
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yueh-Ling Hsieh | + 1680 word(s) | 1680 | 2021-09-24 05:53:21 | | | |
| 2 | Jason Zhu | Meta information modification | 1680 | 2021-09-24 10:19:28 | | | | |
| 3 | Jason Zhu | Meta information modification | 1680 | 2021-09-27 05:32:11 | | | | |
| 4 | Jason Zhu | Meta information modification | 1680 | 2021-09-27 05:32:56 | | | | |
| 5 | Jason Zhu | -75 word(s) | 1605 | 2021-10-27 11:40:26 | | |
Video Upload Options
Ultra-low frequency transcutaneous electrical nerve stimulation (ULF-TENS) is an active therapeutic device that affects relaxation of masticatory and mandibular postural muscles through applying low-frequency, low current stimulation of the mandibular division of the trigeminal nerve and a branch of the superficial facial nerve.
1. Introduction
According to available literature[1][2][3][4], Ultra-low frequency transcutaneous electrical nerve stimulation (ULF-TENS) seems to be a valid support in the management of TMD patients with more ‘relaxed’ muscles [5][6][7][8], but some patients get worse after ULF-TENS, presenting an increase in electromyographic activity[9][10]. Currently, the mechanisms responsible for the analgesia produced by ULF-TENS remain unclear, especially regarding the involvement of connections in central pain-modulating neurons.
Orofacial pain resulted from TMD may involve the parabrachial nucleus that forms ascending trigemino-parabrachial nociceptive pathways to convey the MMP-induced nociception to higher brain circuits for developing the affective dimension of pain, emotional, and autonomic disturbances[11][12][13]. However, there is growing evidence that the parabrachial nucleus is one of the main connections with the descending pain-modulating systems, best characterized by abundant projections of parabrachial nucleus to the rostral ventromedial medulla involved in pain modulation [14]. An alteration in the descending inhibitory or excitatory influences from some structures such as the rostral ventromedial medulla and central opioid pathway seems to be the most powerful in reducing pain behavior and nociceptive neuronal activity[15]. Therefore, modulation of both parabrachial nucleus and rostral ventromedial medulla that are involved in pain-modulatory circuits can be possible mechanisms behind therapy for MMP.
Substance P (SP) is one such biochemical richly distributed in the parabrachial nucleus and thought to be released from primary afferent terminals by noxious or painful stimuli. Its neuromodulation on transmission in the parabrachial nucleus has been reported [16][17]. Activation of μ-opiate receptors (MOR) in interneurons produces hyperpolarization of neurons, leading to inhibition of firing and modulation of responses to SP, thereby blocking pain transmission. Increased expression of SP in the parabrachial nucleus after tetanic contraction-induced MMP in rat model has been previously identified. In view of these results, this study hypothesizes that ULF-TENS at myofascial trigger points activates neurons in the rostral ventromedial medulla affecting its expression of c-Fos, enhances MOR expression in the parabrachial nucleus, as well as reduces SP expression in the parabrachial nucleus, thus alleviating MMP. Therefore, the aim of this study is to examine the effects of ULF-TENS on electrophysiological activities and functional movements of masticatory muscles, as well as the biochemical alterations in both parabrachial nucleus and rostral ventromedial medulla in animal models of MMP.
2. Effects of ULF-TENS on Electrophysiology of Masseter Muscle after MMP Induction
Figure 1A–D show serial changes of EPN activities from myofascial trigger points of masseter muscle recorded at the focal hypoechoic area (Figure 1E) under ultrasonic guidance before, after MMP/sham-MMP induction, and after ULF-TENS/sham ULF-TENS treatment in the four groups. Before MMP induction, there was no significant difference in EPN prevalence among the groups (χ2(3) = 7.32, p = 0.06, Table 1). Significant differences among the four groups were found after MMP induction at both time points of pre-treatment (χ2(3) = 29.37, p = 0.000002) and post-treatment (χ2(3) = 25.87, p = 0.00001). After MMP induction, EPN prevalence in both MU and MsU groups were significantly increased compared with that in sMU and sMsU group, indicating marked increase in mean EPN prevalence in masseter muscle after chronic maximum tetanic eccentric contraction (all p < 0.0083, Figure 1F, Table 1). After treatment, the MMP-induced increment of EPN prevalence was reduced in the MU group, indicating no statistically significant difference compared with that in sMU and sMsU groups (both p > 0.0083, Figure 1F). However, EPN prevalence was still significantly higher in the MsU group than in the other groups (all p < 0.0083, Figure 1F). There were significant differences between the MU and MsU groups (Z = −3.82, p = 0.00013). Significant difference was found in the difference of improvement from pre-treatment to post-treatment time points between MU and MsU groups (Z = −3.82, p = 0.00014, Cohen’s d. = −4.097).
There were significant differences in EPN prevalence among those recorded before induction, before treatment, and after treatment conditions in both MU (χ2(2) = 15.79, p < 0.017) and MsU (χ2(2) = 15.73, p < 0.017) groups (Table 1).
| Pre-Induction | Pre-Treatment | Post-Treatment | 2 Differences among Timepoints, p Value | ||
|---|---|---|---|---|---|
| EPN prevalence | MU | 27.30 ± 5.68 | 52.60 ± 4.77 *†§ | 28.10 ± 6.03 ‡ | 0.00037 |
| (%) | MsU | 29.40 ± 7.07 | 51.20 ± 8.94 *†§ | 54.50 ± 6.17 *†§|| | 0.00038 |
| sMU | 22.70 ± 4.64 | 25.30 ± 6.00 | 24.80 ± 4.52 | 0.04214 (NS) | |
| sMsU | 28.10 ± 4.01 | 27.4 ± 4.76 | 28.20 ± 2.53 | 0.86687 (NS) | |
| 1 Differences among groups, p value | 0.0623 (NS) | 1.87 × 10−6 | 1.02 × 10−5 | ||
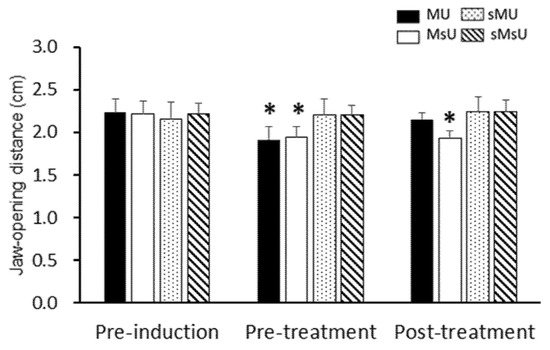
| Jaw-opening distance | MU | 2.23 ± 0.16 | 1.92 ± 0.16 *†§ | 2.15 ± 0.08 ‡ | 0.00183 |
| (cm) | MsU | 2.21 ± 0.15 | 1.95 ± 0.12 *†§ | 1.93 ± 0.09 *†§|| | 0.00037 |
| sMU | 2.16 ± 0.19 | 2.20 ± 0.19 | 2.24 ± 0.18 | 0.13904 (NS) | |
| sMsU | 2.22 ± 0.12 | 2.20 ± 0.11 | 2.26 ± 0.15 | 0.04436 (NS) | |
| 1 Differences among groups, p value | 0.88 (NS) | 3.03 × 10−5 | 1.61 × 10−4 | ||
3. Effects of ULF-TENS on Maximal Jaw-Opening Distance after MMP Induction
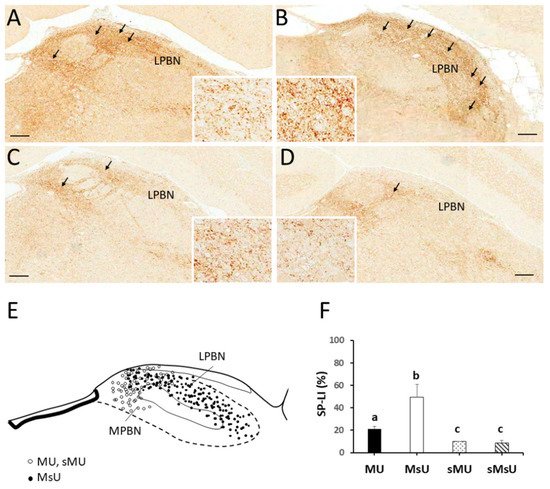
4. Expressions of SP-like and MOR-like Immunoreactivity in Parabrachial Nuclei
| MU | MsU | sMU | sMsU | 1 Differences among Groups, p Value | |
|---|---|---|---|---|---|
| Parabrachial nucleus (%) | |||||
| SP | 21.18 ± 2.19 *†‡ | 49.33 ± 11.42 †‡ | 9.89 ± 0.35 | 8.49 ± 2.63 | p < 0.0001 |
| MOR | 18.63 ± 5.15 *†‡ | 2.10 ± 0.11 †‡ | 9.43 ± 2.85 | 6.09 ± 3.18 | p < 0.0001 |
| Rostral ventromedial medulla (%) | |||||
| c-Fos | 43.39 ± 10.73 *‡ | 13.19 ± 2.04 †‡ | 26.33 ± 5.08 ‡ | 10.97 ± 1.15 | p < 0.0001 |
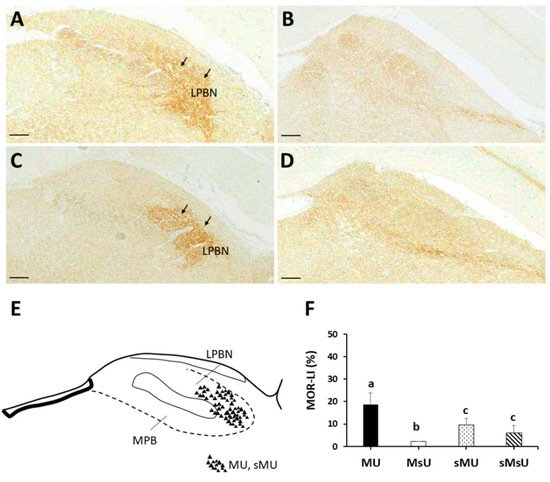
5. Conclusions
References
- Chou, L.W.; Hsieh, Y.L.; Kuan, T.S.; Hong, C.Z. Needling therapy for myofascial pain: Recommended technique with multiple rapid needle insertion. Biomedicine 2014, 4, 13. [Google Scholar] [CrossRef]
- Manfredini, D.; Guarda-Nardini, L.; Winocur, E.; Piccotti, F.; Ahlberg, J.; Lobbezoo, F. Research diagnostic criteria for temporomandibular disorders: A systematic review of axis I epidemiologic findings. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martínez, A.; Paris-Alemany, A.; López-de-Uralde-Villanueva, I.; La Touche, R. Management of pain in patients with temporomandibular disorder (TMD): Challenges and solutions. J. Pain Res. 2018, 11, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.C. Temporomandibular disorders: A position paper of the International College of Cranio-Mandibular Orthopedics (ICCMO). Cranio 2011, 29, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Bazzotti, L. Electromyography tension and frequency spectrum analysis at rest of some masticatory muscles, before and after TENS. Electromyogr. Clin. Neurophysiol. 1997, 37, 365–378. [Google Scholar] [PubMed]
- Cooper, B.C.; Kleinberg, I. Establishment of a temporomandibular physiological state with neuromuscular orthosis treatment affects reduction of TMD symptoms in 313 patients. Cranio 2008, 26, 104–117. [Google Scholar] [CrossRef]
- Kamyszek, G.; Ketcham, R.; Garcia, R., Jr.; Radke, J. Electromyographic evidence of reduced muscle activity when ULF-TENS is applied to the Vth and VIIth cranial nerves. Cranio 2001, 19, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Monaco, A.; Sgolastra, F.; Ciarrocchi, I.; Cattaneo, R. Effects of transcutaneous electrical nervous stimulation on electromyographic and kinesiographic activity of patients with temporomandibular disorders: A placebo-controlled study. J. Electromyogr. Kinesiol. 2012, 22, 463–468. [Google Scholar] [CrossRef]
- Chipaila, N.; Sgolastra, F.; Spadaro, A.; Pietropaoli, D.; Masci, C.; Cattaneo, R.; Monaco, A. The effects of ULF-TENS stimulation on gnathology: The state of the art. Cranio 2014, 32, 118–130. [Google Scholar] [CrossRef]
- Konchak, P.A.; Thomas, N.R.; Lanigan, D.T.; Devon, R.M. Freeway space measurement using mandibular kinesiograph and EMG before and after TENS. Angle Orthod. 1988, 58, 343–350. [Google Scholar]
- De Rossi, S.S.; Stern, I.; Sollecito, T.P. Disorders of the masticatory muscles. Dent. Clin. N. Am. 2013, 57, 449–464. [Google Scholar] [CrossRef]
- Allen, G.V.; Barbrick, B.; Esser, M.J. Trigeminal-parabrachial connections: Possible pathway for nociception-induced cardiovascular reflex responses. Brain Res. 1996, 715, 125–135. [Google Scholar] [CrossRef]
- Gauriau, C.; Bernard, J.F. Pain pathways and parabrachial circuits in the rat. Exp. Physiol. 2002, 87, 251–258. [Google Scholar] [CrossRef]
- Chen, Q.; Roeder, Z.; Li, M.H.; Zhang, Y.; Ingram, S.L.; Heinricher, M.M. Optogenetic evidence for a direct circuit linking nociceptive transmission through the parabrachial complex with pain-modulating neurons of the rostral ventromedial medulla (RVM). eNeuro 2017, 4, e0202-17. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef] [PubMed]
- Nomura, H.; Konno, H.; Takase, S.; Saito, H. Decrease of substance P in the parabrachial nucleus of multiple system atrophy. Auton. Neurosci. 2001, 92, 86–91. [Google Scholar] [CrossRef]
- Saleh, T.M.; Kombian, S.B.; Zidichouski, J.A.; Pittman, Q.J. Peptidergic modulation of synaptic transmission in the parabrachial nucleus in vitro: Importance of degradative enzymes in regulating synaptic efficacy. J. Neurosci. 1996, 16, 6046–6055. [Google Scholar] [CrossRef] [PubMed]





