
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chiara Nediani | + 3639 word(s) | 3639 | 2021-07-28 04:44:07 | | | |
| 2 | Vivi Li | Meta information modification | 3639 | 2021-09-27 03:53:22 | | | | |
| 3 | Vivi Li | Meta information modification | 3639 | 2021-09-27 05:25:11 | | |
Video Upload Options
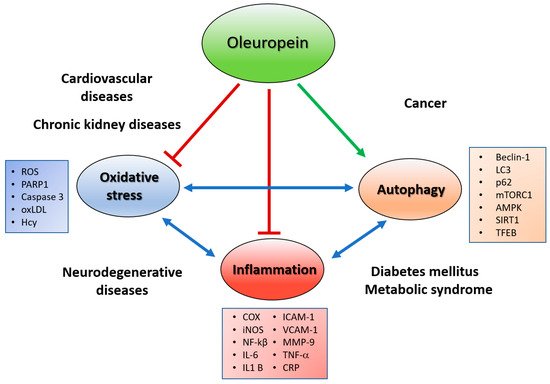
Growing scientific literature data suggest that the intake of natural bioactive compounds plays a critical role in preventing or reducing the occurrence of human chronic non-communicable diseases (NCDs), such as neuro- and cardiovascular diseases, diabetes mellitus and cancer. Oleuropein, the main phenolic component of Olea europaea L., has attracted scientific attention for its several health beneficial properties such as antioxidant, anti-inflammatory, cardio- and neuro-protective, and anti-cancer. This entry contains data from the current literature concerning the effect of oleuropein in NCDs not only due to its putative antioxidant and anti-inflammatory activities, but also to its other peculiar actions such as autophagy inducer and amyloid fibril growth inhibitor and, finally, as anti-cancer agent. Despite the increasing number of published studies, looking at the health effects of oleuropein, there is limited clinical evidence focused on the benefits of this polyphenol as a nutraceutical product in humans, and many problems are still to be resolved about its bioavailability, bioaccessibility, and dosage. Thus, future clinical randomized trials are needed to establish the relation between the beneficial effects and the mechanisms of action occurring in the human body in response to the intake of oleuropein.
1. Introduction
2. Oleuropein As an Antioxidant
3. Oleuropein As an Anti-Inflammatory and CVD Protective Agent
Lipid-Regulating, Anti-Hypertensive and Antidiabetic Effects of Oleuropein
CVD is a group of disorders, affecting heart and/or blood vessels, including coronary cerebrovascular and peripheral arterial diseases. The cardiovascular protective effect of oleuropein is supported by many in vivo animal studies and human clinical trials that showed, in addition to its antioxidant and anti-inflammatory properties, its lipid-lowering activity, anti-hypertensive, and hypoglycemic action [43][44][45][46][47].
Insulin resistance is a systemic disorder, in which there is a reduced action of insulin despite an “hyperinsulinaemia” condition, that affects many organs, in particular the liver and adipose tissue, and leads to development of two NCDs, type 2 diabetes mellitus (T2DM) and metabolic syndrome, well known cardiovascular risk factors. Recent research has described the beneficial properties of OleA and Ole-enriched olive leaf extracts against T2DM, and other metabolic syndrome associated conditions. Therefore, many studies conducted in animal and cell models have reported that Ole has the property of decreasing blood glucose and cholesterol levels, and improving oral glucose tolerance and insulin sensitivity [48][49][50]. These findings were confirmed by human clinical trial results showing that Ole treatment improved glucose homeostasis, reduced glycated hemoglobin and fasting insulin levels, suggesting a significant anti-diabetic effect [51][52][53]. Interestingly, in the context of these latter metabolic disorders, both characterized by insulin-resistance, de BocK et al. [52] showed a recovery of insulin sensitivity and pancreatic β-cell secretion capacity, in a group of overweight middle-aged men that received capsules of oleuropein-leaf extracts for 12 weeks, corroborating previous findings on the hypoglycemic effect of oleuropein [48][45][54]. Non-alcoholic fatty liver disease is another disease highly associated with insulin resistance and the metabolic syndrome, that affects about 25% of the world population, and leads non-alcoholic steatohepatitis. Research on cell and animal models have reported that oleuropein may counteract these conditions through different actions, including (i) an anti-lipidemic activity [55], (ii) protection and prevention of liver damage [56][57][58], and (iii) by interfering with signaling pathways involved in lipogenesis and in the onset of fatty liver disease [43]. Unfortunately, today these findings are not adequately supported by human studies, and remains unproven.
Therefore, in addition to the above reported properties, the ability of oleuropein to inhibit endothelial activation, monocyte cell adhesion and platelet aggregation within the concentration range expected after the nutritional intake from MD, suggest that oleuropein may also be considered an anti-atherogenic agent, reflecting its CVD protective activity [59][60][61][62][63].
4. Oleuropein As an Autophagy Inducer
Autophagy is a process by which the cells remove damaged organelles, malformed proteins or amyloid aggregate accumulation through lysosomal degradation. This is a process highly conserved and is required to maintain cellular homeostasis. Dysregulated autophagy is a common feature in NCDs implicated in NDD, metabolic syndrome, diabetes, CVDs, gastrointestinal diseases, and cancer [1]. As a master regulator of protein, lipid and carbohydrate metabolism, altered autophagy may concomitantly promote metabolic disorders and diseases associated with ageing, unhealthy diets, and inflammation [64][65][66][67][68][69][70]. In the context of CDV, several studies show that autophagy might have beneficial or detrimental roles depending on the stage and type of the considered cardiovascular disease [2]. However, the majority of studies on cardiac disorders show that autophagy may be a common cellular pathway that can be targeted for therapeutic gain, and the growing number of cardioprotective therapies affecting autophagic activity confirms this evidence [1][71][72]. In cancer cells, it is still debated whether autophagy induction or inhibition may represent the most promising approach for future cancer treatments. Interestingly, cancer cells may also use autophagy as a resistance mechanism against chemotherapy [73]. Also in the contest of NDD, such as Alzheimer’s disease (AlzD) many studies reported that the autophagy process was impaired with accumulation of extracellular protein aggregates, mainly composed by polymeric Aβ42 peptide, a product of proteolysis of APP, one of the main responsible for neurological damage and cognitive deficit [74].
All these data support that autophagy is a key factor in the pathogenesis and regulation of various kinds of diseases, serving as a potential and effective target for their intervention. Therefore, the use of substances, such as polyphenols, including OleA, that modulate autophagy and minimize the collateral effect, may be a valid therapeutic approach [75]. Indeed, several studies, some of which conducted in our laboratory, contributed to demonstrating the healthful actions of oleuropein against pathologies involving autophagy dysfunction, acting as an autophagy enhancer through several mechanisms, In our previous study performed in neuroblastoma cell lines, we found that OleA induced autophagy by activation of the Ca2+/Calmodulin Protein Kinase Kinase β (CaMKKβ)/AMPK/mTOR signalling axis [76].The effects of OleA as an autophagy inducer have also been investigated in animal transgenic models. Grossi et al.[77] using a wildtype and TgCRND8 transgenic mouse model for human Aβ pathology, demonstrated that a diet supplemented with OleA restored the defective autophagic flux by inhibition of the mTOR pathway, resulting in a remarkable cortex plaque reduction, and a recovery of the mice cognitive performance. Another mechanism through which OleA may modulate autophagy is activation of NAD-dependent deacetylase sirtuin-1 (SIRT-1). SIRT-1 influences autophagy directly (but also oxidative stress and apoptosis), via deacetylation of key components of this pathway. It showed a functional crosstalk with Poly (ADP-ribose) polymerase-1 (PARP-1) through NAD+ cofactor availability, and so any changes in levels of intracellular NAD+ and/or PARP-1 activity may influence SIRT-1 activity [78]. Luccarini et al.[79] found that PARP-1 activation matched with a significant accumulation of PAR polymers in the cortex of TgCRND8 mice at the early (3.5 month) and intermediate (six month) stages of Aβ deposition. The same TgCRND8 mice fed with a supplementation of OleA showed a rescue of both PARP-1 activation, the accumulation of its product, and increased SIRT-1 expression. Miceli et al. [80] studied the effect of OleA as an autophagy enhancer in a cardiomyocyte model, characterized by autophagy dysfunction induced by oxidative stress due to a monoamine oxidase-A (MAO-A) overexpression. They found that OleA conferred cardioprotection, not simply by its antioxidant action, but also through restoration of defective autophagic flux autophagy, due to translocation of transcriptional factor EB (TFEB) to the nucleus; this latter modulated the transcription of autophagy genes, prevented by MAO-A activation, reducing its transcriptional activity. Interestingly, TFEB translocation and autophagy recovery induced by OleA did not affect ROS status in cardiomyocytes, further highlighting its peculiarity as an autophagy inducer.5. Oleuropein as Anti–Amyloid Tool
Many neurodegenerative pathologies, among which AlzD and Parkinson’ disease (PD), together with T2DM, are amyloid diseases (AD), and belong to the NCD group. In general, AD are diseases potentially fatal, defined by the occurrence of deposition of insoluble fibrillar polymeric material, grown from misfolded proteins (amyloid) in several organs. The core of these amyloids is made of unbranched polymeric fibrils of characteristic protein or peptides, typical for each disease, such as Aß peptides for AlzD, a-synuclein for PD, amylin (hIAPP) for T2DM, and transthyretin (TTR) for familial amyloid cardiomyopathy [81][82][83]. Amyloidogenic process involves the formation of an intermediate (soluble) oligomer form, following insoluble protofibril growth. Recently, some authors have demonstrated that the cytotoxicity of different amyloidogenic proteins is due to soluble, intermediate oligomeric species, rather than to insoluble fibrillary amyloids [84]. Their cytotoxicity involves the disruption of calcium homeostasis, destabilization of membranes, ROS production, and apoptosis induction, all factors that determine cell suffering and death [85]. So, the research of compounds interfering with aggregation of amyloid proteins is recognized as a valuable approach to build new therapeutic molecules. OleA has been found, in vitro experiments, to decrease toxic oligomers formation of Aß peptide, hIAPP and α-synuclein amyloid aggregation, as well as to promote fibril and plaque disaggregation [86][87][88][89]. These actions reflect Ole beneficial effects against amyloid toxicity to cultured cells [86][90] and in transgenic model organisms [91][92]suggesting its possible use as a novel and promising pharmacological tool, acting directly on amyloid formation via protein self-assembly pathway, for prevention and therapy of systemic amyloidosis
6. Oleuropein as an Anticancer Agent and Chemotherapy Enhancer
Current protocols for cancer treatment are dependent on the condition of the tumor at time of diagnosis. If diagnosed early, the tumor mass may be removed by surgery, but if it has spread to lymph nodes, surgery will be more intensive, and chemotherapy and immunotherapy will likely be added to the treatment. Up-to-now, chemotherapy and immunotherapy represent a promising route for a more effective, life-saving cure for most human cancers. Despite advancements in these therapies, many patients with metastatic lesions still face a significant mortality risk. Furthermore, chemotherapy and immunotherapy may result in patient resistance, and generate host side effects. Therefore, new strategies that target cancer cells and also reduce resistance and patient side effects, may help the development of new treatments.
Ole has been deeply investigated in oncology for its anti-neoplastic properties; it may contribute to therapy in several ways, including its inhibitory role in some crucial cancer cell activities, which are summarized in the Figure3.
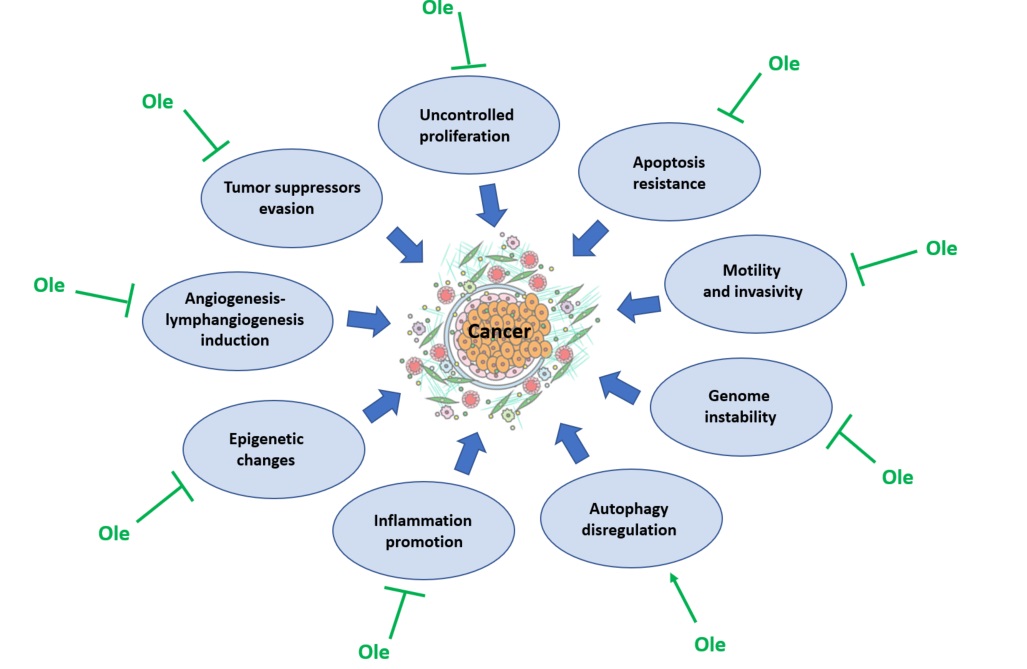
Figure 3. Effect of oleuropein (Ole) on the factors contributing to cancer development.
This polyphenol was found to inhibit two of the most important features of cancer cells, uncontrolled proliferation and resistance to apoptosis, in many types of cancers, as breast, prostate, cervical, hepatoma, neuroblastoma, colon and leukemia[93][94][95][96][97][98][99][100][101].
The main cellular pathways affected by Ole are the same implicated in cellular survival, ERK 1/2 (148), JAKs/STATs and AKT/S6 [102][103]; but they are not the only to be targeted by Ole. Ca2+ channels are very important to mantain cellular homeostasis, and Ole is able to affect them in mesothelioma [104]. Tumor invasiveness is a marker of tumor malignancy and its is closely linked with tumor dissemination and metastatization; Ole was reported to express a potent inhibitory activity on tumor xenografts, disrupting actin filaments, thus abrogating proliferation, motility, and invasiveness [105] and to inhibit angiogenesis and lymphangiogenesis in mouse melanoma cells [106]. Ole effects resulted also in decreasing the activity of MMPs, implicated in invading extracellular tissues [107][108].
Ole was found to reduce dysplasia and genome instability in colon [109], to modulate tumor suppressor genes, such as onco-miRNAs (miRNA-21 and miR-155 in breast cancer [110] and miR-137, -145, and -153, in glioblastoma multiforme cancer stem cells [111] and to enhance p53 activity in human colon cells [112].
Another aspect to consider about tumor arising and development is inflammation; Elamin et al. [113] demonstrated Ole ability to abrogate NF-kB expression in breast cancer cells and this evidence was also confirmed by Liu et al. [114].
Finally Ole exerts its anti-cancer activity by acting as autophagy disregulator, as found in breast [115] and in prostate [116] cancer cells, and by epigenetic changes through histone deacetylases (HDAC2 and HDAC3) inhibition [117].
The use of nutraceutics in oncology is also aimed to enhance the efficacy of conventional treatments and to reduce drug resistance through the combination of standard treatments with biological agents (the so called complementary therapies). Due to the several anticancer properties of Ole, it might represent an effective agent for complementary cancer therapy. This new therapeutical approach has been tested on several types of cancer with encourageous results [116][118][119][120][121][122].
Data from our laboratory [103] demonstrated that Ole enhances chemotherapy of BRAF melanoma cells, by downregulating the pAKT/pS6 pathway. Of a particular significance, the finding that Ole was able to promote the death effect of Everolimus, a mTOR inhibitor, in Vemurafenib-resistant BRAF melanoma cells, points to a possibility for its use in treating resistant melanoma cells. Ole also contributed to the cytotoxic effect of dacarbazine against BRAF melanoma cells. Furthemore in resistant melanoma cells, exposure to Ole was found to reverse trastuzumab resistance in HER2-overexpressing breast cancer cells[123].
7. Conclusions
In conclusion, several in vitro and in vivo studies have showed the ability of oleuropein (and its derivatives) to counteract oxidative stress and inflammation, to modulate the autophagy pathway, to act as amyloid inhibitor and anticancer agent suggesting its use, not only in the prevention, but also as a complementary therapy of NCD. Despite the low bioavailabilty of oleuropein [124], some clinical trials reported several beneficial effects after administration of this compound, confirming the results obtained in vitro and in vivo studies. The effective daily dose of oleuropein to be administered in humans to achieve a theraputic effect is not known, but clinical and experimental evidence suggest that regular intake of this compound can be effective in the long term, representing a continuous low-intensity stimulus to the cellular defence against NCDs [125]
References
- Daniel Peña-Oyarzun; Roberto Bravo-Sagua; Alexis Díaz Vegas; Larissa Aleman; Mario Chiong; Lorena Garcia; Claudia Bambs; Rodrigo Troncoso; Mariana Cifuentes; Eugenia Morselli; et al.Catterina FerreccioAndrew F.G. QuestAlfredo CriolloSergio Lavandero Autophagy and oxidative stress in non-communicable diseases: A matter of the inflammatory state?. Free Radical Biology and Medicine 2018, 124, 61-78, 10.1016/j.freeradbiomed.2018.05.084.
- Sergio Lavandero; Mario Chiong; Beverly A. Rothermel; Joseph A. Hill; Autophagy in cardiovascular biology. Journal of Clinical Investigation 2015, 125, 55-64, 10.1172/jci73943.
- Mariam El Assar; Javier Angulo; Leocadio Rodríguez-Mañas; Oxidative stress and vascular inflammation in aging. Free Radical Biology and Medicine 2013, 65, 380-401, 10.1016/j.freeradbiomed.2013.07.003.
- Beth Levine; Noboru Mizushima; Herbert W. Virgin; Autophagy in immunity and inflammation. Nature 2011, 469, 323-335, 10.1038/nature09782.
- Giuseppe Grosso; Francesca Bella; Justyna Godos; Salvatore Sciacca; Daniele Del Rio; Sumantra Ray; Fabio Galvano; Edward L. Giovannucci; Possible role of diet in cancer: systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutrition Reviews 2017, 75, 405-419, 10.1093/nutrit/nux012.
- Giuseppe Grosso; Justyna Godos; Rosa Lamuela-Raventos; Sumantra Ray; Agnieszka Micek; Andrzej Pajak; Salvatore Sciacca; Nicolantonio D'Orazio; Daniele Del Rio; Fabio Galvano; et al. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Molecular Nutrition & Food Research 2016, 61, 1600930, 10.1002/mnfr.201600930.
- José J. Gaforio; Francesco Visioli; Catalina Alarcón-De-La-Lastra; Olga Castañer; Miguel Delgado-Rodríguez; Monserrat Fitó; Antonio F. Hernández; Jesús R. Huertas; Miguel A. Martínez-González; Javier A. Menendez; et al.Jesús De La OsadaAngeliki PapadakiTesifón ParrónJorge E. PereiraMaría A. RosilloCristina Sánchez-QuesadaLukas SchwingshacklEstefanía ToledoAristidis M. Tsatsakis Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018. Nutrients 2019, 11, 2039, 10.3390/nu11092039.
- Maurizio Servili; Beatrice Sordini; Sonia Esposto; Stefania Urbani; Gianluca Veneziani; Ilona Di Maio; Roberto Selvaggini; Agnese Taticchi; Biological Activities of Phenolic Compounds of Extra Virgin Olive Oil. Antioxidants 2013, 3, 1-23, 10.3390/antiox3010001.
- Marie Josephe Amiot; Annie Fleuriet; Jean Jacques Macheix; Importance and evolution of phenolic compounds in olive during growth and maturation. Journal of Agricultural and Food Chemistry 1986, 34, 823-826, 10.1021/jf00071a014.
- Bernard Le Tutour; Didier Guedon; Antioxidative activities of Olea europaea leaves and related phenolic compounds. Phytochemistry 1992, 31, 1173-1178, 10.1016/0031-9422(92)80255-d.
- Marta Piroddi; Adriana Albini; Roberto Fabiani; Lisa Giovannelli; Cristina Luceri; Fausta Natella; Patrizia Rosignoli; Teresa Rossi; Agnese Taticchi; Maurizio Servili; et al.Francesco Galli Nutrigenomics of extra-virgin olive oil: A review. BioFactors 2016, 43, 17-41, 10.1002/biof.1318.
- Annalisa Romani; Stefano Mulas; Daniela Heimler; Polyphenols and secoiridoids in raw material (Olea europaea L. leaves) and commercial food supplements. European Food Research and Technology 2016, 243, 429-435, 10.1007/s00217-016-2756-3.
- Javier A. Menendez; Jorge Joven; Gerard Aragonès; Enrique Barrajón-Catalán; Raúl Beltrán-Debón; Isabel Borrás-Linares; Jordi Camps; Bruna Corominas-Faja; Sílvia Cufí; Salvador Fernández-Arroyo; et al.Anabel García-HerediaAnna Hernández-AguileraMaria Herranz-LopezCecilia Jiménez-SánchezEugeni López-BonetJesús Lozano-SánchezFedra Luciano-MateoBegoña Martín-CastilloVicente Martin ParederoAlmudena Pérez-SánchezCristina Oliveras-FerrarosMarta Riera-BorrullEsther Rodríguez-GallegoRosa Quirantes-PinéAnna RullLaura Tomás-MenorAlejandro Vazquez-MartinCarlos Alonso-VillaverdeVicente MicolAntonio Segura-Carretero Xenohormetic and anti-aging activity of secoiridoid polyphenols present in extra virgin olive oil. Cell Cycle 2013, 12, 555-578, 10.4161/cc.23756.
- Francesco Visioli; Giorgio Bellomob; Claudio Gallib; Free Radical-Scavenging Properties of Olive Oil Polyphenols. Biochemical and Biophysical Research Communications 1998, 247, 60-64, 10.1006/bbrc.1998.8735.
- Francesco Visioli; Giorgio Bellomo; Gianfranco Montedoro; Claudio Galli; Low density lipoprotein oxidation is inhibited in vitro by olive oil constituents. Atherosclerosis 1995, 117, 25-32, 10.1016/0021-9150(95)05546-9.
- E. Coni; R. Di Benedetto; Mauro Di Pasquale; Roberta Masella; D. Modesti; R. Mattei; Elisaldo Carlini; Protective effect of oleuropein, an olive oil biophenol, on low density lipoprotein oxidizability in rabbits. Lipids 2000, 35, 45-54, 10.1007/s11745-000-0493-2.
- Francesco Visioli; Donatella Caruso; Claudio Galli; Serena Viappiani; Giovanni Galli; Angelo Sala; Olive Oils Rich in Natural Catecholic Phenols Decrease Isoprostane Excretion in Humans. Biochemical and Biophysical Research Communications 2000, 278, 797-799, 10.1006/bbrc.2000.3879.
- Caterina Manna; Valentina Migliardi; Paolo Golino; Annalisa Scognamiglio; Patrizia Galletti; Massimo Chiariello; Vincenzo Zappia; Oleuropein prevents oxidative myocardial injury induced by ischemia and reperfusion. The Journal of Nutritional Biochemistry 2004, 15, 461-466, 10.1016/j.jnutbio.2003.12.010.
- Andrzej Parzonko; Monika Czerwińska; Anna Kiss; Marek Naruszewicz; Oleuropein and oleacein may restore biological functions of endothelial progenitor cells impaired by angiotensin II via activation of Nrf2/heme oxygenase-1 pathway. Phytomedicine 2013, 20, 1088-1094, 10.1016/j.phymed.2013.05.002.
- Hedya Jemai; Abdelfattah El Feki; Sami Sayadi; Antidiabetic and Antioxidant Effects of Hydroxytyrosol and Oleuropein from Olive Leaves in Alloxan-Diabetic Rats. Journal of Agricultural and Food Chemistry 2009, 57, 8798-8804, 10.1021/jf901280r.
- D. Kotyzová; A. Hodková; V. Eybl; The effect of olive oil phenolics – Hydroxytyrosol and oleuropein on antioxidant defence status in acute arsenic exposed rats. Toxicology Letters 2011, 205, S222, 10.1016/j.toxlet.2011.05.761.
- M. Dessì; Annalisa Noce; A. Agnoli; S. De Angelis; L. Fuiano; Carmela Tozzo; M. Taccone-Gallucci; G. Fuiano; G. Federici; The usefulness of the prognostic inflammatory and nutritional index (PINI) in a haemodialysis population. Nutrition, Metabolism and Cardiovascular Diseases 2009, 19, 811-815, 10.1016/j.numecd.2009.01.009.
- Kimberly D. Jacob; Nicole Noren Hooten; Andrzej R. Trzeciak; Michele K. Evans; Markers of oxidant stress that are clinically relevant in aging and age-related disease. Mechanisms of Ageing and Development 2013, 134, 139-157, 10.1016/j.mad.2013.02.008.
- Joanna Collerton; Carmen Martin-Ruiz; Karen Davies; Catharien M. Hilkens; John Isaacs; Claire Kolenda; Craig Parker; Michael Dunn; Michael Catt; Carol Jagger; et al.Thomas von ZglinickiThomas B.L. Kirkwood Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: Cross-sectional findings from the Newcastle 85+ Study. Mechanisms of Ageing and Development 2012, 133, 456-466, 10.1016/j.mad.2012.05.005.
- Karen Bandeen-Roche; Jeremy D. Walston; Yi Huang; Richard D. Semba; Luigi Ferrucci; Measuring Systemic Inflammatory Regulation in Older Adults: Evidence and Utility. Rejuvenation Research 2009, 12, 403-410, 10.1089/rej.2009.0883.
- Imededdine Hassen; Hervé Casabianca; Karim Hosni; Biological activities of the natural antioxidant oleuropein: Exceeding the expectation – A mini-review. Journal of Functional Foods 2015, 18, 926-940, 10.1016/j.jff.2014.09.001.
- Ramon Estruch; Miguel A. Martinez-Gonzalez; Dolores Corella; Jordi Salas-Salvadó; Valentina Ruiz-Gutiérrez; María Isabel Covas; Miguel Fiol; Enrique Gómez-Gracia; Mari Carmen López-Sabater; Ernest Vinyoles; et al.Fernando ArósManuel CondeCarlos LahozJosé LapetraGuillermo SáezEmilio Ros Effects of a Mediterranean-Style Diet on Cardiovascular Risk Factors. Annals of Internal Medicine 2006, 145, 1-11, 10.7326/0003-4819-145-1-200607040-00004.
- Ramon Estruch; Anti-inflammatory effects of the Mediterranean diet: the experience of the PREDIMED study. Proceedings of the Nutrition Society 2010, 69, 333-340, 10.1017/s0029665110001539.
- Mari-Pau Mena; Emilio Sacanella; Mónica Vazquez-Agell; Mercedes Morales; Montserrat Fitó; Rosa Escoda; Manuel Serrano-Martínez; Jordi Salas-Salvadó; Neus Benages; Rosa Casas; et al.Rosa M Lamuela-RaventosFerran MasanesEmilio RosRamon Estruch Inhibition of circulating immune cell activation: a molecular antiinflammatory effect of the Mediterranean diet. The American Journal of Clinical Nutrition 2008, 89, 248-256, 10.3945/ajcn.2008.26094.
- Mireia Urpi-Sarda; Rosa Casas; Gemma Chiva-Blanch; Edwin Saúl Romero-Mamani; Palmira Valderas-Martínez; Jordi Salas-Salvadó; María Isabel Covas; Estefania Toledo; Cristina Andres-Lacueva; Rafael Llorach; et al.Ana García-ArellanoMònica BullóValentina Ruiz-GutierrezRosa M Lamuela-RaventosRamon Estruch The Mediterranean Diet Pattern and Its Main Components Are Associated with Lower Plasma Concentrations of Tumor Necrosis Factor Receptor 60 in Patients at High Risk for Cardiovascular Disease. The Journal of Nutrition 2012, 142, 1019-1025, 10.3945/jn.111.148726.
- Nicola Di Daniele; Laura Di Renzo; Annalisa Noce; Leonardo Iacopino; Pietro Manuel Ferraro; Mariagiovanna Rizzo; Francesca Sarlo; Emidio Domino; Antonino De Lorenzo; Effects of Italian Mediterranean organic diet vs. low-protein diet in nephropathic patients according to MTHFR genotypes. Journal of Nephrology 2014, 27, 529-536, 10.1007/s40620-014-0067-y.
- Evangelos J. Giamarellos-Bourboulis; Taxiarchis Geladopoulos; Michael Chrisofos; Pantelis Koutoukas; John Vassiliadis; Ioannis Alexandrou; Thomas Tsaganos; Labros Sabracos; Vassiliki Karagianni; Emilia Pelekanou; et al.Ira TzepiHariklia KranidiotiVassilios KoussoulasHelen Giamarellou OLEUROPEIN. Shock 2006, 26, 410-416, 10.1097/01.shk.0000226342.70904.06.
- Daniela Impellizzeri; Emanuela Esposito; Emanuela Mazzon; Irene Paterniti; Rosanna Di Paola; Placido Bramanti; Valeria Maria Morittu; Antonio Procopio; Domenico Britti; Salvatore Cuzzocrea; et al. The effects of oleuropein aglycone, an olive oil compound, in a mouse model of carrageenan-induced pleurisy. Clinical Nutrition 2011, 30, 533-540, 10.1016/j.clnu.2011.02.004.
- Elizabeth A. Miles; Pinelope Zoubouli; Philip Calder; Differential anti-inflammatory effects of phenolic compounds from extra virgin olive oil identified in human whole blood cultures. Nutrition 2005, 21, 389-394, 10.1016/j.nut.2004.06.031.
- Rocio de la Puerta; Valentina Ruiz Gutierrez; J.Robin S. Hoult; Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochemical Pharmacology 1999, 57, 445-449, 10.1016/s0006-2952(98)00320-7.
- Su-Jung Ryu; Hyeon-Son Choi; Kye-Yoon Yoon; Ok-Hwan Lee; Kui-Jin Kim; Boo-Yong Lee; Oleuropein Suppresses LPS-Induced Inflammatory Responses in RAW 264.7 Cell and Zebrafish. Journal of Agricultural and Food Chemistry 2015, 63, 2098-2105, 10.1021/jf505894b.
- Francesca Margheri; Beatrice Menicacci; Anna Laurenzana; Mario Del Rosso; Gabriella Fibbi; Maria Grazia Cipolleschi; Jessica Ruzzolini; Chiara Nediani; Alessandra Mocali; Lisa Giovannelli; et al. Oleuropein aglycone attenuates the pro-angiogenic phenotype of senescent fibroblasts: A functional study in endothelial cells. Journal of Functional Foods 2018, 53, 219-226, 10.1016/j.jff.2018.12.026.
- Elisa Giner; María-Carmen Recio; José-Luis Ríos; Rosa-María Giner; Oleuropein Protects against Dextran Sodium Sulfate-Induced Chronic Colitis in Mice. Journal of Natural Products 2013, 76, 1113-1120, 10.1021/np400175b.
- Bombi Lee; Insop Shim; Hyejung Lee; Dae-Hyun Hahm; Effect of oleuropein on cognitive deficits and changes in hippocampal brain-derived neurotrophic factor and cytokine expression in a rat model of post-traumatic stress disorder. Journal of Natural Medicines 2017, 72, 44-56, 10.1007/s11418-017-1103-8.
- Ali Reza Khalatbary; Gh R Zarrinjoei; Anti-Inflammatory Effect of Oleuropein in Experimental Rat Spinal Cord Trauma. Iranian Red Crescent Medical Journal 2012, 14, 229-234.
- Caroline Puel; Jacinthe Mathey; Apostolis Agalias; Séraphin Kati-Coulibaly; Julie Mardon; Christiane Obled; Marie-Jeanne Davicco; Patrice Lebecque; Marie-Noelle Horcajada; Alexios L. Skaltsounis; et al.Véronique Coxam Dose–response study of effect of oleuropein, an olive oil polyphenol, in an ovariectomy/inflammation experimental model of bone loss in the rat. Clinical Nutrition 2006, 25, 859-868, 10.1016/j.clnu.2006.03.009.
- Tiziana LaRussa; Manuela Oliverio; Evelina Suraci; Marta Greco; Roberta Placida; Serena Gervasi; Raffaella Marasco; Maria Imeneo; Donatella Paolino; Luigi Tucci; et al.Elio GullettaMassimo FrestaAntonio ProcopioFrancesco Luzza Oleuropein Decreases Cyclooxygenase-2 and Interleukin-17 Expression and Attenuates Inflammatory Damage in Colonic Samples from Ulcerative Colitis Patients. Nutrients 2017, 9, 391, 10.3390/nu9040391.
- Soyoung Park; Youngshim Choi; Soo-Jong Um; Seung Kew Yoon; Taesun Park; Oleuropein attenuates hepatic steatosis induced by high-fat diet in mice. Journal of Hepatology 2010, 54, 984-993, 10.1016/j.jhep.2010.08.019.
- Hemant Poudyal; Fiona Campbell; Lindsay Brown; Olive Leaf Extract Attenuates Cardiac, Hepatic, and Metabolic Changes in High Carbohydrate–, High Fat–Fed Rats. The Journal of Nutrition 2010, 140, 946-953, 10.3945/jn.109.117812.
- Kazutoshi Murotomi; Aya Umeno; Mayu Yasunaga; Mototada Shichiri; Noriko Ishida; Taisuke Koike; Toshiki Matsuo; Hiroko Abe; Yasukazu Yoshida; Yoshihiro Nakajima; et al. Oleuropein-Rich Diet Attenuates Hyperglycemia and Impaired Glucose Tolerance in Type 2 Diabetes Model Mouse. Journal of Agricultural and Food Chemistry 2015, 63, 6715-6722, 10.1021/acs.jafc.5b00556.
- Stacey Lockyer; Ian Rowland; Jeremy Paul Edward Spencer; Parveen Yaqoob; Welma Stonehouse; Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: a randomised controlled trial. European Journal of Nutrition 2016, 56, 1421-1432, 10.1007/s00394-016-1188-y.
- Endang Susalit; Nafrialdi Agus; Imam Effendi; Raymond Tjandrawinata; Dwi Nofiarny; Tania Perrinjaquet-Moccetti; Marian Verbruggen; Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: Comparison with Captopril. Phytomedicine 2011, 18, 251-258, 10.1016/j.phymed.2010.08.016.
- Hasan Fayadh Al-Azzawie; Mohamed-Saiel Saeed Alhamdani; Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sciences 2006, 78, 1371-1377, 10.1016/j.lfs.2005.07.029.
- A. Eidi; Maryam Eidi; R. Darzi; Antidiabetic effect ofOlea europaeaL. in normal and diabetic rats. Phytotherapy Research 2009, 23, 347-350, 10.1002/ptr.2629.
- Fatma Hadrich; Marie Garcia; Amina Maalej; Marthe Moldes; Hiroko Isoda; Bruno Feve; Sami Sayadi; Oleuropein activated AMPK and induced insulin sensitivity in C2C12 muscle cells. Life Sciences 2016, 151, 167-173, 10.1016/j.lfs.2016.02.027.
- Roberto Carnevale; Romano Silvestri; Lorenzo Loffredo; Marta Novo; Vittoria Cammisotto; Valentina Castellani; Simona Bartimoccia; Cristina Nocella; Francesco Violi; Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. British Journal of Clinical Pharmacology 2018, 84, 1566-1574, 10.1111/bcp.13589.
- Martin De Bock; José Derraik; Christine M. Brennan; Janene B. Biggs; Philip Morgan; Steven C. Hodgkinson; Paul L. Hofman; Wayne S. Cutfield; Olive (Olea europaea L.) Leaf Polyphenols Improve Insulin Sensitivity in Middle-Aged Overweight Men: A Randomized, Placebo-Controlled, Crossover Trial. PLOS ONE 2013, 8, e57622, 10.1371/journal.pone.0057622.
- Julio Wainstein; Tali Ganz; Mona Boaz; Yosefa Bar Dayan; Eran Dolev; Zohar Kerem; Zecharia Madar; Olive Leaf Extract as a Hypoglycemic Agent in Both Human Diabetic Subjects and in Rats. Journal of Medicinal Food 2012, 15, 605-610, 10.1089/jmf.2011.0243.
- Hakam Alkhateeb; Mohammed Al-Duais; Esam Qnais; Beneficial effects of oleuropein on glucose uptake and on parameters relevant to the normal homeostatic mechanisms of glucose regulation in rat skeletal muscle. Phytotherapy Research 2018, 32, 651-656, 10.1002/ptr.6012.
- Ioanna Andreadou; Efstathios K. Iliodromitis; Emmanuel Mikros; Maria Constantinou; Apostolos Agalias; Prokopios Magiatis; Alexios Leandros Skaltsounis; Elli Kamber; Anna Tsantili-Kakoulidou; Dimitrios Th Kremastinos; et al. The Olive Constituent Oleuropein Exhibits Anti-Ischemic, Antioxidative, and Hypolipidemic Effects in Anesthetized Rabbits. The Journal of Nutrition 2006, 136, 2213-2219, 10.1093/jn/136.8.2213.
- Wonhee Hur; Sung Woo Kim; Young Ki Lee; Jung Eun Choi; Sung Woo Hong; Myeong Jun Song; Si Hyun Bae; Taesun Park; Soo-Jong Um; Seung Kew Yoon; et al. Oleuropein reduces free fatty acid-induced lipogenesis via lowered extracellular signal-regulated kinase activation in hepatocytes. Nutrition Research 2012, 32, 778-786, 10.1016/j.nutres.2012.06.017.
- Robert Domitrović; Hrvoje Jakovac; Vanja Vasiljev Marchesi; Ivana Šain; Željko Romić; Dario Rahelić; Preventive and therapeutic effects of oleuropein against carbon tetrachloride-induced liver damage in mice. Pharmacological Research 2012, 65, 451-464, 10.1016/j.phrs.2011.12.005.
- Sung Woo Kim; Wonhee Hur; Tian Zhu Li; Young Ki Lee; Jung Eun Choi; Sung Woo Hong; Kwang-Soo Lyoo; Chan Ran You; Eun Sun Jung; Chan Kun Jung; et al.Taesun ParkSoo-Jong UmSeung Kew Yoon Oleuropein prevents the progression of steatohepatitis to hepatic fibrosis induced by a high-fat diet in mice.. Experimental & Molecular Medicine 2014, 46, e92-e92, 10.1038/emm.2014.10.
- José Manuel Lou Bonafonte; Carmen Arnal; María A. Navarro; Jesús Osada; Efficacy of bioactive compounds from extra virgin olive oil to modulate atherosclerosis development. Molecular Nutrition & Food Research 2012, 56, 1043-1057, 10.1002/mnfr.201100668.
- Maria Annunziata Carluccio; Luisa Siculella; Maria Assunta Ancora; Marika Massaro; Egeria Scoditti; Carlo Storelli; Francesco Visioli; Alessandro Distante; Raffaele De Caterina; Olive Oil and Red Wine Antioxidant Polyphenols Inhibit Endothelial Activation. Arteriosclerosis, Thrombosis, and Vascular Biology 2003, 23, 622-629, 10.1161/01.atv.0000062884.69432.a0.
- Mario Dell'Agli; Rossana Fagnani; Nico Mitro; Samuele Scurati; Maura Masciadri; Luciana Mussoni; Germana V. Galli; Enrica Bosisio; Maurizio Crestani; Emma De Fabiani; et al.Elena TremoliDonatella Caruso Minor Components of Olive Oil Modulate Proatherogenic Adhesion Molecules Involved in Endothelial Activation. Journal of Agricultural and Food Chemistry 2006, 54, 3259-3264, 10.1021/jf0529161.
- Stacey Lockyer; Giulia Corona; Parveen Yaqoob; Jeremy P. E. Spencer; Ian Rowland; Secoiridoids delivered as olive leaf extract induce acute improvements in human vascular function and reduction of an inflammatory cytokine: a randomised, double-blind, placebo-controlled, cross-over trial. British Journal of Nutrition 2015, 114, 75-83, 10.1017/s0007114515001269.
- Mario Dell'Agli; Omar Maschi; Germana V. Galli; Rossana Fagnani; Esther Dal Cero; Donatella Caruso; Enrica Bosisio; Inhibition of platelet aggregation by olive oil phenols via cAMP-phosphodiesterase. British Journal of Nutrition 2008, 99, 945-951, 10.1017/s0007114507837470.
- Shigeto Sato; Nobutaka Hattori; Dopaminergic Neuron-Specific Autophagy-Deficient Mice. Methods in Molecular Biology 2018, 1759, 173-175, 10.1007/7651_2018_156.
- Masaaki Komatsu; Satoshi Waguri; Takashi Ueno; Junichi Iwata; Shigeo Murata; Isei Tanida; Junji Ezaki; Noboru Mizushima; Yoshinori Ohsumi; Yasuo Uchiyama; et al.Eiki KominamiKeiji TanakaTomoki Chiba Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. Journal of Cell Biology 2005, 169, 425-434, 10.1083/jcb.200412022.
- Takeshi Yoshizaki; Chisato Kusunoki; Motoyuki Kondo; Mako Yasuda; Shinji Kume; Katsutaro Morino; Osamu Sekine; Satoshi Ugi; Takashi Uzu; Yoshihiko Nishio; et al.Atsunori KashiwagiHiroshi Maegawa Autophagy regulates inflammation in adipocytes. Biochemical and Biophysical Research Communications 2012, 417, 352-357, 10.1016/j.bbrc.2011.11.114.
- Ling Yang; Ping Li; Suneng Fu; Ediz Calay; Gökhan S. Hotamisligil; Defective Hepatic Autophagy in Obesity Promotes ER Stress and Causes Insulin Resistance. Cell Metabolism 2010, 11, 467-478, 10.1016/j.cmet.2010.04.005.
- Hwan-Woo Park; Haeli Park; Ian A. Semple; Insook Jang; Seung-Hyun Ro; Myungjin Kim; Victor A Cazares; Edward Stuenkel; Jung-Jae Kim; Jeong Sig Kim; et al.Jun Hee Lee Pharmacological correction of obesity-induced autophagy arrest using calcium channel blockers. Nature Communications 2014, 5, 1-12, 10.1038/ncomms5834.
- Susmita Kaushik; Jose Antonio Rodriguez-Navarro; Esperanza Arias; Roberta Kiffin; Srabani Sahu; Gary J. Schwartz; Ana Maria Cuervo; Rajat Singh; Autophagy in Hypothalamic AgRP Neurons Regulates Food Intake and Energy Balance. Cell Metabolism 2011, 14, 173-183, 10.1016/j.cmet.2011.06.008.
- Shakeel U.R. Mir; Nicholas M. George; Lubna Zahoor; Robert Harms; Zachary Guinn; Nora E. Sarvetnick; Inhibition of Autophagic Turnover in β-Cells by Fatty Acids and Glucose Leads to Apoptotic Cell Death. Journal of Biological Chemistry 2015, 290, 6071-6085, 10.1074/jbc.m114.605345.
- Andriy Nemchenko; Mario Chiong; Aslan Turer; Sergio Lavandero; Joseph A. Hill; Autophagy as a therapeutic target in cardiovascular disease. Journal of Molecular and Cellular Cardiology 2011, 51, 584-593, 10.1016/j.yjmcc.2011.06.010.
- Oktay F. Rifki; Joseph A. Hill; Cardiac Autophagy. Journal of Cardiovascular Pharmacology 2012, 60, 248-252, 10.1097/fjc.0b013e3182646cb1.
- Li-Mei Fang; Bin Li; Jun-Jie Guan; Hai-Dong Xu; Gen-Hai Shen; Quan-Gen Gao; Zheng-Hong Qin; Transcription factor EB is involved in autophagy-mediated chemoresistance to doxorubicin in human cancer cells. Acta Pharmacologica Sinica 2017, 38, 1305-1316, 10.1038/aps.2017.25.
- Joaquín G. Cordero; Ramon Garcia-Escudero; Jesús Avila; Ricardo Gargini; Vega García-Escudero; Benefit of Oleuropein Aglycone for Alzheimer’s Disease by Promoting Autophagy. Oxidative Medicine and Cellular Longevity 2018, 2018, 1-12, 10.1155/2018/5010741.
- Rocío M. de Pablos; Ana María Espinosa-Oliva; Ruth Hornedo-Ortega; Mercedes Cano; Sandro Arguelles; Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases. Pharmacological Research 2019, 143, 58-72, 10.1016/j.phrs.2019.03.005.
- Stefania Rigacci; Caterina Miceli; Chiara Nediani; Andrea Berti; Roberta Cascella; Daniela Pantano; Pamela Nardiello; Ilaria Luccarini; Fiorella Casamenti; Massimo Stefani; et al. Oleuropein aglycone induces autophagy via the AMPK/mTOR signalling pathway: a mechanistic insight. Oncotarget 2015, 6, 35344-35357, 10.18632/oncotarget.6119.
- Cristina Grossi; Stefania Rigacci; Stefano Ambrosini; Teresa Ed Dami; Ilaria Luccarini; Chiara Traini; Paola Failli; Andrea Berti; Fiorella Casamenti; Massimo Stefani; et al. The Polyphenol Oleuropein Aglycone Protects TgCRND8 Mice against Aß Plaque Pathology. PLOS ONE 2013, 8, e71702, 10.1371/journal.pone.0071702.
- Sangwoon Chung; Hongwei Yao; Samuel Caito; Jae-Woong Hwang; Gnanapragasam Arunachalam; Irfan Rahman; Regulation of SIRT1 in cellular functions: Role of polyphenols. Archives of Biochemistry and Biophysics 2010, 501, 79-90, 10.1016/j.abb.2010.05.003.
- Ilaria Luccarini; Daniela Pantano; Pamela Nardiello; Leonardo Cavone; Andrea Lapucci; Caterina Miceli; Chiara Nediani; Andrea Berti; Massimo Stefani; Fiorella Casamenti; et al. The Polyphenol Oleuropein Aglycone Modulates the PARP1-SIRT1 Interplay: An In Vitro and In Vivo Study. Journal of Alzheimer's Disease 2016, 54, 737-750, 10.3233/JAD-160471.
- Caterina Miceli; Yohan Santin; Nicola Manzella; Raffaele Coppini; Andrea Berti; Massimo Stefani; Angelo Parini; Jeanne Mialet-Perez; Chiara Nediani; Oleuropein Aglycone Protects against MAO-A-Induced Autophagy Impairment and Cardiomyocyte Death through Activation of TFEB. Oxidative Medicine and Cellular Longevity 2018, 2018, 1-13, 10.1155/2018/8067592.
- Massimo Stefani; Christopher M. Dobson; Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. Journal of Molecular Medicine 2003, 81, 678-699, 10.1007/s00109-003-0464-5.
- Fabrizio Chiti; Christopher M. Dobson; Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annual Review of Biochemistry 2017, 86, 27-68, 10.1146/annurev-biochem-061516-045115.
- Stefania Rigacci; Massimo Stefani; Nutraceutical Properties of Olive Oil Polyphenols. An Itinerary from Cultured Cells through Animal Models to Humans. International Journal of Molecular Sciences 2016, 17, 843, 10.3390/ijms17060843.
- Viviane L. Ndam Ngoungoure; Jan Schluesener; Paul F. Moundipa; Hermann Schluesener; Natural polyphenols binding to amyloid: A broad class of compounds to treat different human amyloid diseases. Molecular Nutrition & Food Research 2014, 59, 8-20, 10.1002/mnfr.201400290.
- Fiorella Malchiodi-Albedi; Silvia Paradisi; Andrea Matteucci; Claudio Frank; Marco Diociaiuti; Amyloid Oligomer Neurotoxicity, Calcium Dysregulation, and Lipid Rafts. International Journal of Alzheimer’s Disease 2011, 2011, 1-17, 10.4061/2011/906964.
- Stefania Rigacci; Valentina Guidotti; Monica Bucciantini; Daniela Nichino; Annalisa Relini; Andrea Berti; Massimo Stefani; Aβ(1-42) Aggregates into Non-Toxic Amyloid Assemblies in the Presence of the Natural Polyphenol Oleuropein Aglycon. Current Alzheimer Research 2011, 8, 841-852, 10.2174/156720511798192682.
- Luana Palazzi; Elena Bruzzone; Giovanni Bisello; Manuela Leri; Massimo Stefani; Monica Bucciantini; Patrizia Polverino De Laureto; Oleuropein aglycone stabilizes the monomeric α-synuclein and favours the growth of non-toxic aggregates. Scientific Reports 2018, 8, 1-17, 10.1038/s41598-018-26645-5.
- Hossein Mohammad-Beigi; Farhang Aliakbari; Cagla Sahin; Charlotte Lomax; Ahmed Tawfike; Nicholas P. Schafer; Alireza Amiri-Nowdijeh; Hoda Eskandari; Ian Max Møller; Mehdi Hosseini-Mazinani; et al.Gunna ChristiansenJane L. WardDina MorshediDaniel E. Otzen Oleuropein derivatives from olive fruit extracts reduce α-synuclein fibrillation and oligomer toxicity. Journal of Biological Chemistry 2019, 294, 4215-4232, 10.1074/jbc.ra118.005723.
- Stefania Rigacci; Valentina Guidotti; Monica Bucciantini; Matteo Parri; Chiara Nediani; Elisabetta Cerbai; Massimo Stefani; Andrea Berti; Oleuropein aglycon prevents cytotoxic amyloid aggregation of human amylin. The Journal of Nutritional Biochemistry 2010, 21, 726-735, 10.1016/j.jnutbio.2009.04.010.
- Elisabetta Borchi; Valentina Bargelli; Valentina Guidotti; Andrea Berti; Massimo Stefani; Chiara Nediani; Stefania Rigacci; Mild exposure of RIN-5F β-cells to human islet amyloid polypeptide aggregates upregulates antioxidant enzymes via NADPH oxidase-RAGE: an hormetic stimulus.. Redox Biology 2013, 2, 114-22, 10.1016/j.redox.2013.12.005.
- Luisa Diomede; Stefania Rigacci; Margherita Romeo; Massimo Stefani; Mario Salmona; Oleuropein Aglycone Protects Transgenic C. elegans Strains Expressing Aβ42 by Reducing Plaque Load and Motor Deficit. PLOS ONE 2013, 8, e58893, 10.1371/journal.pone.0058893.
- Ilaria Luccarini; Teresa Ed Dami; Cristina Grossi; Stefania Rigacci; Massimo Stefani; Fiorella Casamenti; Oleuropein aglycone counteracts Aβ42 toxicity in the rat brain. Neuroscience Letters 2014, 558, 67-72, 10.1016/j.neulet.2013.10.062.
- Zouhaier Bouallagui; Junkuy Han; Hiroko Isoda; Sami Sayadi; Hydroxytyrosol rich extract from olive leaves modulates cell cycle progression in MCF-7 human breast cancer cells. Food and Chemical Toxicology 2010, 49, 179-184, 10.1016/j.fct.2010.10.014.
- Junkyu Han; Terence P. N. Talorete; Parida Yamada; Hiroko Isoda; Anti-proliferative and apoptotic effects of oleuropein and hydroxytyrosol on human breast cancer MCF-7 cells. Cytotechnology 2009, 59, 45-53, 10.1007/s10616-009-9191-2.
- Rosa Sirianni; Adele Chimento; Arianna De Luca; Ivan Casaburi; Pietro Rizza; Arianna Onofrio; Domenico Iacopetta; Francesco Puoci; Sebastiano Andò; Marcello Maggiolini; et al.Vincenzo Pezzi Oleuropein and hydroxytyrosol inhibit MCF-7 breast cancer cell proliferation interfering with ERK1/2 activation. Molecular Nutrition & Food Research 2009, 54, 833-840, 10.1002/mnfr.200900111.
- Luca Vanella; Rosaria Acquaviva; Claudia Di Giacomo; Valeria Sorrenti; Fabio Galvano; Rosa Santangelo; Venera Cardile; Silvia Gangia; Nicolantonio D'Orazio; Nader G. Abraham; et al. Antiproliferative effect of oleuropein in prostate cell lines. International Journal of Oncology 2012, 41, 31-38, 10.3892/ijo.2012.1428.
- Jie Yao; Jibing Wu; Xue Yang; Jiaxi Yang; Yuxing Zhang; Linfang Du; Oleuropein Induced Apoptosis in HeLa Cells via a Mitochondrial Apoptotic Cascade Associated With Activation of the c-Jun NH2-Terminal Kinase. Journal of Pharmacological Sciences 2014, 125, 300-311, 10.1254/jphs.14012fp.
- Chun-Mei Yan; Er-Qing Chai; Hong-Yi Cai; Guo-Ying Miao; Wen Ma; Oleuropein induces apoptosis via activation of caspases and suppression of phosphatidylinositol 3-kinase/protein kinase B pathway in HepG2 human hepatoma cell line. Molecular Medicine Reports 2015, 11, 4617-4624, 10.3892/mmr.2015.3266.
- Mücahit Seçme; Canan Eroğlu; Yavuz Dodurga; Gülseren Bağcı; Investigation of anticancer mechanism of oleuropein via cell cycle and apoptotic pathways in SH-SY5Y neuroblastoma cells. Gene 2016, 585, 93-99, 10.1016/j.gene.2016.03.038.
- Wafa Zeriouh; Abdelhafid Nani; Meriem Belarbi; Adélie Dumont; Charlotte De Rosny; Ikram Aboura; Fatima Zahra Ghanemi; Babar Murtaza; Danish Patoli; Charles Thomas; et al.Lionel ApetohCédric RébéMinique DelmasNaim Akhtar KhanFrançois GhiringhelliMickaël RiallandAziz Hichami Correction: Phenolic extract from oleaster (Olea europaea var. Sylvestris) leaves reduces colon cancer growth and induces caspase-dependent apoptosis in colon cancer cells via the mitochondrial apoptotic pathway. PLOS ONE 2017, 12, e0176574, 10.1371/journal.pone.0176574.
- Jaouad Anter; Zahira Fernández-Bedmar; Myriam Villatoro; Sebastián Demyda-Peyrás; Miguel Moreno-Millán; Ángeles Alonso-Moraga; Andrés Muñoz-Serrano; Maria D. Luque de Castro; A pilot study on the DNA-protective, cytotoxic, and apoptosis-inducing properties of olive-leaf extracts. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 2011, 723, 165-170, 10.1016/j.mrgentox.2011.05.005.
- Saeideh Momtaz; Kamal Niaz; Faheem Maqbool; Mohammad Abdollahi; Luca Rastrelli; Seyed Mohammad Nabavi; STAT3 targeting by polyphenols: Novel therapeutic strategy for melanoma. BioFactors 2016, 43, 347-370, 10.1002/biof.1345.
- Jessica Ruzzolini; Silvia Peppicelli; Elena Andreucci; Francesca Bianchini; Arianna Scardigli; Annalisa Romani; Giancarlo La Marca; Chiara Nediani; Lido Calorini; Oleuropein, the Main Polyphenol of Olea europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients 2018, 10, 1950, 10.3390/nu10121950.
- Carla Marchetti; Marco Clericuzio; Barbara Borghesi; Laura Cornara; Stefania Ribulla; Fabio Gosetti; Emilio Marengo; Bruno Burlando; Oleuropein-Enriched Olive Leaf Extract Affects Calcium Dynamics and Impairs Viability of Malignant Mesothelioma Cells. Evidence-Based Complementary and Alternative Medicine 2015, 2015, 1-9, 10.1155/2015/908493.
- Hamdi K. Hamdi; Raquel Castellon; Oleuropein, a non-toxic olive iridoid, is an anti-tumor agent and cytoskeleton disruptor. Biochemical and Biophysical Research Communications 2005, 334, 769-778, 10.1016/j.bbrc.2005.06.161.
- Hyerim Song; Do Young Lim; Jae In Jung; Han Jin Cho; So Young Park; Gyoo Taik Kwon; Young-Hee Kang; Ki Won Lee; Myung-Sook Choi; Jung Han Yoon Park; et al. Dietary oleuropein inhibits tumor angiogenesis and lymphangiogenesis in the B16F10 melanoma allograft model: a mechanism for the suppression of high-fat diet-induced solid tumor growth and lymph node metastasis. Oncotarget 2017, 8, 32027-32042, 10.18632/oncotarget.16757.
- Ming Liu; Jian Wang; Bin Huang; Anjing Chen; Xingang Li; Oleuropein inhibits the proliferation and invasion of glioma cells via suppression of the AKT signaling pathway. Oncology Reports 2016, 36, 2009-2016, 10.3892/or.2016.4978.
- Zeinab K. Hassan; Maha H. Elamin; Maha H. Daghestani; Sawsan A. Omer; Ebtesam M. Al-Olayan; Mai A. Elobeid; Promy Virk; Osama Mohammed; Oleuropein Induces Anti-metastatic Effects in Breast Cancer. Asian Pacific Journal of Cancer Prevention 2012, 13, 4555-4559, 10.7314/apjcp.2012.13.9.4555.
- Maria Vittoria Sepporta; Raffaela Fuccelli; Patrizia Rosignoli; Giovanni Ricci; Maurizio Servili; Roberto Fabiani; Oleuropein Prevents Azoxymethane-Induced Colon Crypt Dysplasia and Leukocytes DNA Damage in A/J Mice. Journal of Medicinal Food 2016, 19, 983-989, 10.1089/jmf.2016.0026.
- Maryam Abtin; Mohammad R. Alivand; Mahmoud S. Khaniani; Milad Bastami; Mohammad Zaeifizadeh; Sima M. Derakhshan; Simultaneous downregulation of miR‐21 and miR‐155 through oleuropein for breast cancer prevention and therapy. Journal of Cellular Biochemistry 2018, 119, 7151-7165, 10.1002/jcb.26754.
- Gulcin Tezcan; Berrin Tunca; Ahmet Bekar; Ferah Budak; Saliha Sahin; Gulsah Cecener; Unal Egeli; Mevlut Ozgur Taskapılıoglu; Hasan Kocaeli; Sahsine Tolunay; et al.Hulusi MalyerCevdet DemirGulendam Tumen Olea europaea leaf extract improves the treatment response of GBM stem cells by modulating miRNA expression. American journal of cancer research 2014, 4, 572-590.
- Ana Cárdeno; Marina Sánchez-Hidalgo; Maria Angeles Rosillo; Catalina Alarcon De La Lastra; Oleuropein, a Secoiridoid Derived from Olive Tree, Inhibits the Proliferation of Human Colorectal Cancer Cell Through Downregulation of HIF-1α. Nutrition and Cancer 2013, 65, 147-156, 10.1080/01635581.2013.741758.
- Maha H. Elamin; Maha H. Daghestani; Sawsan A. Omer; Mai A Elobeid; Promy Virk; Ebtesam M. Al-Olayan; Zeinab K. Hassan; Osama B. Mohammed; Abdelilah Aboussekhra; Olive oil oleuropein has anti-breast cancer properties with higher efficiency on ER-negative cells. Food and Chemical Toxicology 2013, 53, 310-316, 10.1016/j.fct.2012.12.009.
- Lian Liu; Kwang Seok Ahn; Muthu K Shanmugam; Hong Wang; Hongyuan Shen; Frank Arfuso; Arunachalam Chinnathambi; Sulaiman Ali Alharbi; Yung Chang; Gautam Sethi; et al.Feng Ru Tang Oleuropein induces apoptosis via abrogating NF‐κB activation cascade in estrogen receptor–negative breast cancer cells. Journal of Cellular Biochemistry 2018, 120, 4504-4513, 10.1002/jcb.27738.
- Hui-Yuan Lu; Jian-Sheng Zhu; Zhan Zhang; Wei-Jian Shen; Shan Jiang; Yun-Feng Long; Bin Wu; Tao Ding; Fei Huan; Shou-Lin Wang; et al. Hydroxytyrosol and Oleuropein Inhibit Migration and Invasion of MDA-MB-231 Triple-Negative Breast Cancer Cell via Induction of Autophagy. Anti-Cancer Agents in Medicinal Chemistry 2020, 19, 1983-1990, 10.2174/1871520619666190722101207.
- Anastasia Papachristodoulou; Magafoula Tsoukala; Dimitra Benaki; Sarantos Kostidis; Katerina Gioti; Nektarios Aligiannis; Harris Pratsinis; Dimitris Kletsas; Alexios-Leandros Skaltsounis; Emmanuel Mikros; et al.Roxane Tenta Oleuropein is a Powerful Sensitizer of Doxorubicin-mediated Killing of Prostate Cancer Cells and Exerts Its Action via Induction of Autophagy. Journal of Cancer Research and Treatment 2016, 4, 61-68, 10.12691/jcrt-4-4-2.
- Sahar Bayat; Mahmoud Shekari Khaniani; Jalal Choupani; Mohammad Reza Alivand; Sima Mansoori Derakhshan; HDACis (class I), cancer stem cell, and phytochemicals: Cancer therapy and prevention implications. Biomedicine & Pharmacotherapy 2018, 97, 1445-1453, 10.1016/j.biopha.2017.11.065.
- Ioanna Andreadou; Emmanuel Mikros; Konstantinos Ioannidis; Fragiska Sigala; Katerina Naka; Sarantos Kostidis; Dimitrios Farmakis; Roxane Tenta; Nikolaos Kavantzas; Sofia-Iris Bibli; et al.Evangelos GikasLeandros SkaltsounisDimitrios Th. KremastinosEfstathios K. Iliodromitis Oleuropein prevents doxorubicin-induced cardiomyopathy interfering with signaling molecules and cardiomyocyte metabolism. Journal of Molecular and Cellular Cardiology 2014, 69, 4-16, 10.1016/j.yjmcc.2014.01.007.
- Gulcin Tezcan; Mevlut Ozgur Taskapilioglu; Berrin Tunca; Ahmet Bekar; Hilal Demirci; Hasan Kocaeli; Secil Ak Aksoy; Unal Egeli; Gulsah Cecener; Sahsine Tolunay; et al. Olea europaea leaf extract and bevacizumab synergistically exhibit beneficial efficacy upon human glioblastoma cancer stem cells through reducing angiogenesis and invasion in vitro. Biomedicine & Pharmacotherapy 2017, 90, 713-723, 10.1016/j.biopha.2017.04.022.
- Larisa Ryskalin; Anderson Gaglione; Fiona Limanaqi; Francesca Biagioni; Pietro Familiari; Alessandro Frati; Vincenzo Esposito; Francesco Fornai; The Autophagy Status of Cancer Stem Cells in Gliobastoma Multiforme: From Cancer Promotion to Therapeutic Strategies. International Journal of Molecular Sciences 2019, 20, 3824, 10.3390/ijms20153824.
- Iman O. Sherif; Mohammed M.H. Al-Gayyar; Oleuropein potentiates anti-tumor activity of cisplatin against HepG2 through affecting proNGF/NGF balance. Life Sciences 2018, 198, 87-93, 10.1016/j.lfs.2018.02.027.
- Ting Xu; Dajiang Xiao; Oleuropein enhances radiation sensitivity of nasopharyngeal carcinoma by downregulating PDRG1 through HIF1α-repressed microRNA-519d.. Journal of Experimental & Clinical Cancer Research 2017, 36, 3, 10.1186/s13046-016-0480-2.
- Javier A Menendez; Alejandro Vazquez-Martin; Ramón Colomer; Joan Brunet; Alegria Carrasco-Pancorbo; Rocio Garcia-Villalba; Alberto Fernández-Gutiérrez; Antonio Segura-Carretero; Olive oil's bitter principle reverses acquired autoresistance to trastuzumab (Herceptin™) in HER2-overexpressing breast cancer cells. BMC Cancer 2007, 7, 80-19, 10.1186/1471-2407-7-80.
- Martin De Bock; Eric B. Thorstensen; José Derraik; Harold Henderson; Paul L. Hofman; Wayne S. Cutfield; Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Molecular Nutrition & Food Research 2013, 57, 2079-2085, 10.1002/mnfr.201200795.
- Liguri, Gianfranco; Stefani, Massimo; Recent advances in basic and clinical research on the prevention and treatment of the metabolic syndrome and related disorders by the use of olive polyphenols. Journal of Gerontology and Geriatrics 2017, 65, 48-58.




