
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Philippe Gérard | + 2716 word(s) | 2716 | 2021-09-24 04:21:26 | | | |
| 2 | Catherine Yang | Meta information modification | 2716 | 2021-09-26 03:25:02 | | |
Video Upload Options
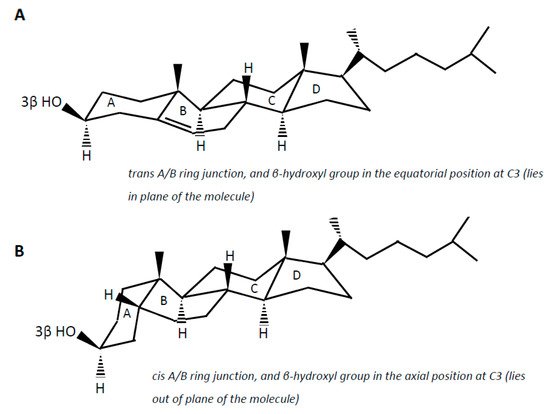
Cholesterol (examples of synonyms 3β-hydroxy-5-cholestene or 5-cholesten-3β-ol) in the intestine may be either absorbed or undergo microbial conversion to different metabolites, of which non-absorbable coprostanol (examples of synonyms 5β-cholestanol or 5β-cholestan-3β-ol) is the end and predominant product found in feces. Note that multiple synonyms can be employed for a same steroid molecule, which does not help the reader to find his way. Cholesterol is a 27-carbon molecule with a structure formed by a polycyclic ring skeleton with a trans A/B ring junction, a β-hydroxyl group in the equatorial position at C3 (i.e., in plane of the molecule), a double bond at C5 (Δ5double bond), two methyl groups at C10 and C13, and a side chain at C17 (A).
1. Introduction
Cholesterol sustains life of most higher animals and birds as a main element of cell membrane architecture, a unique natural precursor for the synthesis of all five classes of steroid hormones (glucocorticoids, mineralocorticoids, progestins, androgens and oestrogens), vitamin D, and bile acids. Yet, an excess of cholesterol in blood is also detrimental in humans since it is recognized as a major risk of cardiovascular disease, which is a leading cause of mortality in developed countries [1]. Excess cholesterol in blood and other organs comes from an imbalance between input and output. Input originates from endogenous synthesis mainly in the liver and the small intestine, plus exogenous food intake of animal origin. Output proceeds via bioconversion to bile acids in the liver, steroid hormones in diverse tissues, mainly the adrenal cortex, testis, and ovary, cell renewal, plus enteric metabolism into virtually non-absorbable microbial derivatives, which are eliminated in the feces. Among them, non-absorbable coprostanol is by far the most predominant and of highest clinical interest for removal of cholesterol from the body. Figure 1 gives a rough estimation of daily cholesterol input and output in the healthy adult.
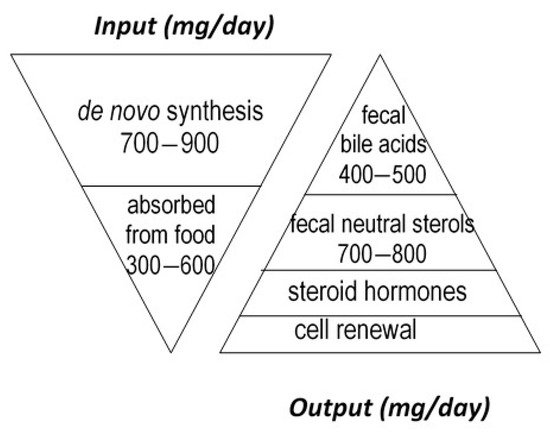
Considerable effort has thus been devoted to develop, question, and update reliable clinical interventional strategies aimed at lowering blood cholesterol through either depleting input, stimulating output, or both. A simple way of getting there would be to limit cholesterol input by lowering its dietary supply from animal products and/or its intestinal absorption by sequestering or competing ingredients [2], which unfortunately may not be sufficient in a number of cases where an inherited propensity to synthesize too much endogenous cholesterol limits the effectiveness of a healthy diet. For that reason, a number of medicines have been proposed to inhibit synthesis of cholesterol by the liver and intestine, the most popular and widely prescribed being statins. A complementary strategy would be to increase the use of cholesterol for bile acid synthesis. This could be done through oral intake of bile acid sequestering agents, which partially remove bile acids from the enterohepatic circulation and boost their compensatory de novo synthesis from the unique precursor cholesterol. A fourth and last strategy, which was proposed several decades ago, but failed to be put into practice up to now, would be to increase the bioconversion of cholesterol in the gut lumen to its end, non-absorbable bacterial metabolite coprostanol, which could be achieved through supplementation with either cholesterol-metabolizing microbes or microbial enzymes.
2. Gut Microbial Metabolites of Cholesterol and Suspected Pathways
Cholesterol (examples of synonyms 3β-hydroxy-5-cholestene or 5-cholesten-3β-ol) in the intestine may be either absorbed or undergo microbial conversion to different metabolites, of which non-absorbable coprostanol (examples of synonyms 5β-cholestanol or 5β-cholestan-3β-ol) is the end and predominant product found in feces. Note that multiple synonyms can be employed for a same steroid molecule, which does not help the reader to find his way. Cholesterol is a 27-carbon molecule with a structure formed by a polycyclic ring skeleton with a trans A/B ring junction, a β-hydroxyl group in the equatorial position at C3 (i.e., in plane of the molecule), a double bond at C5 (Δ 5 double bond), two methyl groups at C10 and C13, and a side chain at C17 ( Figure 2 A). Cholesterol metabolism by gut bacteria seems to be limited to the end product coprostanol and its intermediates, with no degradation of the side chain, and no fission of the steroid rings, which, in aerobic soil bacteria, lead to the complete degradation of the steroid molecule up to CO 2 [3][4]. This could be interpreted as a symbiotic relationship between the gut cholesterol-metabolizing microbiota and the host, which participates in the elimination of excess cholesterol from the body without altering the integrity of the intestinal cell membranes. On the other hand, the significance of cholesterol-to-coprostanol reduction to the physiology of the bacteria remains to be elucidated. Eyssen et al. [5] speculated that cholesterol acted as a terminal electron acceptor in cholesterol-reducing bacteria, thereby supplying energy by means of electron transport. However, several active strains do not require cholesterol for growth, indicating that some cholesterol reducing microorganisms can use alternate electron acceptors, or generate energy by other ways [6].
Coprostanol is the saturated analogue of cholesterol. In addition to the saturation of the Δ 5 double bond, the structure of coprostanol differs from that of cholesterol by a cis A/B ring junction, which causes the A ring to be bent into a second plane at approximately a right angle to the B:C:D rings, and the 3-hydroxy group to be in the axial position, i.e., out of plane of the molecule ( Figure 2 B). Such a structure would be mainly responsible for the poor ability of coprostanol to be esterified within the intestinal mucosa, and therefore to be absorbed [7][8][9].
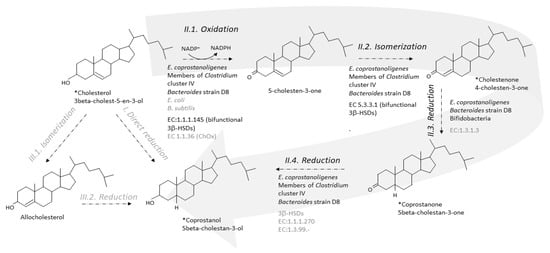
Two metabolic pathways have been proposed to explain the conversion of cholesterol to coprostanol by intestinal microorganisms [10][11]. One pathway is the direct stereospecific reduction of the Δ 5 double bond [10] ( Figure 3 ). The other pathway involves the intermediate formation of Δ 4-cholelesten-3-one (or 4-cholelesten-3-one, or cholestenone) and coprostanone [11] ( Figure 3 ). A series of complex in vivo and in vitro experiments using labeled and double-labeled steroid molecules was conducted over almost two decades, to follow the outcome of the hydrogen atom of the 3-hydroxy group [10][11][12][13] and prove the formation of the intermediates 4-cholesten-3-one and coprostanone [11][14]. This led to the incontrovertible evidence that conversion of cholesterol to coprostanol proceeds at least in part, if not largely, via the intermediate-involving pathway, while participation of a direct pathway is not proved but cannot be excluded [11]. Since then, many patterns of neutral fecal sterols by gas liquid chromatography, coupled or not with mass spectrometry, have referred to the presence of coprostanol, 4-cholesten-3-one and coprostanone in feces from humans and conventional animals, while all three metabolites are totally absent in germ-free models [15][16][17][18].
3. Step-by-Step Conversion of Cholesterol to Coprostanol
Evidence of cholesterol reduction via the indirect pathway being established, efforts have been made to characterize each step involved, and to identify and purify the corresponding enzymes. Here is where it gets tricky, as limited progress has been made since the isolation of the first active strains.
The first step of the indirect pathway is the oxidation of cholesterol to 4-cholesten-3-one. Two types of known enzymes are able to catalyse this reaction, cholesterol oxidase (ChOx, EC 1.1.36) and 3β-hydroxy-Δ 5-steroid dehydrogenase (3β-hydroxy-Δ 5-HSD, EC 1.1.1.145) [19]. Both are bifunctional enzymes, which carry out two separate, sequential reactions. The enzymes first oxidize the 3β-hydroxy group of cholesterol into the 3-keto group of 5-cholesten-3-one, and then isomerize the double bond in the steroid ring backbone, from Δ5 to Δ4, giving 4-cholesten-3-one (EC 5.3.3.1, steroid Delta-isomerase) ( Figure 3 ).
Importantly, ChOx are oxygen-dependent enzymes, and they are found in hundreds of various aerobic cholesterol-degrading soil microorganisms, including bacteria, molds, and yeasts [20][21]. ChOx is the first enzyme in the oxic pathway of cholesterol mineralization to carbon dioxide [22]. To our knowledge, only two inhabitants of the gastrointestinal tract, both facultative anaerobes, namely, one Escherichia coli strain isolated from the feces of a colon-cancer patient [23], and one Bacillus subtilis strain isolated from fecal tiger excreta [24], were reported to support ChOx activity in aerobic cultures. We also found genes that share 30–60% sequence identity with ChOx in the genome of Bacteroides strain D8 (unpublished data). Although it is unlikely that ChOx could participate in the conversion of cholesterol to 4-cholesten-3-one in the anaerobic environment of the lumen, it cannot be ruled out that the mucosa-associated microbiota could oxidize cholesterol in an oxygen-dependent enzymatic reaction. No homologs of any queried ChOx could be found in the genome of E. coprostanoligenes or among almost six million non-redundant complete genes assembled from the metagenomic sequencing of more than three thousand human fecal samples from various countries of the world [25]. In contrast, within the same study [25], a 3β-HSD was found in E. coprostanoligenes ATCC 5122. The enzyme, named IsmA for “Intestinal steroid metabolism A,” was overexpressed in E. coli , purified using HisPur™ Ni-NTA Resin, and partially characterized [25]. It is an NADP+ dependent, oxygen independent, and cholesterol inducible HSD that oxidizes cholesterol to 4-cholesten-3-one and coprostanol to coprostanone, but does not accept the 3α-hydroxy bile acids cholic and chenodeoxycholic acids as substrates. Based on massive amount of correlations combining huge metagenomics and metabolomics datasets from several cohorts in the world, six homologs of IsmA were also tracked from uncultivated members of the human gut microbiota [25]. They were overexpressed in E. coli , and all six lysates were confirmed to oxidize cholesterol to 4-cholesten-3-one as well as coprostanol to coprostanone. When binning co-abundant genes into metagenomic species (MSPs) across more than 3000 metagenomics datasets, 20 different MSPs containing ismA genes were identified. They formed a coherent clade in the phylogenetic neighbourhood of Clostridium cluster IV, which contains species linked to host health, including short-chain fatty acid producers. IsmA-encoding MSPs had a high relative abundance (average 1.4%) in human metagenomes, and the percentage of metagenomes containing at least one IsmA-encoding MSP varied from 37% to 92%, which supports the idea that IsmA-encoding bacteria are prevalent constituents of the human gut microbiome [25]. However, they do not exclude implication of other enzymes, since functional predictions based on sequence similarity may be difficult in the particular case of enzymes where the actual enzymatic reactions or substrates may differ even at high sequence similarity, and vice versa [26][27].
In 1973, Björkhem and coworkers [14] obtained a crude enzyme preparation from supernatants of freeze-pressed cecal contents of rats, which, when incubated with [4- 14C]cholesten-3-one, produced coprostanone as the only product. The 3-oxo-Δ 4-steroid 5β-reductase activity required NADH as cofactor. The semi-purified enzyme preparation also catalyzed the reduction of the Δ 4-double bond of progesterone and testosterone but not the Δ 5-double bond of cholesterol, pregnenolone, or dehydroepiandrosterone. The mechanism of reduction of Δ 4-double bonds in 3-oxo-Δ 4-steroids by the microbial 3-oxo-Δ 4-steroid 5β-reductase was found to involve transfer of hydrogen from the 4 B -position of NADH to the 5β-position of the steroid. We could not find any recent proof of 3-oxo-Δ 4-steroid 5β-reductase activity in the gut microbiota, and no bacterial 3-oxo-Δ 4-steroid 5β-reductase; EC:1.3.1.3, has never been purified nor can be found in the databases ( Figure 3 ). However, three genes predicted to encode cholestenone 5β-reductase, EC 1.3.1.3, were found to be upregulated when B. bifidum PRL2010 was grown in presence of cholesterol [28] ( Figure 3 ). One of them had a sequence that was found to be highly conserved (identity greater than 80 % and coverage 100 %) in the genomes of ten other bifidobacteria, all isolated from the mammalian gut [28]. When a large daily inoculum is administered (10 9 cells per day of overnight cultures over 20 days) to the hypercholesterolemic apoE-KO mice, B. bifidum PRL2010 tended to lower plasma cholesterol in this small-sized experiment (5 mice treated and 5 untreated). Genes sharing 30–60% sequence identity with cholestenone 5β-reductase, EC 1.3.1.3, were also found in the genome of Bacteroides strain D8 (unpublished data).
4. Dietary Regulation
Any dietary strategy aimed at decreasing cholesterol absorption in the proximal intestine and increasing cholesterol-to-coprostanol biotransformation in the hindgut would be beneficial for blood cholesterol lowering, provided that the coprostanol producing phenotype is present. Many studies report the results of dietary strategies to lower blood cholesterol in man or diverse animal models. However, few of them include a profile of fecal sterol excretion among which we can gather little significant information on cholesterol-to-coprostanol transformation and excretion rates. Importantly, the purpose of this paragraph is not to summarize the extensively documented dietary regulation of blood cholesterol, but to bring together what we know about the effect of the diet on biotransformation of cholesterol in the gut. Some effects of dietary sugars, fatty acids, proteins, and amino acids, as well as specific diets, are reported below.
Fecal concentration of cholesterol and its microbial metabolites, either expressed per g of dry [29] or wet [30] feces, is generally reported to be highest in omnivores, lowest in vegans, and intermediate in lacto-ovovegetarians, but total excretion of neutral sterols per 24 h would be higher in vegetarians [29]. Interestingly, mean efficiency of microbial cholesterol metabolism, expressed as the ratio of cholesterol to its microbial products, would not essentially differ between dietary habits [29][30], and the same percentage of low converters (around 20%) would be found in omnivores and vegetarians [29]. In a way similar to cholesterol, plant sterols are metabolized by the gut microbiota. In this respect, main bacterial metabolites are ethylcoprostanone, ethylcoprostanol, and sitostanol for β-sitosterol, methylcoprostanone and campestanol for campesterol, and stigmastenol and ethylcoprostenol for stigmasterol [31]. Independent studies agree that cholesterol metabolism by the gut microbiota is less efficient when high supraphysiological doses of plant sterols are consumed daily by healthy human volunteers [31][32], suggesting that the gut microbiota could preferably use plant sterols as substrates when present in greater proportions than cholesterol [31]. The same was observed in hamsters fed a hypercholesterolemic diet [33], providing evidence that the higher plant sterol consumption, the lesser microbial cholesterol metabolism. At the higher dose (0.2% by weight of the diet), plant sterols were effective in reducing cholesterol absorption in the intestine as proved by increased excretion of untransformed cholesterol, decreased total and non-HDL blood cholesterol, decreased liver cholesterol, and reduction of the atherosclerotic plaque by more than 50% [33]. Since the plant sterols had no effect on gene expression of the different transporters and enzymes involved in intestinal cholesterol absorption, it was concluded and in vitro experienced that plant sterols could displace cholesterol from micelles and therefore inhibit its intestinal absorption [33].
In pigs, high cholesterol intake led to a quadrupled neutral sterols stool excretion, but the ratio of coprostanol to cholesterol in stools was shifted from more than ten to less than one. This provides evidence that the efficiency of cholesterol to coprostanol reduction was limited in this animal model [34]. In parallel, total and LDL blood cholesterol markedly increased when moving from the low to the high cholesterol diet. In the same study, the addition of a cholesterol sequestering agent, β-cyclodextrin, to the hypercholesterolemic diet, brought back cholesterolemia to normal values, while still increasing excretion of neutral sterols in stools, mainly in the form of untransformed cholesterol [34]. Limited bioreduction of cholesterol to coprostanol when dietary cholesterol supply increased was also observed in rats [35]. At last, in three healthy male volunteers, exclusive consumption of a liquid diet for 10 days was accompanied by a drastic drop in fecal excretion of neutral steroids and conversion of cholesterol to coprostanol [36].
Clearly, many, if not all, dietary ingredients have an impact on the biotransformation of cholesterol in the hindgut, but mechanisms are far from all being elucidated. In particular, these changes in cholesterol biotransformation have never been put in perspective with structural and functional modulations of the gut microbiota.
References
- Barquera, S.; Pedroza-Tobías, A.; Medina, C.; Hernández-Barrera, L.; Bibbins-Domingo, K.; Lozano, R.; Moran, A.E. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch. Med. Res. 2015, 46, 328–338.
- Catapano, A.L.; Farnier, M.; Foody, J.M.; Toth, P.P.; Tomassini, J.E.; Brudi, P.; Tershakovec, A.M. Combination therapy in dyslipidemia: Where are we now? Atherosclerosis 2014, 237, 319–335.
- Nagasawa, M.; Bae, M.; Tamura, G.; Arima, K. Microbial transformation of sterols. Part II. Cleavage of sterol side chains by microorganisms. Agric. Biol. Chem. 1969, 33, 1644–1650.
- Giorgi, V.; Menéndez, P.; García-Carnelli, C. Microbial transformation of cholesterol: Reactions and practical aspects—An update. World J. Microbiol. Biotechnol. 2019, 35, 131.
- Eyssen, H.; Parmentier, G. Biohydrogenation of sterols and fatty acids by the intestinal microflora. Am. J. Clin. Nutr. 1974, 27, 1329–1340.
- Brinkley, A.W.; Gottesman, A.R.; Mott, G.E. Isolation and characterization of new strains of cholesterol-reducing bacteria from baboons. Appl. Environ. Microbiol. 1982, 43, 86–89.
- Rosenfeld, R.S.; Zumoff, B.; Hellman, L. Metabolism of coprostanol-C14 and cholestanol-4-C14 in man. J. Lipid Res. 1963, 4, 337–340.
- Treadwell, C.R.; Vahouny, G.V. Handbook of Physiology. Alimentary Canal; American Physiological Society: Washington, DC, USA, 1977; Volume Ill, pp. 1407–1438.
- Bhattacharyya, A.K. Differences in uptake and esterification of saturated analogues of cholesterol by rat small intestine. Am. J. Physiol. 1986, 251, G495–G500.
- Rosenfeld, R.S.; Fukushima, D.K.; Hellman, L.; Gallagher, T.F. The transformation of cholesterol to coprostanol. J. Biol. Chem. 1954, 211, 301–311.
- Björkhem, I.; Gustafsson, J.A. Mechanism of microbial transformation of cholesterol into coprostanol. Eur. J. Biochem. 1971, 21, 428–432.
- Gallagher, T.F.; Hellman, L.; Rosenfeld, R.S. The transformation of cholesterol-3d to coprostanol-d. Location of deuterium in coprostanol. J. Biol. Chem. 1956, 222, 321–323.
- Rosenfeld, R.A.; Gallagher, T.F. Further studies of the biotransformation of cholesterol to coprostanol. Steroids 1964, 4, 515–520.
- Björkhem, I.; Gustafsson, J.A.; Wrange, O. Microbial transformation of cholesterol into coprostanol. Properties of a 3-oxo-Δ4-steroid-5β-reductase. Eur. J. Biochem. 1973, 37, 143–147.
- Danielsson, H.; Gustafsson, B. On serum-cholesterol levels and neutral fecal sterols in germ-free rats. Bile acids and steroids 59. Arch. Biochem. Biophys. 1959, 83, 482–485.
- Evrard, E.; Hoet, P.P.; Eyssen, H.; Charlier, H.; Sacquet, E. Faecal lipids in germ-free and conventional rats. Br. J. Exp. Pathol. 1964, 45, 409–414.
- Gustafsson, B.E.; Gustafsson, J.A.; Sjövall, J. Intestinal and fecal sterols in germfree and conventional rats. Bile acids and steroids 172. Acta Chem. Scand. 1966, 20, 1827.
- Kellogg, T.F.; Wostmann, B.S. Fecal neutral steroids and bile acids from germfree rats. J. Lipid Res. 1969, 10, 495–503.
- Kreit, J. Microbial catabolism of sterols: Focus on the enzymes that transform the sterol 3β-hydroxy-5-en into3-keto-4-en. FEMS Microbiol. Lett. 2017, 364, fnx007.
- Arima, K.; Nagasawa, M.; Bae, M.; Tamura, G. Microbial transformation of sterols. Part I. Decomposition of cholesterol by microorganisms. Agric. Biol. Chem. 1969, 33, 1636–1643.
- Kieslich, K. Microbial side-chain degradation of sterols. J. Basic Microbiol. 1985, 25, 461–474.
- Chiang, Y.R.; Ismail, W.; Heintz, D.; Schaeffer, C.; Van Dorsselaer, A.; Fuchs, G. Study of anoxic and oxic cholesterol metabolism by Sterolibacterium denitrificans. J. Bacteriol. 2008, 190, 905–914.
- Owen, R.W.; Tenneson, M.E.; Bilton, R.F.; Mason, A.N. The degradation of cholesterol by Escherichia coli isolated from human faeces. Biochem. Soc. Trans. 1978, 6, 377–379.
- Kumari, L.; Kanwar, S.S. Purification and characterization of an extracellular cholesterol oxidase of Bacillus subtilis isolated from Tiger excreta. Appl. Biochem. Biotechnol. 2016, 178, 353–367.
- Kenny, D.J.; Plichta, D.R.; Shungin, D.; Koppel, N.; Hall, A.B.; Fu, B.; Vasan, R.S.; Shaw, S.Y.; Vlamakis, H.; Balskus, E.P.; et al. Cholesterol metabolism by uncultured human gut bacteria influences host cholesterol level. Cell Host Microbe 2020, 28, 245–257.
- Rost, B. Enzyme function less conserved than anticipated. J. Mol. Biol. 2002, 318, 595–608.
- Tian, W.; Skolnick, J. How well is enzyme function conserved as a function of pairwise sequence identity? J. Mol. Biol. 2003, 333, 863–882.
- Zanotti, I.; Turroni, F.; Piemontese, A.; Mancabelli, L.; Milani, C.; Viappiani, A.; Prevedini, G.; Sanchez, B.; Margolles, A.; Elviri, L.; et al. Evidence for cholesterol-lowering activity by Bifidobacterium bifidum PRL2010 through gut microbiota modulation. Appl. Microbiol. Biotechnol. 2015, 99, 6813–6829.
- Korpela, J.T.; Adlercreutz, H. Fecal neutral sterols in omnivorous and vegetarian women. Scand. J. Gastroenterol. 1985, 20, 1180–1184.
- van Faassen, A.; Bol, J.; van Dokkum, W.; Pikaar, N.A.; Ockhulzen, T.; Hermus, J.J. bile acids, neutral steroids, and bacteria in feces as affected by a mied, lacto-ovovegetarian, and a vegan diet. Am. J. Clin. Nutr. 1987, 46, 962–967.
- Cuevas-Tena, M.; Bermudez, J.; de los Angeles Silvestre, R.; Alegría, A.; Lagarda, M.J. Impact of colonic fermentation on sterols after the intake of a plant sterol-enriched beverage: A randomized, double-blind crossover trial. Clin. Nutr. 2019, 38, 1549–1560.
- Weststrate, J.A.; Ayesh, R.; Bauer-Plank, C.; Drewitt, P.N. Safety evaluation of phytosterol esters. Part 4. Faecal concentrations of bile acids and neutral sterols in healthy normolipidaemic volunteers consuming a controlled diet either with or without a phytosterol ester-enriched margarine. Food Chem. Toxicol. 1999, 37, 1063–1071.
- Zhu, H.; Chen, J.; He, Z.; Hao, W.; Liu, J.; Kwek, E.; Ma, K.Y.; Bi, Y. Plasma cholesterol-lowering activity of soybean germ phytosterols. Nutrients 2019, 11, 2784.
- Férézou, J.; Riottot, M.; Sérougne, C.; Cohen-Solal, C.; Catala, I.; Alquier, C.; Parquet, M.; Juste, C.; Lafont, H.; Mathé, D.; et al. Hypocholesterolemic action of beta-cyclodextrin and its effects on cholesterol metabolism in pigs fed a cholesterol-enriched diet. J. Lipid Res. 1997, 38, 86–100.
- Sulpice, J.C.; Férézou, J.; Lutton, C.; Mathé, D.; Chevallier, F. Diet and sterol biohydrogenation in the rat: Occurrence of epicoprostanol. Lipids 1978, 13, 217–224.
- Crowther, J.S.; Drasar, B.S.; Goddard, P.; Hill, M.J.; Johnson, K. The effect of a chemically defined diet on the faecal flora and faecal steroid concentration. Gut 1973, 14, 790–793.




