacter. Poult. Sci. 2019, 98, 6572–6578.
- Smialek, M.; Burchardt, S.; Koncicki, A. The influence of probiotic supplementation in broiler chickens on population and carcass contamination with Campylobacter spp.—Field study. Res. Vet. Sci. 2018, 118, 312–316.
- Olnood, C.G.; Beski, S.S.M.; Choct, M.; Iji, P.A. Use of Lactobacillus johnsonii in broilers challenged with Salmonella sofia. Anim. Nutr. 2015, 1, 203–212.
- Grosu-Tudor, S.-S.; Stancu, M.-M.; Pelinescu, D.; Zamfir, M. Characterization of some bacteriocins produced by lactic acid bacteria isolated from fermented foods. World J. Microbiol. Biotechnol. 2014, 30, 2459–2469.
- Reuben, R.C.; Sarkar, S.L.; Ibnat, H.; Setu, M.A.A.; Roy, P.C.; Jahid, I.K. Novel multistrain probiotics reduces Pasteurella multocida induced fowl cholera mortality in broilers. Sci. Rep. 2021, 11, 8885.
- Knap, P.W.; Wang, L. Pig breeding for improved feed efficiency. In Feed Efficiency in Swine; Patience, J.F., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2012.
- Willing, B.P.; Malik, G.; van Kessel, A.G. Nutrition and Gut Health in Swine. In Sustainable Swine Nutrition; Chiba, L.I., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2012; pp. 197–213.
- Dong, X.; Zhang, N.; Zhou, M.; Tu, Y.; Deng, K.; Diao, Q. Effects of dietary probiotics on growth performance, faecal microbiota and serum profiles in weaned piglets. Anim. Prod. Sci. 2014, 54, 616.
- Nguyen, D.H.; Nyachoti, C.M.; Kim, I.H. Evaluation of effect of probiotics mixture supplementation on growth performance, nutrient digestibility, faecal bacterial enumeration, and noxious gas emission in weaning pigs. Ital. J. Anim. Sci. 2019, 18, 466–473.
- Zhao, P.Y.; Kim, I.H. Effect of direct-fed microbial on growth performance, nutrient digestibility, fecal noxious gas emission, fecal microbial flora and diarrhea score in weanling pigs. Anim. Feed Sci. Technol. 2015, 200, 86–92.
- Kong, Q.; He, G.-Q.; Jia, J.-L.; Zhu, Q.-L.; Ruan, H. Oral Administration of Clostridium butyricum for Modulating Gastrointestinal Microflora in Mice. Curr. Microbiol. 2011, 62, 512–517.
- Zhang, Z.F.; Kim, I.H. Effects of multistrain probiotics on growth performance, apparent ileal nutrient digestibility, blood characteristics, cecal microbial shedding, and excreta odor contents in broilers. Poult. Sci. 2014, 93, 364–370.
- Agazzi, A. The Beneficial Role of Probiotics in Monogastric Animal Nutrition and Health. J. Dairy Vet. Anim. Res. 2015, 2.
- Liu, W.C.; Ye, M.; Liao, J.H.; Zhao, Z.H.; Kim, I.H.; An, L.L. Application of Complex Probiotics in Swine Nutrition—A Review. Ann. Anim. Sci. 2018, 18, 335–350.
- Ray, S.M.; Ghule, S.; Muthukumar, S.; Banik, A.; Maji, C. Effects of Dietary Supplementation of a Single-and a Multi-Strain Probiotic on Growth Performance and Intestinal Histomorphology of Commercial Broiler Chickens. Int. J. Poult. Sci. 2020, 19, 363–371.
- Ramlucken, U.; Ramchuran, S.O.; Moonsamy, G.; Lalloo, R.; Thantsha, M.S.; van Rensburg, C.J. A novel Bacillus based multistrain probiotic improves growth performance and intestinal properties of Clostridium perfringens challenged broilers. Poult. Sci. 2020, 99, 331–341.
- Biswas, A.; Dev, K.; Tyagi, P.K.; Mandal, A. The effect of multistrain probiotics as feed additives on performance, immunity, expression of nutrient transporter genes and gut morphometry in broiler chickens. Anim. Biosci. 2021.
- Chung, S.H.; Lee, J.; Kong, C. Effects of Multi Strain Probiotics on Egg Production and Quality in Laying Hens Fed Diets Containing Food Waste Product. Int. J. Poult. Sci. 2014, 14, 19–22.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
3 times
(View History)
Update Date:
09 Oct 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




