
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ajay Vikram Singh | + 3495 word(s) | 3495 | 2021-09-24 03:55:25 | | | |
| 2 | Jason Zhu | Meta information modification | 3495 | 2021-09-26 05:11:15 | | |
Video Upload Options
Nanopriming can be a potential future target for sustainable agriculture with the current global supply versus demand trend. A fortification program consists of adding vitamins and minerals to processed foods as a public health measure to improve the nutritional quality of the food supply. The goal is to enhance the nutrient intake by the population.
1. Introduction
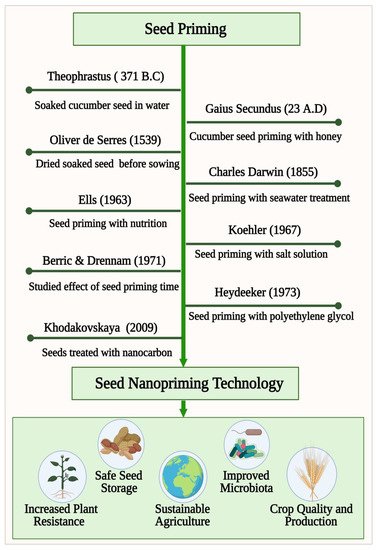
World food production must increase 50% by 2050 to meet the needs of 9 billion people [1]. The growing food demand and rapidly changing climatic conditions across the world motivates us to look for technological solutions that can provide food security for the future generations. Seed is a primary requirement for crop production, which carries the genetic potential of the variations and determines the ultimate productivity. Therefore, seed production is always the basic pre-requisite of any food security undertaking. A resilient and climate hardened seed can give the maximum output to the farmers. To increase the seed vigor and crop production, different chemical-based fertilizers and pesticides are used extensively in agriculture. In light of leaching, degradation, hydrolysis, and pollution associated with conventional chemical-based practices, they are being discouraged [2].There is an urgent need to develop a sustainable technology that can contribute to the green revolution to address these growing concerns and to restore the damage caused to the ecosystem [3]. Seed priming is an innovative sustainable seed technology to increase the seed vigor and crop production without harming the ecosystem [4][5][6][7]. As mentioned in Figure 1 , priming of seeds dates back to Theophrastus, (371–287 B.C.) who, observed during an investigation that soaking cucumber seeds in water causes their germination to be faster and more uniform than unprimed seeds (enquiry into plants, book VII, I.6). A similar preparation of cucumber seeds in honey and water for seed germination was reported by the Roman naturalist Gaius Plinius Secundus (23–79 A.D.) in Gaius’ Encyclopedia (Gaius 1949–1954). The French botanist Oliver de Serres, in 1539–1619, found that soaking seeds in manure water for 2 days and drying them prior to sowing was an effective cure for poor crop growth. The osmo-priming process was tested on lettuce and cress seeds in seawater by Charles Darwin, who observed that the primed seeds germination was higher than that of non-primed seeds. By identifying the critical parameters of seed treatment, Ells (1963) presented the modern concept of seed priming. His experiments with nutrient solutions showed a high germination rate. Khodakovskaya et al. published one of the first studies to demonstrate the potential for nanomaterials to affect seed germination [8]. The latest innovative technique with potential application in seed priming is seed nanopriming, an important emerging seed technology that blends seed priming science with updated nanotechnology [9][10]. A lot of attention has recently been brought to the development and optimization of nanomaterials for application in agriculture, including improved growth, plant protection, and overall performance. Agricultural nanomaterials are still in a juvenile state in terms of their application to sustainable agriculture [11]. Nanomaterials will be applied to multiple functions in agriculture as the understanding of nanotechnology increases. It has been noticed that most agricultural lands are affected by abiotic stress factors such as salinity and drought, which is limiting plant distribution in the habitat [12]. In order to combat abiotic stress factors, engineered nanoparticles have been found to be a promising alternative [13]. Recent studies have shown that nanomaterials can significantly impact plant metabolism, genes expression, and antioxidant enzyme activity [14]. Abiotic stress can be improved by nanoparticles such as halloysite, cerium oxide, chitosan-selenium and titanium dioxide by enabling their antioxidant system to perform better [15][16][17][18]. The use of nanoparticles as seed priming agents has demonstrated encouraging results in the field of crop productivity and seed germination [19]. By using nanotechnology, it is possible to release priming agents at specific sites and in controlled ways, which is revolutionary for ecosystems, crop improvement, animal health, and pesticide use. Nanopriming is an ongoing effort to create nano-agrochemicals for releasing specific nutrients in a controlled manner, which maintains soil fertility. Using high quality seed detection technologies makes it more likely to identify a variety of better crops since seed quality is an important factor in crop production. An increasing number of effective methods are needed that can detect nanoprimed seed quality in a non-destructive, objective manner as quickly as possible. The presented review describes the potential application of engineered nanomaterials in seed nanopriming for sustainable agriculture and machine learning technology for detection and classification of nanoprimed seeds to improve crop production.
2. Seed Nanopriming Technology
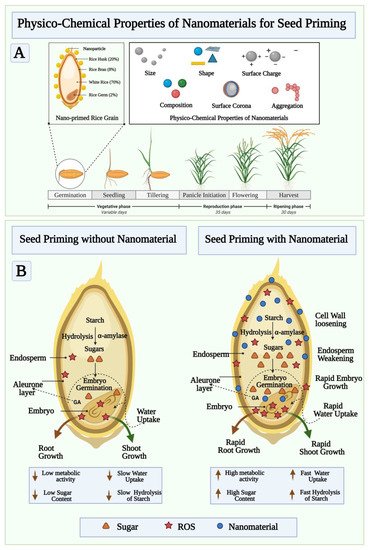
Seeds are miniature plants which, when fertilized, hatch into ovules containing both a germinating embryo and enough food to grow. A variety of treatments can be applied to a seed before planting in order to enhance its quality and potential yield. The process of priming seeds, in its broadest sense, involves soaking them in a solution where enough water is absorbed so that germination of the seed becomes possible, but not enough to allow the radicle to protrude through the seed coat [20]. Using this technique, seeds are advanced to an equal stage of germination to facilitate emergence from seed quickly and uniformly [21]. Thus, priming of seeds is a very important practice in order to ensure seed productivity [22]. Seed priming methods include hydro-priming, halo-priming, osmo-priming, hormonal priming, solid matrix priming, and bio-priming to stimulate seeds, encourage germination, and reduce environmental stress. Among the most well-known and cost-effective pre-sowing seed priming methods, hydro-priming involves treating seeds with water before sowing. The seeds are soaked in water, then re-dried to their original moisture content [23]. The process of halo priming involves treating seeds with inorganic solutions such as sodium chloride, potassium nitrate, calcium chloride, and calcium sulphate to improve germination. A known application of halopriming during the germination, seedling emergence, emergence of plants, and growth of plants is well known [24]. In osmo-priming, seeds are soaked in different concentrations of osmotic solutions. Different osmotic solutions are used depending on the species, including sugar, polyethylene glycol, glycerol, sorbitol, and mannitol and then air drying is followed by sowing. Solutions containing low water potential facilitate seed imbibitions and, therefore, early stages of germination occur [25]. Hormonal-priming involves the use of hormone solutions to prime seeds. During hormonal priming, plant growth regulators are used to imbibe seeds that have direct effects on seed metabolism. Hormo-priming uses various regulators such as abscisic acid, salicylic acid, ascorbic acid, cytokinins, auxins, gibberellins, kinetin, ethylene, and polyamines [26]. In solid matrix priming (SMP or Matri-conditioning), seeds are mixed in known proportions with a solid material and water. Water-absorbing seeds will reach a point of equilibrium, precisely at which priming will take place. A thorough washing and drying follow the separated seeds from the matrix. A hydrated, metabolically active seed can then be achieved, which is an important germination step [27]. As an ecological approach, bio-priming involves seed inoculation with beneficial organisms to protect seed from disease. Seed treatment with this new trend involves hydrating seeds with beneficial microorganisms and improving germination procedures. In terms of disease management, biopriming provides better results than pelleting or coating [28]. Nanopriming, a technique that involves seeds soaking in nanomaterials, has been shown to facilitate germination and growth by allowing nanoparticles to penetrate the seed coat and increase water uptake. When compared with unprimed seeds or seeds treated with other priming agents, nanopriming improves storage, quality, seedling emergence, yields, and tolerance to environmental stress [7]. In addition, nanopriming of seeds can help prevent diseases caused by pathogens present in seeds. A high percentage of nanomaterials are retained on the seed surface as coatings when nanoparticles are absorbed, and a tortuous pathway prevents uncontrolled water uptake while reducing gas permeability, which leads to more stable seed storage. A nanopriming process can alter the metabolism of seeds and signalling pathways, thereby influencing their germination and growth. In addition to affecting almost every existing scientific field, nanoparticles can gain significant impact on agricultural sustainability by being introduced to the agricultural domain [7]. Studies have demonstrated that nanoparticle application can stimulate germination and growth of plants in numerous ways. Nanoparticles are effective because of their small size and unique physio-chemical properties, which make them an ideal seed priming agent [21]. Nanoparticles are molecular or atomic aggregates that have a measured dimension between 1 nm and 100 nm and which may produce significantly higher chemical and physical properties than typical bulk materials [29]. It is important to note that nanomaterials have a wide range of physio-chemical properties depending on the shape, size, surface area, surface/volume ratio, chemical behavior, particle charge, production method, coating, and so forth (as shown in Figure 2 A). The unique features of nanomaterials, such as their high surface to mass ratio, enables them to enhance catalysis and deliver materials of interest, as well as adsorb substances of interest. The nanopriming process triggers a special metabolic reaction that is naturally triggered during the early stages of germination, as shown in Figure 2 B. It increases seed germination, by modulating the metabolism of seed, which leads to enhanced water uptake, starch hydrolysis rate, cell wall loosening, endosperm weakening, rapid embryo growth, and rapid root-shoot development. The nanopriming method improves the emergence of seedlings, their growth, production, and quality [30]. The cellular and molecular mechanisms of plant interaction with the environment are also modulated by seed nanopriming. The goal of using nanoparticles in agriculture and natural ecosystems is to increase the performance and sustainability of plants and soil by using less of the input parameters defined above [31][32]. It is important to include different factors that affect priming success, such as the amount of water in the soil, the kind of priming treatment, the amount of time the seeds are exposed to the treatment, and the conditions in which the seeds are stored. Numerous studies have demonstrated the ability of nanoparticles to penetrate seed coats and enhance water uptake over time, which facilitates germination and flowering [33][34]. As seed pretreatment agents, several metal-metal oxide nanoparticles and carbon-based nanoparticles have been applied to enhance germination and seedling growth of some crops and to strengthen their stress tolerance [35][36][37]. Seed priming, in which seeds are re-dried to their original moisture content before planting, has been used in only a few studies, but it is not widely used. In this case, seed nanopriming would provide a different mechanism to that of pre-sowing seed treatment without drying. Additionally, comprehensive studies have not been conducted on the physiological and molecular mechanisms of nanopriming on seed germination, thus there are many unanswered questions, particularly concerning the mechanism behind nanoparticles-induced seed germination. A summary of the types of nanoparticles that are used for seed priming is shown in Table 1 , with their potential effects as stimulants or protective against biotic and abiotic stress.
3. Effects of Seed Nanopriming under Abiotic and Biotic Stresses
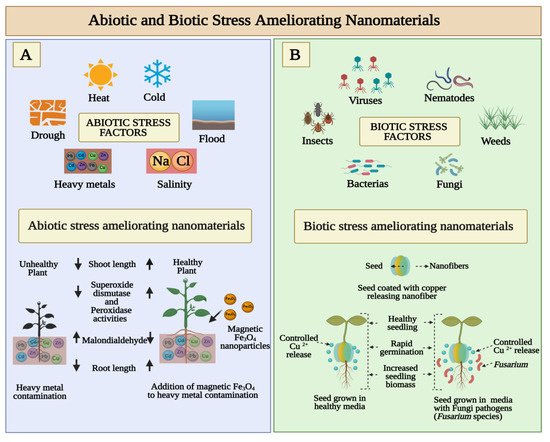
Abiotic and biotic stresses cause damage to seed growth and eventually lead to economic losses. In the wake of global warming and climate change, seeds are subject to an increased number of biotic and abiotic stress combinations, which negatively affect their growth and yield [38]. The simultaneous presence of abiotic stress factors such as drought, flood, salinity, heavy mineral contamination, cold and heat has been shown to severely deter seed germination ( Figure 3 A). The biotic stress that is exhibited in Figure 3 B includes a variety of plant pathogens including bacteria, fungi, viruses, nematodes, insects, and others. As a result of pathogen infection, changes in plant physiology often result in reduced biomass, early flowering, decreased seed set, accumulation of protective metabolites, and many other changes [39]. Researchers have reported that a variety of nanomaterials reduce biotic and abiotic stress and improve seed germination [40][41].
By seed priming with cerium oxide nanoparticles, An et al. studied the molecular mechanisms behind plant salinity stress tolerance. Poly (acrylic acid)-coated cerium oxide nanoparticles (PNC) show morphological, physiological, biochemical, and transcriptomic effects on cotton seedling priming under salinity stress, according to An et al. An advantage of PNC nanoparticle seed priming is that it can be used to increase a crop tolerance to stress during the early seedling stage in a sustainable, practical, and scalable way. These results showed promising molecular mechanisms that could be synergistically operated to enhance plant salt tolerance [42]. According to a recent study published by Baz et al., nanopriming with water-soluble carbon nanoparticles (CNPs) significantly increases seed vigor and seedling growth of lettuce under salinity stress. Under high temperatures and salinity stress, CNPs have been shown to significantly promote seed germination. A CNP-assisted nanopriming treatment enhanced lateral root growth but slightly inhibited the elongation of primary roots, resulting in a balanced accumulation of chlorophyll in high salinity stress [43]. The effects of manganese (III) oxide nanoparticles (MnNPs) on the salinity stress of capsicum annum L were studied by Ye et al. It showed that MnNPs can penetrate seed coats and form corona complexes. The results of the study demonstrate that MnNPs can modulate biochemical interactions in seeds that exhibit salinity tolerance in order to promote sustainable agriculture [44]. The study showed that nano-silica primed zea mays seeds had a higher rate of germination and a higher seedling vigor index. The priming process helps to increase the activity of antioxidant enzymes, which can suppress lipid peroxidation by suppressing the production of ROS under salinity stress. Additionally, priming reduces abscisic acid content while increasing gibberellin content. As a result of this hormonal balance, the hydrolysis enzymes (amylase and lipase) are activated. It was found that nano-silica priming enhanced the metabolic activity of maize seeds when exposed to salinity [45]. Khan et al. investigated the effect of nano ZnO and nano Fe on cadmium accumulation in wheat. Nanoprimed seeds show increased length of spikes, shoots, and roots, as well as increased grain size. Superoxide dismutase activity and electrolyte leakage were reduced in nanoprimed seeds. Nanoparticles in seeds increase wheat biomass and nutrient content as well as decreasing Cd toxicity overall [46]. The role of bulk and nanosized SiO 2 particles in fenugreek germination has been investigated by R. Ivani et al. A study concluded nanosized particles of SiO 2 protect fenugreek seeds from salt stress and improve growth attributes [47]. A study by S. Hojjat et al. examined the effects of silver nanoparticles on the germination of fenugreek seeds under salinity conditions. AgNPs improved salinity tolerance in fenugreek seedlings. This may increase various plant defense mechanisms that reduce salt stress [48]. In a study by Konate et al. , magnetic (Fe 3O 4) nanoparticles were shown to mitigate heavy metals uptake and toxicity in wheat seedlings. Physiological mechanisms were investigated in wheat seedlings to determine how magnetic nanoparticles (nano-Fe 3O 4) mitigated the toxic effects of heavy metals (Pb, Zn, Cd, and Cu) [49].
As a bioprotectant against rice borne pathogens P. grisea and X. oryzae , Sathayabama and Muthukumar investigated the antimicrobial activity of chitosan guar nanoparticles (CGNP) . The treated rice showed no symptoms of blast disease. Rice plants with CGNP-applied antimicrobial protection displayed an enhanced rate of seed germination and growth [50]. T. Xu et al. demonstrated enhanced seedling development and agrochemical delivery by biodegradable, biopolymer-based nanofiber seed coatings. The germination and subsequent growth of nanofiber-coated tomato and lettuce seeds were studied in a greenhouse, with and without a fungal pathogen ( Fusarium species) as shown in Figure 2 B. Based on recent observations, it appears to be possible to use nanomaterials to deliver active ingredients at precise locations. Nanofiber seed coatings provide precise agrochemical delivery and significantly improve seedling germination and biomass in comparison to conventional film coating techniques used in the industry due to their nanofibrous structure and controlled release kinetics [51]. The effects of cinnamaldehyde encapsulated mesoporous silica nanoparticles (MSNP) on seed borne diseases were studied by Bravo Cadena et al. Based on the results of this study, it is clear that MSNP significantly enhanced the antimicrobial activity of plant products, which allows the use of volatile biocides such as essential oils at very low concentrations to prevent microbial diseases in crop plants [52]. Choudhary et al. evaluated the effect of zinc chitosan nanoparticles as a seed priming agent and foliar application to maize plants. An in vitro study showed that seed nanopriming with Zn-chitosan nanoparticles enhanced seed germination and inhibited fungal growth. These results showed that Zn-chitosan nanoparticles have strong fungicidal activity, are an effective micronutrient fortifier, and could stimulate maize crop growth [53].
4. Limitations of Nanopriming Techniques and Future Prospects
It is recognized that despite remarkable progress, scientists do not have a complete understanding of how these nanomaterials can affect the macro- and micro-environments of seeds. It is alarming that there is still a lack of basic understanding regarding the possible health and safety effects of engineered nanomaterials on both human and non-human receptors considering the actual and projected levels of exposure [54]. A general rule concerning seed nanopriming does not exist, and there is no clear trend regarding priming responses depending on the taxonomic position of the species. As a result of some nanopriming treatments, there is a possibility of contamination of the medium with fungi and bacteria, which may greatly hinder subsequent seed germination [7]. A seed that has been nanoprimed is dried back to its original moisture content, but this process is done faster than the dehydration of mature seeds. Several researchers have hypothesized that brutal desiccation procedures alter the effects of nanopriming [7]. As a consequence, nanoprimed seed material can be less stable, and higher maintenance costs for seed companies and farmers are consequently incurred. In some cases, repeated nanopriming treatments can partly prevent seed viability losses, whereas in others, such losses are permanent and cannot be reversed [55]. Possibly requiring an additional treatment may be both an extra cost and a source of variability because germination potential may not be fully restored.
Nanopriming can be a potential future target for sustainable agriculture with the current global supply versus demand trend. A fortification program consists of adding vitamins and minerals to processed foods as a public health measure to improve the nutritional quality of the food supply. The goal is to enhance the nutrient intake by the population [56]. As a result, biofortification reduces the runoff of fertilizers and other agrochemicals and their inputs into the environment. As a result of seed biofortification, stands have been established and plant production, yields, nutrient content, and water utilization have increased as well as plant tolerance to biotic and abiotic stresses have increased [57]. The technique has been found to be a simple, practical, and cost-effective approach to improving the quality of seed and crops in resource-constrained regions. Compared to conventional seed priming, seed priming using engineered nanomaterial produced higher germination rates at equivalent or lower life cycle embodied energy. Nanotechnology is also an exceptionally potent way to deliver nutrients effectively due to its ability to deliver a wide range of engineered nanomaterials in a more efficient manner than before [34][58]. By introducing nano-bio-fortification into seeds, less fertilizer potentially may be consumed. This results in the need to use less water and less resources to cultivate the same volume of nutrient-rich food. Nano-bio fortification of seeds could also assist in shortening the growth cycle of plants (e.g., requiring less time for them to germinate and to mature), allowing the land to grow crops faster in the future. In similar ways to other agronomic methods, the success of seed nano-biofortification will depend on how well it is adapted to the setting, including the type of soil, the type of crop and the climate, and the location [59]. The advantages of nanotechnology need to be compared to other biofortification procedures that may provide long-term and cost-effective solutions, including plant breeding and CRISPR/transgenic technology [59]. In addition to assessing the overall environmental implications, it is important to determine if nano-based solutions are practical compared to conventional practices [60]. Nanomaterials continue to be manufactured in larger scales, which lead to further decreases in production costs. It is highly likely that nanotechnology will integrate with seed biofortification practices in the future, especially as highly specialized and tunable nanomaterials emerge. Nano-enabled seed biofortification is an important topic that merits intense scrutiny and greater attention given its potential benefits and the increasing global food insecurity that we will face in the coming decades.
References
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. The Challenge of Feeding the World. Sustainability 2019, 11, 5816.
- Umesha, S.; Manukumar, H.M.G.; Chandrasekhar, B. Chapter 3—Sustainable Agriculture and Food Security. In Biotechnology for Sustainable Agriculture; Singh, R.L., Mondal, S., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 67–91.
- Durán-Lara, E.F.; Valderrama, A.; Marican, A. Natural Organic Compounds for Application in Organic Farming. Agriculture 2020, 10, 41.
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293.
- Waqas, M.; Korres, N.E.; Khan, M.D.; Nizami, A.-S.; Deeba, F.; Ali, I.; Hussain, H. Advances in the Concept and Methods of Seed Priming. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer Singapore: Singapore, 2019; pp. 11–41.
- Marthandan, V.; Geetha, R.; Kumutha, K.; Renganathan, V.G.; Karthikeyan, A.; Ramalingam, J. Seed Priming: A Feasible Strategy to Enhance Drought Tolerance in Crop Plants. Int. J. Mol. Sci. 2020, 21, 8258.
- do Espirito Santo Pereira, A.; Caixeta Oliveira, H.; Fernandes Fraceto, L.; Santaella, C. Nanotechnology Potential in Seed Priming for Sustainable Agriculture. Nanomaterials 2021, 11, 267.
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Biris, A.S. Carbon Nanotubes Are Able To Penetrate Plant Seed Coat and Dramatically Affect Seed Germination and Plant Growth. ACS Nano 2009, 3, 3221–3227.
- Abbasi Khalaki, M.; Moameri, M.; Asgari Lajayer, B.; Astatkie, T. Influence of nano-priming on seed germination and plant growth of forage and medicinal plants. Plant Growth Regul. 2021, 93, 13–28.
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558.
- Ioannou, A.; Gohari, G.; Papaphilippou, P.; Panahi Rad, S.; Akbari, A.; Dadpour, M.; Krasia-Christoforou, T.; Fotopoulos, V. Advanced nanomaterials in agriculture under a changing climate: The way to The future? Environ. Exp. Bot. 2020, 176.
- Gohari, G.; Panahirad, S.; Sadeghi, M.; Akbari, A.; Zareei, E.; Zahedi, S.M.; Bahrami, M.K.; Fotopoulos, V. Putrescine-functionalized carbon quantum dot (put-CQD) nanoparticles effectively prime grapevine (Vitis vinifera cv. ‘Sultana’) against salt stress. BMC Plant Biol. 2021, 21, 120.
- Gohari, G.; Zareei, E.; Rostami, H.; Panahirad, S.; Kulak, M.; Farhadi, H.; Amini, M.; Martinez-Ballesta, M.d.C.; Fotopoulos, V. Protective effects of cerium oxide nanoparticles in grapevine (Vitis vinifera L.) cv. Flame Seedless under salt stress conditions. Ecotoxicol. Environ. Saf. 2021, 220, 112402.
- Azimi, F.; Oraei, M.; Gohari, G.; Panahirad, S.; Farmarzi, A. Chitosan-selenium nanoparticles (Cs–Se NPs) modulate the photosynthesis parameters, antioxidant enzymes activities and essential oils in Dracocephalum moldavica L. under cadmium toxicity stress. Plant Physiol. Biochem. 2021, 167, 257–268.
- Masoudniaragh, A.; Oraei, M.; Gohari, G.; Akbari, A.; Faramarzi, A. Using halloysite nanotubes as carrier for proline to alleviate salt stress effects in sweet basil (Ocimum basilicum L.). Sci. Hortic. 2021, 285, 110202.
- Sheikhalipour, M.; Esmaielpour, B.; Behnamian, M.; Gohari, G.; Giglou, M.T.; Vachova, P.; Rastogi, A.; Bresti, M.; Skalicky, M.J.N. Chitosan Selenium Nanoparticle (C“ Se NP) Foliar Spray Alleviates Salt Stress in Bitter Melon. Nanomaterials 2021, 11, 684.
- Sheikhalipour, M.; Esmaielpour, B.; Gohari, G.; Haghighi, M.; Jafari, H.; Farhadi, H.; Kulak, M.; Kalisz, A. Salt Stress Mitigation via the Foliar Application of Chitosan-Functionalized Selenium and Anatase Titanium Dioxide Nanoparticles in Stevia (Stevia rebaudiana Bertoni). Molecules 2021, 26, 4090.
- Mohammadi, M.H.Z.; Panahirad, S.; Navai, A.; Bahrami, M.K.; Kulak, M.; Gohari, G. Cerium oxide nanoparticles (CeO2-NPs) improve growth parameters and antioxidant defense system in Moldavian Balm (Dracocephalum moldavica L.) under salinity stress. Plant Stress 2021, 1, 100006.
- Sanzari, I.; Leone, A.; Ambrosone, A. Nanotechnology in Plant Science: To Make a Long Story Short. Front. Bioeng. Biotechnol. 2019, 7, 120.
- Kaur, R.; Chandra, J.; Keshavkant, S. Nanotechnology: An efficient approach for rejuvenation of aged seeds. Physiol. Mol. Biol. Plants 2021, 27, 399–415.
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-Based Sustainable Agriculture and Food Science: Recent Advances and Future Outlook. Front. Bioeng. Biotechnol. 2020, 2, 9954.
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23.
- Damalas, C.A.; Koutroubas, S.D.; Fotiadis, S. Hydro-Priming Effects on Seed Germination and Field Performance of Faba Bean in Spring Sowing. Agriculture 2019, 9, 201.
- Patade, V.Y.; Bhargava, S.; Suprasanna, P. Halopriming imparts tolerance to salt and PEG induced drought stress in sugarcane. Agric. Ecosyst. Environ. 2009, 134, 24–28.
- Chen, K.; Arora, R. Dynamics of the antioxidant system during seed osmopriming, post-priming germination, and seedling establishment in Spinach (Spinacia oleracea). Plant Sci. Int. J. Exp. Plant Biol. 2011, 180, 212–220.
- Moori, S.; Ahmadi-Lahijani, M.J. Hormopriming instigates defense mechanisms in Thyme (Thymus vulgaris L.) seeds under cadmium stress. J. Appl. Res. Med. Aromat. Plants 2020, 19, 100268.
- Sen, S.K.; Chouhan, D.; Das, D.; Ghosh, R.; Mandal, P. Improvisation of salinity stress response in mung bean through solid matrix priming with normal and nano-sized chitosan. Int. J. Biol. Macromol. 2020, 145, 108–123.
- Rakshit, A.; Sunita, K.; Pal, S.; Singh, A.; Singh, H. Bio-Priming Mediated Nutrient Use Efficiency of Crop Species; Springer: New Delhi, India, 2015; pp. 181–191.
- Nasrollahzadeh, M.; Sajadi, S.M.; Sajjadi, M.; Issaabadi, Z. Chapter 1—An Introduction to Nanotechnology. In Interface Science and Technology; Nasrollahzadeh, M., Sajadi, S.M., Sajjadi, M., Issaabadi, Z., Atarod, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28, pp. 1–27.
- García-Gómez, C.; Fernández, M.D. Chapter Four—Impacts of metal oxide nanoparticles on seed germination, plant growth and development. In Comprehensive Analytical Chemistry; Verma, S.K., Das, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 84, pp. 75–124.
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in Sustainable Agriculture: Recent Developments, Challenges, and Perspectives. Front. Microbiol. 2017, 8, 1014.
- Chen, H. Metal based nanoparticles in agricultural system: Behavior, transport, and interaction with plants. Chem. Speciat. Bioavailab. 2018, 30, 123–134.
- Bandala, E.R.; Berli, M. Nanomaterials: New Agrotechnology Tools to Improve Soil Quality? In Agricultural Nanobiotechnology: Modern Agriculture for a Sustainable Future; López-Valdez, F., Fernández-Luqueño, F., Eds.; Springer International Publishing: Cham, The Netherlands, 2018; pp. 127–140.
- Lowry, G.V.; Avellan, A.; Gilbertson, L.M. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat. Nanotechnol. 2019, 14, 517–522.
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263.
- Vera-Reyes, I.; Vázquez-Núñez, E.; Lira-Saldivar, R.H.; Méndez-Argüello, B. Effects of Nanoparticles on Germination, Growth, and Plant Crop Development. In Agricultural Nanobiotechnology: Modern Agriculture for a Sustainable Future; López-Valdez, F., Fernández-Luqueño, F., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 77–110.
- Szőllősi, R.; Molnár, Á.; Kondak, S.; Kolbert, Z. Dual Effect of Nanomaterials on Germination and Seedling Growth: Stimulation vs. Phytotoxicity. Plants 2020, 9, 1745.
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34.
- Chojak-Koźniewska, J.; Kuźniak, E.; Zimny, J. The Effects of Combined Abiotic and Pathogen Stress in Plants: Insights From Salinity and Pseudomonas syringae pv lachrymans Interaction in Cucumber. Front. Plant Sci. 2018, 9, 1691.
- Aslani, F.; Bagheri, S.; Muhd Julkapli, N.; Juraimi, A.S.; Hashemi, F.S.G.; Baghdadi, A. Effects of Engineered Nanomaterials on Plants Growth: An Overview. Sci. World J. 2014, 2014, 641759.
- Ansari, M.H.D.; Lavhale, S.; Kalunke, R.M.; Srivastava, P.L.; Pandit, V.; Gade, S.; Yadav, S.; Laux, P.; Luch, A.; Gemmati, D.; et al. Recent Advances in Plant Nanobionics and Nanobiosensors for Toxicology Applications. Curr. Nanosci. 2020, 16, 27–41.
- An, J.; Hu, P.; Li, F.; Wu, H.; Shen, Y.; White, J.C.; Tian, X.; Li, Z.; Giraldo, J.P. Emerging investigator series: Molecular mechanisms of plant salinity stress tolerance improvement by seed priming with cerium oxide nanoparticles. Environ. Sci. Nano 2020, 7, 2214–2228.
- Baz, H.; Creech, M.; Chen, J.; Gong, H.; Bradford, K.; Huo, H. Water-Soluble Carbon Nanoparticles Improve Seed Germination and Post-Germination Growth of Lettuce under Salinity Stress. Plants 2020, 10, 1192.
- Ye, Y.; Cota-Ruiz, K.; Hernández-Viezcas, J.A.; Valdés, C.; Medina-Velo, I.A.; Turley, R.S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Manganese Nanoparticles Control Salinity-Modulated Molecular Responses in Capsicum annuum L. through Priming: A Sustainable Approach for Agriculture. ACS Sustain. Chem. Eng. 2020, 8, 1427–1436.
- Naguib, D.M.; Abdalla, H. Metabolic Status during Germination of Nano Silica Primed Zea mays Seeds under Salinity Stress. J. Crop Sci. Biotechnol. 2019, 22, 415–423.
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Javed, M.R.; Adrees, M. The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ. Sci. Pollut. Res. Int. 2019, 26, 19859–19870.
- Ivani, R.; Sanaei Nejad, S.H.; Ghahraman, B.; Astaraei, A.R.; Feizi, H. Role of bulk and Nanosized SiO(2) to overcome salt stress during Fenugreek germination (Trigonella foenum- graceum L.). Plant Signal. Behav. 2018, 13, e1044190.
- Hojjat, S.S.; Kamyab, M. The effect of silver nanoparticle on Fenugreek seed germination under salinity levels. Russ. Agric. Sci. 2017, 43, 61–65.
- Konate, A.; He, X.; Zhang, Z.; Ma, Y.; Zhang, P.; Alugongo, G.M.; Rui, Y. Magnetic (Fe3O4) Nanoparticles Reduce Heavy Metals Uptake and Mitigate Their Toxicity in Wheat Seedling. Sustainability 2017, 9, 790.
- Sathiyabama, M.; Muthukumar, S. Chitosan guar nanoparticle preparation and its in vitro antimicrobial activity towards phytopathogens of rice. Int. J. Biol. Macromol. 2020, 153, 297–304.
- Xu, T.; Ma, C.; Aytac, Z.; Hu, X.; Ng, K.W.; White, J.C.; Demokritou, P. Enhancing Agrichemical Delivery and Seedling Development with Biodegradable, Tunable, Biopolymer-Based Nanofiber Seed Coatings. ACS Sustain. Chem. Eng. 2020, 8, 9537–9548.
- Bravo Cadena, M.; Preston, G.M.; Van der Hoorn, R.A.L.; Flanagan, N.A.; Townley, H.E.; Thompson, I.P. Enhancing cinnamon essential oil activity by nanoparticle encapsulation to control seed pathogens. Ind. Crop. Prod. 2018, 124, 755–764.
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Zinc encapsulated chitosan nanoparticle to promote maize crop yield. Int. J. Biol. Macromol. 2019, 127, 126–135.
- Marmiroli, N.; White, J.C. Editorial: Nanotoxicology and Environmental Risk Assessment of Engineered Nanomaterials (ENMs) in Plants. Front. Plant Sci. 2016, 7, 1370.
- Wang, W.; He, A.; Peng, S.; Huang, J.; Cui, K.; Nie, L. The Effect of Storage Condition and Duration on the Deterioration of Primed Rice Seeds. Plant Sci. 2018, 9, 172.
- Hussain, A.; Rizwan, M.; Ali, Q.; Ali, S. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut. Res. Int. 2019, 26, 7579–7588.
- Farooq, M.; Usman, M.; Nadeem, F.; Rehman, H.; Wahid, A.; Basra, S.; Siddique, K. Seed priming in field crops—Potential benefits, adoption and challenges. Crop Pasture Sci. 2019, 70, 731–771.
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540.
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 5, 12.
- Gilbertson, L.M.; Pourzahedi, L.; Laughton, S.; Gao, X.; Zimmerman, J.B.; Theis, T.L.; Westerhoff, P.; Lowry, G.V. Guiding the design space for nanotechnology to advance sustainable crop production. Nat. Nanotechnol. 2020, 15, 801–810.




