
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tsung-Chieh Shih | + 1485 word(s) | 1485 | 2021-09-27 09:56:08 | | | |
| 2 | Nora Tang | + 44 word(s) | 1529 | 2021-09-29 03:22:26 | | |
Video Upload Options
Genetic alterations are heritable, and as a result the cells harboring these aberrations are subject to Darwinian selection. Immunologically, tumor cells can be regarded as modified self-cells that have eluded typical growth-regulating machinery. The evasion of immune surveillance is an accepted hallmark of tumor progression, and treatment strategies for targeting immune-suppressive pathways have led to enhanced patient survival in multiple cancers.
1. Introduction
In 2020, the range of newly diagnosed cancer incidences was about 19.3 million globally, resulting in about 10 million fatalities. Owing to the world’s increasing population, if the number of incidences continues at this rate, the number of cancer cases worldwide will increase to 28.4 million by the year 2040 [1]. All cancers share the same characteristics in that they are genetic disorders caused by DNA mutations, with most pathogenic mutations either being induced by exposure to mutagens or occurring spontaneously as part of aging. Genetic alterations are heritable, and as a result the cells harboring these aberrations are subject to Darwinian selection. Immunologically, tumor cells can be regarded as modified self-cells that have eluded typical growth-regulating machinery [2]. The evasion of immune surveillance is an accepted hallmark of tumor progression [3], and treatment strategies for targeting immune-suppressive pathways have led to enhanced patient survival in multiple cancers [4].
Cell surface carbohydrates carry out a vast array of roles and are imperative to normal cellular physiology. In addition to serving as ligands of glycan-binding proteins (GBPs), they directly impact glycoprotein function by enabling glycan-dependent signaling on the cell surface. Carbohydrate interactions on the GBP cell surface play key roles in immune responses and in the tumor microenvironment [5]. GBPs were first discovered by Ashwell and Morrell in the 1960s and were designated as vertebrates (the asialoglycoprotein receptor) [6]. In 1975, Teichberg et al. discovered electrolectin from the electric eel ( Electrophorus electricus) [7]. It was the first vertebrate galectin, Galectin-1 (Gal-1). Shortly after that, Gal-1 was isolated in 1976 by Kornfeld et al. from extracts of calf heart and lung [8]. In the same year, Barondes et al. isolated a similar galectin from chick muscle extracts [9]. To date, 15 different galectins have been discovered in mammals, with 11 found in humans, all in subunit size (14–39 kDa) [10].
Galectin expression varies from cell to cell, as it relies on the state of activation of a specific cell. All cellular types express at least one galectin, with different galectins being expressed in high concentrations in different cell types. After synthesis in the cytosolic ribosomes, they are translocated to the nucleus or additional subcellular locations. Galectins are lacking in secretion signal peptides, archetypal transmembrane segments, and N-termini with acetyl groups with features similar to those of other cytosolic proteins [11]. The anomalous expression of galectins is linked to the incidence, advancement, and metastasis of cancers. Galectins also have a broad spectrum of effects on diverse immune cells, promoting inflammation or inhibiting immune responses mediated by T-cells and dependent on the receptors in specific target cells [11]. Gal-1 has been the most studied galectin since it first displayed hemagglutinating activity in 1975. In recent years, Gal-1 has been known to promote cancer cell growth; in addition, tumor-secreted Gal-1 is involved in immune escape by tumors, indicating that Gal-1 is a critical molecular target in cancer and could be a potentially therapeutic target for cancer treatment.
2. Gal-1 in T-Cell Immunodeficiency Diseases
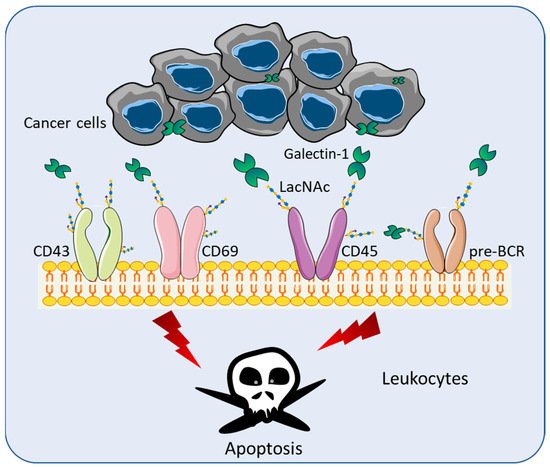
Although Gal-1 lacks a secretion signal peptide or archetypal transmembrane segments, Gal-1 is secreted and found in the extracellular space [12]. Gal-1 recognizes terminal galactose residues β-1,4-linked to N-Acetyllactosamine (LacNAc), which are present in the branch of O- or N -linked glycans on an extensive array of cell receptors including pre-BCR, CD43, CD45, CD69, and vascular endothelial growth factors (VEGF) [13] ( Figure 1 ). Through the binding of LacNAc, Gal-1 can stimulate the apoptosis of effector leukocytes [14] ( Figure 1 ). Various studies have demonstrated that Gal-1 mediates T cell apoptosis through multiple mechanisms, including the loss of mitochondrial membrane potential [15], the activation of the Lck/ZAP-70 signaling pathway [16], the release of cytochrome c [17], the activation of the c-Jun/AP-1 pathway, and the downregulation of Bcl-2 protein expression [18]. The binding of Gal-1 is halted by the modification of LacNAc by the α2,6 sialyltransferase 1 (ST6GAL1), which adds α2,6-linked sialic acid to the terminal galactose of N-linked glycans [13].
Toscano et al. showed that T helper type 2 (Th2) cells were protected from Gal-1 induced cell death through the differential sialylation of cell surface glycoproteins [19]. Consistent with these findings, the treatment of mice with recombinant Gal-1 (rGal-1) has been reported to block the development of T helper type 1 (Th1) cell-mediated diseases [20][21][22]. In broad terms, Th1 cells promote a cellular immune response and Th2 cells produce a humoral immune response [23]. Rabinovich et al. showed that an injection of syngeneic DBA/1 fibroblasts engineered to secrete Gal-1 was able to abrogate clinical and histopathological manifestations of arthritis, and this effect was reproduced by the daily administration of rGal-1 [20]. The cytokine profiles of draining lymph node cells in mice sera showed the inhibition of the proinflammatory response and skewed towards Th2 immunity [20]. Santucci et al. showed that rGal-1 exerts therapeutic activity in Th1-mediated experimental colonic inflammation by eliminating the uncontrolled Th1 response to the hapten [21]. In experimental autoimmune uveitis (EAU), Toscano et al. showed that rGal-1 treatment was sufficient to suppress clinical ocular pathology, inhibit leukocyte infiltration, and counteract pathogenic Th1 cells [22]. The administration of rGal-1 modulates the Th1/Th2 balance toward nonpathogenic Th2 and T-regulatory cytokine profiles [22]. These studies evidenced that rGal-1 suppressed Th1-dependent responses and increased T cell susceptibility to activation-induced cell death.
3. Role of Galectins in Cancer Immune Surveillance
There are two main types of lymphoma: classical Hodgkin lymphoma (cHL) and non-Hodgkin lymphoma (NHL). cHL contains a particular type of cell known as a Reed–Sternberg (RS) cell, which is an abnormal B lymphocyte [24]. NHL cases do not contain Reed–Sternberg cells, and arise from a defect in B cells that express membrane-bound CD20 [25]. In cHL research, Juszczynski et al. found that cHL RS cells overexpressed Gal-1 through an AP1-dependent enhancer [26]. In co-cultures of activated T cells and cHL RS cells, the inhibition of RS Gal-1 via siRNA increased T cell viability and restored the Th1/Th2 balance. In addition, the Gal-1 treatment of activated T cells fostered the secretion of Th2 cytokines and the expansion of CD4 + CD25 high FOXP3 + T regulatory (Treg) cells [26]. Based on these findings, Rodig et al. tested whether the coordinate expression of activated AP1 pathway components and Gal-1 served as a diagnostic signature of cHL [27]. The immunohistochemical results showed that Gal-1 was selectively expressed by malignant RS cells in 92% (66 of 72 cases) of primary cHLs and that Gal-1 expression was concordant with the activated AP1 component, c-Jun. In contrast, diffuse large B-cell lymphoma, primary mediastinal large B-cell lymphoma, and nodular lymphocyte-predominant Hodgkin lymphoma (another Hodgkin-related entity) do not express Gal-1 [27].
In NHL research, Lykken et al. showed that Human NHLs expressed elevated Gal-1 compared with nonmalignant lymphocytes and that Gal-1 expression by lymphoma cells abrogated CD20 immunotherapy in mice. Mechanistically, both exogenous rGal-1 and lymphoma-derived Gal-1 impaired mAb-dependent lymphoma phagocytosis by macrophages in vitro, demonstrating that extracellular Gal-1 can impede macrophage activation and function [28].
PDAC is an extremely aggressive malignancy and is resistant to currently available systemic therapies. Most PDAC is characterized by a prominent oncogenic tumor–stroma reaction around tumor tissue [29]. Pancreatic stellate cells (PSCs), which are stellate-shaped mesenchymal pancreatic cells, are one of the entities in the PDAC stroma. PSCs have been identified as important regulators of desmoplasia in PDAC [30]; Tang et al. showed that Gal-1 is expressed in abundance in activated PSCs. PSCs that overexpressed Gal-1 significantly induced the apoptosis of CD4 + T cells and CD8 + T cells and increased Th2 cytokine secretion (IL-4 and IL-5) from T cells [30]. Qian et al. demonstrated that Gal-1 induces the secretion of stromal cell-derived factor-1 (SDF-1) in PSCs, leading to increases in the migration and invasion of pancreatic cancer cells [31]. Martínez-Bosch et al. showed that the depletion of Gal-1 reduces the in vivo tumorigenicity, leading to significantly increased survival in the Ela-myc mouse pancreatic cancer model [32]. Mechanistically, Gal-1 activates the Hedgehog signaling pathway in PDAC epithelial and fibroblastic cells [32]. In a recent study by Orozco et al. , the genetic deletion of Gal-1 decreased stroma activation, attenuated vascularization, and enhanced T cell infiltration in Kras-driven mouse pancreatic cancer models [33].
Breast cancer develops in breast cells and most breast cancers form in the lobules or the ducts. Dalotto-Moreno et al. found that the expression of Gal-1 correlates with the aggressiveness of human breast tumors and is upregulated in the mouse metastatic 4T1 breast cancer model [34]. The inhibition of Gal-1 expression prevented tumor growth and suppressed the development of lung metastasis. In addition, they showed that tumor-derived Gal-1 promotes an immunosuppressive breast cancer microenvironment by increasing the frequency of CD4 + CD25 + Foxp3 + Treg cells within the tumor, draining lymph nodes, spleen, and lung metastases [34]. Cheng et al. showed that tumor-derived Gal-1 could stimulate tolerogenic DCs differentiation after internalizing into CD14 + monocytes through the caveolae-dependent pathway and activating myosin IIa [35].
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2021, 71, 209–249.
- Houghton, A.N.; Guevara-Patiño, J.A. Immune recognition of self in immunity against cancer. J. Clin. Investig. 2004, 114, 468–471.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 2015, 33, 1974–1982.
- Schnaar, R.L. Glycans and glycan-binding proteins in immune regulation: A concise introduction to glycobiology for the allergist. J. Allergy Clin. Immunol. 2015, 135, 609–615.
- Morell, A.G.; Gregoriadis, G.; Scheinberg, I.H.; Hickman, J.; Ashwell, G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J. Biol. Chem. 1971, 246, 1461–1467.
- Teichberg, V.I.; Silman, I.; Beitsch, D.D.; Resheff, G. A beta-D-galactoside binding protein from electric organ tissue of Electrophorus electricus. Proc. Natl. Acad. Sci. USA 1975, 72, 1383–1387.
- de Waard, A.; Hickman, S.; Kornfeld, S. Isolation and properties of beta-galactoside binding lectins of calf heart and lung. J. Biol. Chem. 1976, 251, 7581–7587.
- Nowak, T.P.; Kobiler, D.; Roel, L.E.; Barondes, S.H. Developmentally regulated lectin from embryonic chick pectoral muscle. Purification by affinity chromatography. J. Biol. Chem. 1977, 252, 6026–6030.
- Liu, F.T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41.
- Chou, F.-C.; Chen, H.-Y.; Kuo, C.-C.; Sytwu, H.-K. Role of galectins in tumors and in clinical immunotherapy. Int. J. Mol. Sci. 2018, 19, 430.
- Leffler, H.; Carlsson, S.; Hedlund, M.; Qian, Y.; Poirier, F. Introduction to galectins. Glycoconj. J. 2002, 19, 433–440.
- Hirabayashi, J.; Hashidate, T.; Arata, Y.; Nishi, N.; Nakamura, T.; Hirashima, M.; Urashima, T.; Oka, T.; Futai, M.; Muller, W.E.; et al. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim. Biophys. Acta 2002, 1572, 232–254.
- Perillo, N.L.; Pace, K.E.; Seilhamer, J.J.; Baum, L.G. Apoptosis of T cells mediated by galectin-1. Nature 1995, 378, 736–739.
- Hahn, H.P.; Pang, M.; He, J.; Hernandez, J.D.; Yang, R.Y.; Li, L.Y.; Wang, X.; Liu, F.T.; Baum, L.G. Galectin-1 induces nuclear translocation of endonuclease G in caspase and cytochrome c-independent T cell death. Cell Death Differ. 2004, 11, 1277–1286.
- Ion, G.; Fajka-Boja, R.; Tóth, G.K.; Caron, M.; Monostori, É. Role of p56lck and ZAP70-mediated tyrosine phosphorylation in galectin-1-induced cell death. Cell Death Differ. 2005, 12, 1145–1147.
- Matarrese, P.; Tinari, A.; Mormone, E.; Bianco, G.A.; Toscano, M.A.; Ascione, B.; Rabinovich, G.A.; Malorni, W. Galectin-1 sensitizes resting human T lymphocytes to Fas (CD95)-mediated cell death via mitochondrial hyperpolarization, budding, and fission. J. Biol. Chem. 2005, 280, 6969–6985.
- Rabinovich, G.A.; Alonso, C.R.; Sotomayor, C.E.; Durand, S.; Bocco, J.L.; Riera, C.M. Molecular mechanisms implicated in galectin-1-induced apoptosis: Activation of the AP-1 transcription factor and downregulation of Bcl-2. Cell Death Differ. 2000, 7, 747–753.
- Toscano, M.A.; Bianco, G.A.; Ilarregui, J.M.; Croci, D.O.; Correale, J.; Hernandez, J.D.; Zwirner, N.W.; Poirier, F.; Riley, E.M.; Baum, L.G.; et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 2007, 8, 825–834.
- Rabinovich, G.A.; Daly, G.; Dreja, H.; Tailor, H.; Riera, C.M.; Hirabayashi, J.; Chernajovsky, Y. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J. Exp. Med. 1999, 190, 385–398.
- Santucci, L.; Fiorucci, S.; Rubinstein, N.; Mencarelli, A.; Palazzetti, B.; Federici, B.; Rabinovich, G.A.; Morelli, A. Galectin-1 suppresses experimental colitis in mice. Gastroenterology 2003, 124, 1381–1394.
- Toscano, M.A.; Commodaro, A.G.; Ilarregui, J.M.; Bianco, G.A.; Liberman, A.; Serra, H.M.; Hirabayashi, J.; Rizzo, L.V.; Rabinovich, G.A. Galectin-1 suppresses autoimmune retinal disease by promoting concomitant Th2- and T regulatory-mediated anti-inflammatory responses. J. Immunol. 2006, 176, 6323–6332.
- Kaiko, G.E.; Horvat, J.C.; Beagley, K.W.; Hansbro, P.M. Immunological decision-making: How does the immune system decide to mount a helper T-cell response? Immunology 2008, 123, 326–338.
- Steidl, C.; Connors, J.M.; Gascoyne, R.D. Molecular pathogenesis of Hodgkin’s lymphoma: Increasing evidence of the importance of the microenvironment. J. Clin. Oncol. 2011, 29, 1812–1826.
- Anderson, K.C.; Bates, M.P.; Slaughenhoupt, B.L.; Pinkus, G.S.; Schlossman, S.F.; Nadler, L.M. Expression of human B cell-associated antigens on leukemias and lymphomas: A model of human B cell differentiation. Blood 1984, 63, 1424–1433.
- Juszczynski, P.; Ouyang, J.; Monti, S.; Rodig, S.J.; Takeyama, K.; Abramson, J.; Chen, W.; Kutok, J.L.; Rabinovich, G.A.; Shipp, M.A. The AP1-dependent secretion of galectin-1 by reed–sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc. Natl. Acad. Sci. USA 2007, 104, 13134–13139.
- Rodig, S.J.; Ouyang, J.; Juszczynski, P.; Currie, T.; Law, K.; Neuberg, D.S.; Rabinovich, G.A.; Shipp, M.A.; Kutok, J.L. AP1-dependent galectin-1 expression delineates classical Hodgkin and anaplastic large cell lymphomas from other lymphoid malignancies with shared molecular features. Clin. Cancer Res. 2008, 14, 3338–3344.
- Lykken, J.M.; Horikawa, M.; Minard-Colin, V.; Kamata, M.; Miyagaki, T.; Poe, J.C.; Tedder, T.F. Galectin-1 drives lymphoma CD20 immunotherapy resistance: Validation of a preclinical system to identify resistance mechanisms. Blood 2016, 127, 1886–1895.
- Apte, M.V.; Park, S.; Phillips, P.A.; Santucci, N.; Goldstein, D.; Kumar, R.K.; Ramm, G.A.; Buchler, M.; Friess, H.; McCarroll, J.A.; et al. Desmoplastic reaction in pancreatic cancer: Role of pancreatic stellate cells. Pancreas 2004, 29, 179–187.
- Hosein, A.N.; Brekken, R.A.; Maitra, A. Pancreatic cancer stroma: An update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 487–505.
- Qian, D.; Lu, Z.; Xu, Q.; Wu, P.; Tian, L.; Zhao, L.; Cai, B.; Yin, J.; Wu, Y.; Staveley-O’Carroll, K.F.; et al. Galectin-1-driven upregulation of SDF-1 in pancreatic stellate cells promotes pancreatic cancer metastasis. Cancer Lett. 2017, 397, 43–51.
- Martínez-Bosch, N.; Fernández-Barrena, M.G.; Moreno, M.; Ortiz-Zapater, E.; Munné-Collado, J.; Iglesias, M.; André, S.; Gabius, H.J.; Hwang, R.F.; Poirier, F.; et al. Galectin-1 drives pancreatic carcinogenesis through stroma remodeling and Hedgehog signaling activation. Cancer Res. 2014, 74, 3512–3524.
- Orozco, C.A.; Martinez-Bosch, N.; Guerrero, P.E.; Vinaixa, J.; Dalotto-Moreno, T.; Iglesias, M.; Moreno, M.; Djurec, M.; Poirier, F.; Gabius, H.-J.; et al. Targeting galectin-1 inhibits pancreatic cancer progression by modulating tumor-stroma crosstalk. Proc. Natl. Acad. Sci. USA 2018, 115, E3769–E3778.
- Dalotto-Moreno, T.; Croci, D.O.; Cerliani, J.P.; Martinez-Allo, V.C.; Dergan-Dylon, S.; Méndez-Huergo, S.P.; Stupirski, J.C.; Mazal, D.; Osinaga, E.; Toscano, M.A.; et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 2013, 73, 1107.
- Cheng, D.-E.; Hung, J.-Y.; Huang, M.-S.; Hsu, Y.-L.; Lu, C.-Y.; Tsai, E.-M.; Hou, M.-F.; Kuo, P.-L. Myosin IIa activation is crucial in breast cancer derived galectin-1 mediated tolerogenic dendritic cell differentiation. Biochim. Biophys. Acta (BBA) 2014, 1840, 1965–1976.




