Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Motomu Shimaoka | + 2415 word(s) | 2415 | 2021-08-13 10:05:36 | | | |
| 2 | Dean Liu | Meta information modification | 2415 | 2021-09-24 04:58:52 | | | | |
| 3 | Dean Liu | Meta information modification | 2415 | 2021-09-24 04:59:39 | | | | |
| 4 | Conner Chen | Meta information modification | 2415 | 2021-10-11 08:41:50 | | | | |
| 5 | Conner Chen | Meta information modification | 2415 | 2021-10-11 08:42:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shimaoka, M. Connexins and Integrins in Exosomes. Encyclopedia. Available online: https://encyclopedia.pub/entry/14500 (accessed on 07 February 2026).
Shimaoka M. Connexins and Integrins in Exosomes. Encyclopedia. Available at: https://encyclopedia.pub/entry/14500. Accessed February 07, 2026.
Shimaoka, Motomu. "Connexins and Integrins in Exosomes" Encyclopedia, https://encyclopedia.pub/entry/14500 (accessed February 07, 2026).
Shimaoka, M. (2021, September 24). Connexins and Integrins in Exosomes. In Encyclopedia. https://encyclopedia.pub/entry/14500
Shimaoka, Motomu. "Connexins and Integrins in Exosomes." Encyclopedia. Web. 24 September, 2021.
Copy Citation
Connexins and integrins, the two structurally and functionally distinct families of transmembrane proteins, have been shown to be inter-connected by various modes of cross-talk in cells, such as direct physical coupling via lateral contact, indirect physical coupling via actin and actin-binding proteins, and functional coupling via signaling cascades.
connexin
pannexin
integrin
exosomes
crosstalk
cancer
inflammation
1. Introduction
Connexins (Cxs) [1][2][3] and integrins [4][5][6] are two structurally and functionally distinct families of membrane proteins that have been shown to play apparently different biological roles in intercellular communications, as detailed in the sections that follow. Briefly, Cxs primarily function as an integral component of channels at the gap junctions, where they mediate inter-cellular exchanges of small molecules [1][2][3], whereas integrins primarily function as cell-adhesion molecules that mediate cell-to-cell and/or cell-to-extracellular matrix adhesive interactions [4][5][6]. Several important modes of crosstalk between integrins and Cxs have been identified [7][8][9], underscoring the important collaborations between these two distinct membrane proteins in regulating cellular functions in both health and diseases, including cancers. Recently, investigations of Cxs and integrins have expanded to exosomes, the lipid bi-layered nanoparticles secreted from cells that are now recognized as a new biological means for intercellular communications under normal physiological and many patho-physiological conditions, such as cancers [10]. Exosomes display many membrane proteins—including Cxs [11] and integrins [12]—that originate from source cells. However, the functions and biological implications of exosomal Cxs and integrins remain little understood. Here, we aim to review recent progress in understanding how Cxs and integrins function in exosomes, and will then theorize how they might crosstalk in exosomes.
2. Connexins and Integrins in Exosomes
Exosomes are taken up by target cells, by which they are internalized in order to deliver any exosomal contents, including small molecules and small RNAs, to the cytoplasm [13][14]. Two major mechasnisms of exosomal uptake have been proposed: fusion to the cellular plasma membrane and endocytosis via several different modes (e.g., macropinocytosis, phagocytosis, clathrin-mediated endocytosis, caveolin-mediated endocytosis, and lipid-raft-mediated endocytosis) [13][14]. As described in the sections that follow, exosomal Cxs and integrins have been shown to play important roles in exosomal binding to, uptake by, and/or delivery of contents to, target cells.
2.1. Connexins in Exosomes
2.1.1. Exosomal Connexin-Mediated Molecular Transfer to Target Cells
Girao and colleagues have demonstrated the presence of the Cx43 protein in the membranes of exosomes secreted by several cultured cell lines that express Cx43 on the cell surface [11]. Exosomes isolated from biological fluids and human plasma also contain the Cx43 protein. Exosomal Cx43 has been shown to assemble and attach to intact oligomers, thereby surviving to form hexameric hemichannels. Of note, exosomal Cx43-based hemichannels have been shown to be functionally active in their ability to transfer small molecules to target cells [11]. Exosomes expressing Cx43 exhibited the capability to transfer, to target cells, a dye luciferin (molecular weight 280) known to penetrate gap junction channels. The transfer of luciferin happens instantaneously, requiring only a few seconds. As the instantaneous nature of the transfer phenomenon is probably dissimilar to the mechanism underlying exosomal internalization, the transfer most likely occurs through a channel pore. Exosomal transfer of luciferin was inhibited by a peptide gap 26 that blocked Cx43-based channel functions [11]. In addition, exosomes containing mutant Cx43 (S368A), which inhibited serine phosphorylation, and thereby facilitated the formation of a constitutively open hemichannel, exhibited higher levels of instantaneous exosomal luciferin transfer [11]. By contrast, exosomes containing another mutant Cx43 (S368D), one that mimics serine phosphorylation at residue 368 and thereby facilitates the formation of a constitutively closed hemichannel, exhibited reduced levels of exosomal luciferin transfer. These results confirmed the Cx channel-dependent exosomal transfer of luciferin [11]. This transfer appears to take place through the docking of the exosomal hemichannel and via targeting of the cellular hemichannel, thus constituting a gap junction-like functional channel between exosomes and target cells [11]. The blocking peptide gap 26, which would disrupt the proper formation of Cx43-based hemichannels, inhibited the channel docking between exosomes and target cells [11].
In addition to the internalization of exosomes via fusion to the plasma membrane and endocytosis [13][14], this gap junction-like Cx channel-mediated mechanism might represent a novel alternative method for instantaneously delivering at least parts of the exosomal contents to target cells [11]. Cx channel-mediated transfer would precede, and/or simultaneously occur in tandem with, the uptake of exosomes via internalization. Thus, it is interesting to speculate that those small molecules instantaneously delivered via Cx channels might alter the metabolism of target cells, thereby preconditioning them to either promote or suppress subsequent exosomal uptake.
Another interesting point of speculation concerns the possibility that exosomes might form Cx43-based channels not only with target cells that endogenously express Cx43, but also with those cells that do not. As shown by the exosomal transfer of integrin proteins to target cells [15][16], the membrane fusion of Cx43-containig exosomes to target cells could lead to the incorporation of Cx43 into the plasma membranes of target cells. Therefore, the ability of exosomes to transfer the Cx43 protein could enable Cx43 channel-mediated exosomal delivery to virtually any target cell population, regardless of the endogenous expression of target cellular Cx43. This might entail a generalized model in which the Cx channel-mediated instantaneous delivery of exosomal contents occurs in tandem with exosomal uptake via internalization and membrane fusion during the course of ongoing intercellular exosomal communication.
2.1.2. Cx-Mediated Transfer of Therapeutically and Biologically Active Exosomal Contents
Using a model involving molecular luciferin dye, Cx channel-mediated transfer of exosomal contents was shown to occur with proof-of-principal validity [11]. However, it remains to be elucidated exactly what type of therapeutically and/or biologically active exosomal contents would be delivered via the Cx channels to target cells.
A sequel study conducted by Girao and colleagues aimed to investigate the ability of exosomal Cx43 to enhance the delivery of the chemotherapeutic drug doxorubicin to tumors [17]. Although the presence of Cx43 on doxorubicin-loaded exosomes did not enhance the anti-tumor effects in vivo, exosomal Cx43 ameliorated the cardiotoxicity of doxorubicin [17]. Whereas the heart expresses Cx43, cardiac Cx43 is thought to locate mainly at the intercalated discs, forming gap junctions therein. Thus, cardiac Cx43 is not readily available for exosomal Cx43 to dock, thereby partly illustrating the molecular mechanism underlying exosomal Cx43-mediated amelioration of doxorubicin-induced cardiotoxicity, which remains unclear [17]. A possible additional explanation could be that Cx43-positive, doxorubicin-loaded exosomes are taken up by non-cardiac organs that express high levels of Cx43, such as the bone marrow, endocrine tissues, and skin [18].
MicroRNAs (miRNAs) are among the most biologically active components of exosomes, and have been extensively studied in terms of the exosome-induced functional alteration of target cells [19]. Notably, two RNA-binding motifs were predicted in the sequence of Cx43; specifically, at the cytoplasmic loop and C-terminal cytoplasmic regions [20]. Although the functions of RNA-binding motifs still await experimental testing, they are believed to facilitate the packing of small RNAs, including miRNAs, into Cx43-containing exosomes. Currently, the “gap junction-like” Cx channel-mediated exosomal miRNA transfer to target cells remains an attractive idea lacking experimental verification. By contrast, miRNA transfer through an authentic gap junction between cells has been demonstrated [21][22]. Mature miRNAs were transferred and exchanged via gap junctions between the cytoplasmic spaces of adjacent cells [22]. Gap junction-mediated exchange of mature miRNAs has been observed both between homotypic cells and between heterotypic cells [21]. For example, the miRNA exchange that occurs between endothelial cells and cancer cells has been demonstrated in vitro [21]. The physiological significance of gap junction-mediated miRNA exchanges between cells has yet to be elucidated; however, it may trigger bystander effects in gene therapy settings. In such cases, synthetic miRNA mimetics would be delivered not only onto the target cells, but also onto adjacent non-target cells [21]. The miRNA mimetics initially delivered to adjacent non-target cells could subsequently spread, reaching target cells through the gap junctions between adjacent and target cells [21]. In this way, gap-function-mediated bystander effects would enhance the therapeutic effects of miRNA mimetic treatments.
Kalluri and colleagues have demonstrated that cancer exosomes contain all of the necessary components for gene silencing to include miRNAs along with the RNA-induced silencing complexes (RISCs), which contain Dicer, Ago2, and trans-activating response RNA-binding proteins [23]. Thus, miRNA biogenesis (i.e., pri-miRNA processing by Dicer to transform into mature miRNA) [19] could occur within exosomes, which suggests they function somewhat like miRNA factories [24]. The simultaneous transfer of mature miRNAs into RISCs would enable gene silencing as soon as the exosomes were delivered to the target cells, independently of target cellular RISC components. These results need to be reproduced independently and the detailed nature of miRNA processing during exosomal biogenesis requires further and extensive investigation.
2.1.3. Regulation of Exosome Release by Cellular Connexin
Several types of cellular stress (e.g., cellular senescence by irradiation [25]; endoplasmic reticulum stress by cisplatin [26]; and tunicamycin [27]) have been shown to increase the release of exosomes from cells. The regulatory role of Cx43 in exosome release has been shown in traumatic brain injury [28]. Cx43 in astroglias in the hippocampus has been implicated in propagating damage into surrounding brain tissues [28]. Using a rat model, Tong and colleagues have demonstrated that traumatic brain injury induced at the hippocampus results in the release of exosomes, which attempted to restore the injury-induced functional memory defects then present in the hippocampus neurons (i.e., long-term potentiation) [28]. Of note, Cx43 is responsible for promoting injury-induced exosomal release from the hippocampus [28], indicating its important role in both propagating and dampening neuronal damage in traumatic brain injury.
2.2. Integrins in Exosomes
2.2.1. Integrin-Directed Exosomal Homing to Tissues and Remodeling of the Homing Niche
Integrins on leukocytes, such as α4β7 and αLβ2, support the critical adhesive interactions with their ligands MAdCAM-1 (Mucosal Addressin Cell Adhesion Molecule-1) and ICAM-1 (Intercellular Adhesion Molecule-1), respectively, on endothelial cells, thereby mediating migration to specific tissues [29]. This trafficking process is known as tissue-specific leukocyte migration (or homing). It constitutes an integral part of an effective adoptive immune response and of eliciting immune cell accumulation at the site of inflammation [29]. The concept of integrin-mediated cell migration to specific tissues, wherein endothelial cells express corresponding integrin ligands, can be generalized to the metastatic spread of cancer [30][31]. Some cancer cells are known to upregulate the expression of αVβ3, αVβ6, α5β1, and/or α6β4 [31]. These cancer-upregulated integrins bind to specific extracellular matrix protein ligands, such as vitronectin, fibronectin, laminin, and collagens, that are deposited in the cancer microenvironment, thereby promoting the metastatic dissemination of primary cancer cells to distant organs [31].
Exosomes express integrin on the surface. Consistent with the function of cellular integrins to guide cells to specific organs, exosomal integrins have been shown to be capable of guiding exosomes to specific tissues, as was originally shown in cancer exosomes. Using breast cancer and pancreatic cancer exosomes, Lyden and colleagues have shown that those exosomes expressing integrin α6β4 were preferentially distributed to the lung, whereas those expressing integrin αVβ5 were preferentially distributed to the liver [12]. It has been suggested that preferential exosomal distribution to the lung was mediated by the interaction of integrin α6β4 with laminin in the lung, whereas that to the liver was mediated by the interaction of integrin αVβ5 with fibronectin [12]. Interestingly, cancer exosomal homing to the lung and the liver does not simply precede the metastatic dissemination of primary breast and pancreatic tumors to these distant organs, but also preconditions the lung and liver to form pre-metastatic niches, a specialized microenvironment that promotes the homing, retention, and subsequent proliferation of disseminated cancer cells (Figure 1A). The proposed mechanism underlying pre-metastatic niche formation involves the activation of proinflammatory S100 genes in lung and liver tissue cells [12][32]. This process is elicited by the Src activation activity carried out by exosomal integrin proteins transferred to target cells. Integrins themselves do not conduct kinase activities; instead, they associate with Src-family kinases, thereby acting as a hub for establishing signaling complexes [12]. While exosomal transfer of the intact integrin-signaling complex remains to be demonstrated, exosomally transferred integrin proteins could trigger the signals that lead to S100 activation by using Src kinases derived from either exosomes or target cells [12]. In this way, cancer exosomes establish the organotropism driving metastasis.
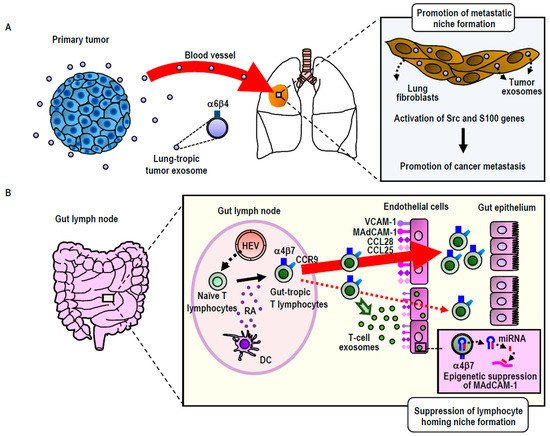
Figure 1. Exosomal remodeling of cancer metastatic (A) and lymphocyte homing (B) niches. (A) Integrin α6β4high exosomes secreted by breast or pancreatic cancers preferentially distribute to the lung, wherein they remodel to promote metastatic niche formation via the activation of Src family kinases and S100 proinflammatory genes. (B) Integrin α4β7high exosomes secreted by gut-tropic T-lymphocytes preferentially distribute to the gut, wherein they remodel to suppress homing niche formation by the down-regulation of the MAdCAM-1 gene via an miRNA-mediated epigenetic mechanism. HEV: High endothelial venules; RA: Retinoic acid; DC: Dendritic cells.
Building upon and extending the study of cancer exosomes to non-cancerous cells (i.e., T-lymphocytes), our group has utilized the concept of integrin-mediated exosomal homing to remodel the microenvironment of target tissues [15] (Figure 2B). Upon activation via contact with antigen-presenting dendritic cells residing in the gut, T-lymphocytes acquire gut-tropism—i.e., the ability to preferentially home to the gut by upregulating integrin α4β7 [33]. Integrin α4β7 binds to its endothelial ligand MAdCAM-1, which is exclusively and constitutively expressed in the gut, thereby playing a central role in gut-specific lymphocyte homing [33]. We have demonstrated that gut-trophic lymphocytes secrete exosomes expressing high levels of integrin α4β7, which guide the exosomes to the gut via binding to MAdCAM-1 [15]. In contrast to cancer exosomes, which promote the formation of pre-homing (or pre-metastatic) niches, gut-tropic T-lymphocytic exosomes diminish gut-homing niches by suppressing the expression of MAdCAM-1 and other homing-supporting molecules [15]. The ability of T-lymphocytic exosomes to diminish gut-homing niches has been attributed to several specific exosomal miRNAs that target those molecules, as well as the transcription factor that controls MAdCAM-1 expression [15]. These results underscore the significance of exosomal regulation to cell homing by modifying the microenvironments of destination tissues [15]. We propose that in certain physiological settings (i.e., non-cancerous cells), this exosomal regulation acts suppressively to balance the excessive accumulation of homed cells. However, in malignantly transformed cells, aberrant exosomal regulation could act in a promotive-like manner, thereby contributing to the pathogenesis of cancer metastasis.
2.2.2. Exosomal Integrin as a Biomarker
Altered expression of integrin subsets on exosomes circulating in the blood has been reported in cancer and non-cancerous inflammatory disorders. In patients suffering from metastatic lung cancers, the levels of β4 integrin in exosomes in the plasma were significantly elevated, regardless of the origin of their cancers [12]. In contrast, in patients who have liver metastasis, the levels of αV integrin in exosomes in the plasma were significantly elevated. These results were in good agreement with the in vivo results showing that α6β4- and αVβ5-expressing cancer exosomes were preferentially distributed to the lung and the liver, respectively, wherein the remodeling of tissues to form premetastatic niches occurs.
References
- Sorgen, P.L.; Trease, A.J.; Spagnol, G.; Delmar, M.; Nielsen, M.S. Protein–Protein Interactions with Connexin 43: Regulation and Function. Int. J. Mol. Sci. 2018, 19, 1428.
- Maes, M.; Decrock, E.; Cogliati, B.; Oliveira, A.G.; Marques, P.E.; Dagli, M.L.; Menezes, G.B.; Mennecier, G.; Leybaert, L.; Vanhaecke, T.; et al. Connexin and pannexin (hemi)channels in the liver. Front. Physiol. 2014, 4, 405.
- Jiang, J.X.; Penuela, S. Connexin and pannexin channels in cancer. BMC Cell Biol. 2016, 17 (Suppl. 1), 12.
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687.
- Shimaoka, M.; Takagi, J.; Springer, T.A. Conformational regulation of integrin structure and function. Annu. Rev. Biophys. Biomol. Struct. 2002, 31, 485–516.
- Shattil, S.J.; Kim, C.; Ginsberg, M.H. The final steps of integrin activation: The end game. Nat. Rev. Mol. Cell Biol. 2010, 11, 288–300.
- Batra, N.; Burra, S.; Siller-Jackson, A.J.; Gu, S.; Xia, X.; Weber, G.F.; DeSimone, D.; Bonewald, L.F.; Lafer, E.M.; Sprague, E.; et al. Mechanical stress-activated integrin α5β1 induces opening of connexin 43 hemichannels. Proc. Natl. Acad. Sci. USA 2012, 109, 3359–3364.
- Zemljic-Harpf, A.E.; Godoy, J.C.; Platoshyn, O.; Asfaw, E.K.; Busija, A.R.; Domenighetti, A.A.; Ross, R.S. Vinculin directly binds zonula occludens-1 and is essential for stabilizing connexin-43-containing gap junctions in cardiac myocytes. J. Cell Sci. 2014, 127, 1104–1116. [Green Version]
- Mortimer, L.; Moreau, F.; Cornick, S.; Chadee, K. The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive Entamoeba histolytica via Activation of α5β1 Integrin at the Macrophage-Amebae Intercellular Junction. PLoS Pathog. 2015, 11, e1004887.
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232.
- Soares, A.R.; Martins-Marques, T.; Ribeiro-Rodrigues, T.; Ferreira, J.V.; Catarino, S.; Pinho, M.J.; Zuzarte, M.; Isabel Anjo, S.; Manadas, B.; P G Sluijter, J.; et al. Gap junctional protein Cx43 is involved in the communication between extracellular vesicles and mammalian cells. Sci. Rep. 2015, 5, 13243. [Green Version]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Green Version]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Green Version]
- Gonda, A.; Kabagwira, J.; Senthil, G.N.; Wall, N.R. Internalization of Exosomes through Receptor-Mediated Endocytosis. Mol. Cancer Res. 2018.
- Park, E.J.; Prajuabjinda, O.; Soe, Z.Y.; Darkwah, S.; Appiah, M.G.; Kawamoto, E.; Momose, F.; Shiku, H.; Shimaoka, M. Exosomal regulation of lymphocyte homing to the gut. Blood Adv. 2019, 3, 1–11.
- Singh, A.; Fedele, C.; Lu, H.; Nevalainen, M.T.; Keen, J.H.; Languino, L.R. Exosome-mediated Transfer of αvβ3 Integrin from Tumorigenic to Nontumorigenic Cells Promotes a Migratory Phenotype. Mol. Cancer Res. 2016, 14, 1136–1146.
- Martins-Marques, T.; Pinho, M.J.; Zuzarte, M.; Oliveira, C.; Pereira, P.; Sluijter, J.P.; Gomes, C.; Girao, H. Presence of Cx43 in extracellular vesicles reduces the cardiotoxicity of the anti-tumour therapeutic approach with doxorubicin. J. Extracell. Vesicles 2016, 5, 32538.
- Thul, P.J.; Lindskog, C. The human protein atlas: A spatial map of the human proteome. Protein Sci. 2018, 27, 233–244.
- Hata, A.; Lieberman, J. Dysregulation of microRNA biogenesis and gene silencing in cancer. Sci. Signal. 2015, 8, re3.
- Varela-Eirin, M.; Varela-Vazquez, A.; Rodríguez-Candela Mateos, M.; Vila-Sanjurjo, A.; Fonseca, E.; Mascareñas, J.L.; Eugenio Vázquez, M.; Mayan, M.D. Recruitment of RNA molecules by connexin RNA-binding motifs: Implication in RNA and DNA transport through microvesicles and exosomes. Biochim. Biophys. Acta Mol. Cell. Res. 2017, 1864, 728–736.
- Thuringer, D.; Jego, G.; Berthenet, K.; Hammann, A.; Solary, E.; Garrido, C. Gap junction-mediated transfer of miR-145-5p from microvascular endothelial cells to colon cancer cells inhibits angiogenesis. Oncotarget 2016, 7, 28160–28168. [Green Version]
- Lemcke, H.; Steinhoff, G.; David, R. Gap junctional shuttling of miRNA—A novel pathway of intercellular gene regulation and its prospects in clinical application. Cell. Signal. 2015, 27, 2506–2514.
- Melo, S.A.; Sugimoto, H.; O’Connell, J.T.; Kato, N.; Villanueva, A.; Vidal, A.; Qiu, L.; Vitkin, E.; Perelman, L.T.; Melo, C.A.; et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 2014, 26, 707–721.
- Tran, N. Cancer Exosomes as miRNA Factories. Trends Cancer 2016, 2, 329–331.
- Lehmann, B.D.; Paine, M.S.; Brooks, A.M.; McCubrey, J.A.; Renegar, R.H.; Wang, R.; Terrian, D.M. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008, 68, 7864–7871.
- Xiao, X.; Yu, S.; Li, S.; Wu, J.; Ma, R.; Cao, H.; Zhu, Y.; Feng, J. Exosomes: Decreased sensitivity of lung cancer A549 cells to cisplatin. PLoS ONE 2014, 9, e89534.
- Kanemoto, S.; Nitani, R.; Murakami, T.; Kaneko, M.; Asada, R.; Matsuhisa, K.; Saito, A.; Imaizumi, K. Multivesicular body formation enhancement and exosome release during endoplasmic reticulum stress. Biochem. Biophys. Res. Commun. 2016, 480, 166–172.
- Chen, W.; Guo, Y.; Yang, W.; Chen, L.; Ren, D.; Wu, C.; He, B.; Zheng, P.; Tong, W. Phosphorylation of connexin 43 induced by traumatic brain injury promotes exosome release. J. Neurophysiol. 2018, 119, 305–311.
- Habtezion, A.; Nguyen, L.P.; Hadeiba, H.; Butcher, E.C. Leukocyte Trafficking to the Small Intestine and Colon. Gastroenterology 2016, 150, 340–354. [Green Version]
- Burger, J.A.; Bürkle, A. The CXCR4 chemokine receptor in acute and chronic leukaemia: A marrow homing receptor and potential therapeutic target. Br. J. Haematol. 2007, 137, 288–296.
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548.
- Lukanidin, E.; Sleeman, J.P. Building the niche: The role of the S100 proteins in metastatic growth. Semin. Cancer Biol. 2012, 22, 216–225.
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698.
More
Information
Subjects:
Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
5 times
(View History)
Update Date:
11 Oct 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




