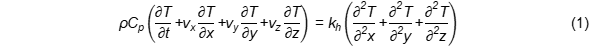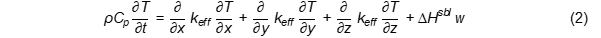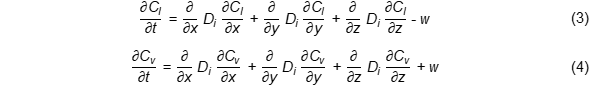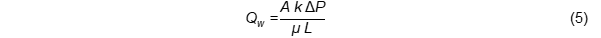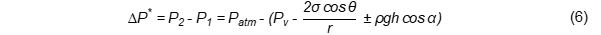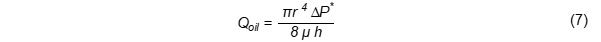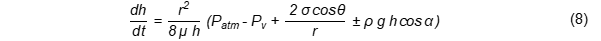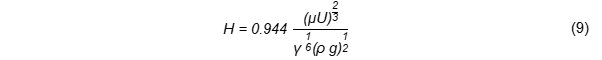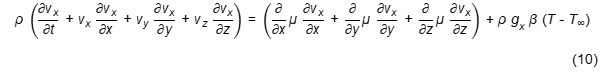| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sze Ying Leong | + 2610 word(s) | 2610 | 2020-07-22 08:41:53 |
Video Upload Options
Frying is one of the oldest unit operations to produce food products with a crisp texture, an aromatic flavour and a golden-brown colour. Deep-fried foods such as French fries, potato/veggie crisps are popular among consumers. Detailed insights into the frying process in terms of heat, mass (water and oil) and momentum transfers are outlined in this entry.
1. Definition
Frying is a very complex process including simultaneous heat, mass and momentum transfers accompanied by a series of physical and chemical reactions [1]. During the frying process, heat is transferred from oil to fried foods leading to mass transfer (e.g., water evaporation and oil uptake). Additionally chemical constituents (e.g., sugars, amino acids and water) within the food material react with each other during frying, and physical reactions (e.g., water evaporation and oil uptake) occur which can lead to structural changes [2].
2. Introduction
Frying (typically at 170 °C or above) is one of the oldest unit operations used by food processors; its goal is to produce final products with a crisp texture, an aromatic flavour and a golden-brown colour. There is a large variety of fried food products available in the market and fried potato products (i.e., French fries and potato crisps) are the most widely consumed around the world [3].
3. Frying Process of Food Materials
Frying is a complex process which involves simultaneous heat, mass and momentum transfers, resulting in the movement of oil and water, phase changes and physicochemical reactions occurring within the raw material [2]. These reactions account for both the beneficial and the deleterious effects of frying on food.
3.1. Heat Transfer
Frying is an efficient and intensive heat transfer process owing to the high heat transfer coefficients and dynamic conditions within the frying system. During frying, heat transferred from the hot oil to the surface of the cold food materials is driven by convection and from the surface to the inside by conduction [4]. As water begins to evaporate from the food, it creates vapour turbulence owing to its rapid evaporation which contributes to the turbulence of the oil around the food [5]. Vapour turbulence enhances the heat transfer rate to its maximum level [6], because when vapour bubbles cannot escape from the food material, they form an insulating layer on its surface limiting heat transfer [7]. During frying, water evaporation dissipates some energy inside the food material, thereby decreasing the available energy for temperature increase. The change of energy for heat transfer within a non homogenous food material can be calculated using the following Equation (1) in Cartesian coordinates [8]:
T: temperature, Cp: specific heat capacity of the material, kh: thermal conductivity of the material, ρ: density, vi: fluid velocity in i-direction. The right side of Equation (1) represents heat transfer due to conduction (dominates in case of solids) while the left side of the equation represents the unsteady term and convective heat-transfer terms.
In terms of frying, the energy balance equation [8] can be described using Equation (2):
keff: effective thermal conductivity, ΔHsbl: latent heat of sublimation, w: water content.
3.2. Mass Transfer
During the frying process, mass transfer mainly refers to the evaporation of water from the food material to the oil as well as the uptake of oil by food material.
3.2.1. Water Transfer
There are four main stages in the frying process which involve heat and mass transfers, namely the initial heating, surface boiling, falling rate and bubble endpoint periods [5]. In the initial heating stage, raw materials (at cold or ambient temperature) are dropped into the hot oil and are heated up gradually to the boiling point of water. During the surface boiling period, water begins to evaporate from the surface of materials along with the formation and release of bubbles leading to a rapid loss of water and the formation of pores on the surface of the food. A crust also begins to form at the outer surface. In the falling rate stage, the humid core region of the food material is heated slowly to the boiling point of water. Meanwhile, crust thickness increases and steam transfer speed decreases during this stage. In the bubble endpoint stage, water evaporation slows until bubbles are no longer being released to the surface.
Water transfer in the form of liquid (Cl) and vapour (Cv) during the unsteady diffusion process at the initial heating stage can be represented by the following Equations (3) and (4) in Cartesian coordinates [8]:
Ci: concentration of liquid (i = l) or vapour (i = v), Di: diffusivity of i-th species in the medium, w: water content.
During frying, the food material is transformed into a porous medium consisting of tiny void spaces (or small pores) that are interconnected and filled with liquid or vapour [9]. Therefore, the movement of water vapour can no longer be described as diffusion. Darcy’s law is considered to be more appropriate in describing the flow of water vapour inside the solid through the porous structure of fried foods [8]. Since raw food materials typically contain a high water content (80–95%), Darcy’s equation takes into account the significant pressure build up inside the porous food caused by the evaporation of internal water during frying [9]. Moreover, the resistance of the porous structure, which is proportional to the thickness of fried foods, can be integrated into Darcy’s law formulating Equation (5) to better describe water loss due to vapour flow through the crust [8].
Qw: flow rate of water vapour, A: cross-sectional area of the fried food, k: permeability of the crust layer (related to porosity), ΔP: pressure difference/drop over a given distance, µ: viscosity of the water vapour, L: thickness of fried foods.
3.2.2. Oil Transfer
There are several mechanisms to explain oil uptake during frying including water replacement, capillarity penetration, the cooling-phase effect and the surface-active agent theory, all of which are associated with water transfer and/or crust formation.
The water replacement mechanism describes how oil enters the food materials through the voids created by water evaporation, so water loss is considered to be the basis of oil uptake [10]. When water bubbles escape from food materials, they form capillary pathways and increase its surface porosity [10].
Capillarity penetration describes how the oil moves upwards through narrow pores in fried materials when the adhesive intermolecular forces between the oil and food materials are stronger than the cohesive intermolecular forces in the oil [11]. The pressure difference (ΔP*) between both ends of the capillary pathways mainly drives the capillarity penetration phenomena [12], as outlined in Equation (6):
Pi: pressure at the point i (P2: pressure at the pore surface, P1: pressure at the deepest pore point inside the food material, Patm: atmospheric pressure, Pv: water vapour pressure), θ: contact angle between the oil and the food material, r: pore radius, σ: surface tension of the oil, ρ: oil density, g: acceleration gravity, h: height of the capillary motion, α: angle between the capillary pathway and vertical direction.
Capillary penetration can also be described by the Washburn equation, as outlined in Equation (7):
Qoil: volumetric flow of laminar oil, p: ratio of an oil circumference to its diameter (3.142), r: pore radius, m: oil viscosity.
The penetration of oil over time [12] can be calculated based on the modification of the above two Equations (6) and (7) to yield Equation (8):
However, the voids or capillary pathways are always filled with water during frying and the inner steam pressure may resist oil penetration. Sometimes, the oil is absorbed after the capillary (food material) is removed from the oil [10][12].
The mechanism of cooling-phase effect describes oil uptake after the food material has been removed from the oil as the water vapour condenses and internal pressure reduces during the cooling period. Oil uptake after removal of the fried food from the oil is a balance between oil drainage and oil adhesion [2]. Adhered oil on the surface of fried food is absorbed due to the “vacuum effect” caused by the condensation of steam during the cooling period. When the fried food is removed from the oil, an oil film is formed on the surface and its thickness (H) can be calculated by the Landau–Levich–Derjaguin equation [13], as outlined in Equation (9):
µ: oil viscosity, γ: surface tension, U: speed of oil removal after frying; ρ: oil density, g: acceleration gravity.
The surface-active agent theory has been proposed in addition to the other oil uptake mechanisms. In this theory surface-active agents (e.g., monoglycerides and diglycerides) produced by oil degradation and hydrolytic reactions during frying enhance the interactions between the oil and the fried food leading to an increase in oil absorption [10]. Such surface-active agents can increase the foaming tendency of oil and reduce the interfacial tension leading to an increase in surface hydrophobicity [14].
3.3. Momentum Transfer
Momentum transfer is a physical phenomenon that involves convection mechanism between molecules or groups of molecules within the food material [15]. It depends upon the interrelation of the fundamental variables of mass, velocity and time and of changes in the velocity per unit mass [15]. During frying, momentum is transferred by convection (i.e. vapour leaving the fried materials) or by molecular forces (i.e. viscous stress or pressure) [16]. Momentum transfer equations are based on the principle that the momentum is conserved in a phase. The momentum balance equation, which contains three velocity components and the x-component equation in Cartesian coordinates [1], as described in Equation (10).
vi: fluid velocity of i component, ρ: fluid density, gx: acceleration gravity of x-component, β: thermal expansion coefficient.
3.4. Maillard Reaction
The Maillard reaction is one of the most important chemical reactions that occur in food during frying because it modifies many quality parameters in the fried product such as colour, flavour, taste, nutritional value and the level of toxic compounds (e.g., acrylamide) [17]. The Maillard reaction refers to the reaction between an amino group (e.g., amino acids) and a carbonyl group (e.g., reducing sugars). Firstly, a Schiff base is formed and rearranged to Amadori or Heyns products, which then undergo enolisation and are subsequently modified to form reactive α-dicarbonyl compounds, the source of brown pigments production [18]. These compounds can react with additional nucleophiles (i.e., guanidines, amines and thiols) and undergo Strecker degradation producing Strecker aldehydes. Furthermore, advanced glycation end products are produced in a series of downstream reactions, and further chemical reactions form a large number of polymerised products, named melanoidins, which result in colour darkening [18]. Temperature, time, reactant type (amino and carbonyl groups) and concentration, water activity and pH are the main factors that influence the Maillard reaction [19].
4. Quality Parameters of Fried Foods
4.1. Colour of Fried Foods
Colour is considered the most important parameter contributing to the visual perception of the quality of foods, and it influences the acceptance and choice of consumers. Apart from Maillard reactions (see Section 3.4), oil degradation may also affect the colour of fried foods. The polymerisation of triglycerides and the products of triglyceride hydrolysis, such as free fatty acids, monoglycerides and diglycerides, during oil degradation may result in changes in colour. Process variables including raw material properties (i.e., reducing sugar content, amino acids content, protein content and product dimensions), frying temperature, time and oil type can also affect the colour of fried food.
4.2. Moisture Content of Fried Foods
When a food material is placed into hot oil, its surface temperature increases rapidly and surface water rapidly evaporates in the form of water bubbles when its temperature rises to 100 °C [5]. As a result, the surface dries quickly and forms a crust. This crust acts as an additional barrier to the escape of water from the inner regions; therefore, the inner region is always moist compared to the outer region [20]. A number of other factors can also affect the moisture content of fried foods, including frying temperature and time, the size and shape of the product, type and thickness of the batter coating and the application of any pre-frying procedures such as pre-drying [21].
4.3. Uptake of Oil by Fried Foods
Oil is the most important ingredient for frying since it drives heat and mass transfer during frying. Most of the oil is absorbed by entering through pores from which moisture escapes during frying. Factors, such as frying conditions (time, temperature, food-to-oil ratio, repeated frying), oil characteristics (quality and type) and food characteristics (size, shape, surface roughness and porosity) and pre-treatments (blanching, edible coating, vacuum drying, PEF, etc.) may influence the amount of oil absorbed [22].
4.4. Texture Properties of Fried Foods
Fried foods are expected to have a crispy crust and a moist and soft interior [2]. Crispness, porosity and shrinkage are regarded as the main textural indicators of fried foods. Crispness describes a quick fracture under small strain stresses owing to the food having a low water content on its surface layer [2]. During frying, some water vapour is unable to move through the food material because of restrictive intercellular diffusion. As a result, superheated vapour distorts the pores and leads to the formation of a porous structure. Normally, intense water evaporation results in the formation of larger pores. There are many factors influencing the porosity of fried foods, including frying conditions (time, temperature, and pre-treatments), food material characteristics (size, shape, component, density) and oil types. Product shrinkage is caused by water loss, resulting in the reduction of open pores and an increase in the density of the fried product [21]. During frying, shrinkage initially occurs at the surface, accompanied by the formation of a rigid outer layer. Shrinkage then moves inwards until the final volume is fixed. Shrinkage phenomena are related to the frying time and temperature, shape, size and density of the food materials [21].
4.5. Generation of Toxic Compounds in Fried Foods
Toxic compounds that may be produced during frying include acrylamide, hydroxymethylfurfural, furan, ethylcarbamate, heterocyclic amines, polycyclic aromatic hydrocarbons and nitrosamines, all of which pose potential health risks to consumers[17]. Most acrylamide in fried foods is formed by the Maillard reaction between reactive carbonyls and asparagine at a temperature of over 120 °C through a series of intermediates [17]. Factors such as frying conditions (temperature, time, pH and pre-treatments) and material characteristics (chemical constituents, water activity) may influence the acrylamide content in fried foods [17].
5. Concluding remarks and future research direction
Frying is a multifaceted process involving the occurrence of heat, mass and momentum transfers that change the physical and chemical states of the food. In the food industry, frying is a popular unit operation as the process is a relatively quick way to produce attractive food products with desirable organoleptic properties (flavourful and crispy crust) and a longer shelf life. The type of oils and batter coating (or breading) used for frying foods, frying procedure, temperature and duration of frying, and how often oils were reused are important determinants of the quality of fried foods. Although frequent consumption of fried foods has been associated with a higher risk of developing coronary disease, diabetes, hypertension and obesity [23], the use of emerging food processing technologies such as Pulsed Electric Fields (PEF) has been explored as viable pre-treatments, particularly on potato tubers, prior to deep-frying to reduce the oil content in French fries [24]. Moreover, this emerging technology is recommended for use in the food industry to reduce production costs (e.g., better water and energy efficiencies) and minimise food waste [24]. Future research should focus on potentials of emerging food processing technologies as pre-treatments prior to frying and the exploration of alternative options for frying media or coatings as strategies to reduce the oil content in fried foods.
6. Contributors
Zihan Xu 1, Sze Ying Leong 1,2, Mohammed Farid 3, Patrick Silcock 1, Phil Bremer 1 and Indrawati Oey 1,2
1Department of Food Science, University of Otago, PO Box 56, Dunedin 9054, New Zealand
2Riddet Institute, Private Bag 11 222, Palmerston North 4442, New Zealand
3Department of Chemical and Materials Engineering, University of Auckland, Private Bag 92019
References
- S. Eichenlaub; C. Koh; Modeling of food-frying processes. Modeling Food Processing Operations 2015, 1, 163-184, 10.1016/b978-1-78242-284-6.00006-4.
- Pedro Bouchon; Jose Aguilera; Microstructural analysis of frying potatoes. International Journal of Food Science and Technology 2001, 36, 669-676, 10.1046/j.1365-2621.2001.00499.x.
- Agnieszka Kita; The effect of frying on fat uptake and texture of fried potato products. European Journal of Lipid Science and Technology 2014, 116, 735-740, 10.1002/ejlt.201300276.
- Harkirat S. Bansal; Pawan S. Takhar; Jirawan Maneerote; Modeling multiscale transport mechanisms, phase changes and thermomechanics during frying. Food Research International 2014, 62, 709-717, 10.1016/j.foodres.2014.04.016.
- Armando Alvis; Carlos Velez; Maite Rada-Mendoza; Mar Villamiel; Héctor S. Villada; Heat transfer coefficient during deep-fat frying. Food Control 2009, 20, 321-325, 10.1016/j.foodcont.2008.05.016.
- Ferruh Erdogdu; T. Koray Palazoglu; Food frying process design. Handbook of Food Process Design 2012, 1, 789-810, 10.1002/9781444398274.ch28.
- Jorge Mir-Bel; Rosa Oria; María L. Salvador; María Luisa Salvador Solano; Influence of temperature on heat transfer coefficient during moderate vacuum deep-fat frying. Journal of Food Engineering 2012, 113, 167-176, 10.1016/j.jfoodeng.2012.06.009.
- Singh, S.V.; Verma, A.K. Mathematical modeling in foods: Review. In Food engineering: Emerging issues, modeling, and applications, Meghwal, M.; Goyal, M.R., Eds. Apple Academic Press: New York, 2016; pp 75-110.
- Ashim K. Datta; Porous media approaches to studying simultaneous heat and mass transfer in food processes. I: Problem formulations. Journal of Food Engineering 2007, 80, 80-95, 10.1016/j.jfoodeng.2006.05.013.
- Muhammad Arslan; Zou Xiaobo; Jiyong Shi; Allah Rakha; Hu Xuetao; Muhammad Zareef; Xiaodong Zhai; Sajid Basheer; Oil uptake by potato chips or French fries: A review. European Journal of Lipid Science and Technology 2018, 120, 1800058, 10.1002/ejlt.201800058.
- Aman Mohammad Ziaiifar; Nawel Achir; Francis Courtois; Isabelle Trezzani; Gilles Trystram; Review of mechanisms, conditions, and factors involved in the oil uptake phenomenon during the deep-fat frying process. International Journal of Food Science and Technology 2008, 43, 1410-1423, 10.1111/j.1365-2621.2007.01664.x.
- P. Bouchon; D.L. Pyle; Modelling oil absorption during post-frying cooling: I: Model development. Food and Bioproducts Processing 2005, 83, 253-260, 10.1205/fbp.05115.
- Joseph W Krozel; Ahmet N Palazoglu; Robert L Powell; Experimental observation of dip-coating phenomena and the prospect of using motion control to minimize fluid retention. Chemical Engineering Science 2000, 55, 3639-3650, 10.1016/s0009-2509(00)00036-1.
- E.J. Pinthus; Pnina Weinberg; I.S. Saguy; Oil uptake in deep fat frying as affected by porosity. Journal of Food Science 1995, 60, 767-769, 10.1111/j.1365-2621.1995.tb06224.x.
- J F Velez-Ruiz; Introductory aspects of momentum transfer phenomena. Food Preservation Technology 2002, 7, 67-90, 10.1201/9781420006261.ch3.
- Mohammed Farid; Riza Kizilel; A new approach to the analysis of heat and mass transfer in drying and frying of food products. Chemical Engineering and Processing - Process Intensification 2009, 48, 217-223, 10.1016/j.cep.2008.03.013.
- Vural Gökmen; Tunc Koray Palazoglu; Acrylamide formation in foods during thermal processing with a focus on frying. Food and Bioprocess Technology 2007, 1, 35-42, 10.1007/s11947-007-0005-2.
- Marianne N. Lund; Colin Ray; Control of Maillard reactions in foods: Strategies and chemical mechanisms. Journal of Agricultural and Food Chemistry 2017, 65, 4537-4552, 10.1021/acs.jafc.7b00882.
- Henry Jäger; A. Janositz; D. Knorr; The Maillard reaction and its control during food processing. The potential of emerging technologies. Pathologie Biologie 2010, 58, 207-213, 10.1016/j.patbio.2009.09.016.
- Betul Isik; Serpil Sahin; Gulum Sumnu; Pore development, oil and moisture distribution in crust and core regions of potatoes during frying. Food and Bioprocess Technology 2016, 9, 1653-1660, 10.1007/s11947-016-1748-4.
- M. K. Krokida; V. Oreopoulou; Zacharias Maroulis; Dimitrios Marinos-Kouris; Deep fat frying of potato strips—Quality issues. Drying Technology 2001, 19, 879-935, 10.1081/drt-100103773.
- Michel Mellema; Mechanism and reduction of fat uptake in deep-fat fried foods. Trends in Food Science and Technology 2003, 14, 364-373, 10.1016/s0924-2244(03)00050-5.
- Taraka V. Gadiraju; Yash R. Patel; J. Michael Gaziano; Luc Djoussé; Fried Food Consumption and Cardiovascular Health: A Review of Current Evidence. Nutrients 2015, 7, 8424-8430, 10.3390/nu7105404.
- T. Fauster; D. Schlossnikl; F. Rath; R. Ostermeier; F. Teufel; S. Toepfl; H. Jaeger; Impact of pulsed electric field (PEF) pretreatment on process performance of industrial French fries production. Journal of Food Engineering 2018, 235, 16-22, 10.1016/j.jfoodeng.2018.04.023.