
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Navin Kumar | + 3271 word(s) | 3271 | 2021-09-23 10:29:58 | | | |
| 2 | Catherine Yang | Meta information modification | 3271 | 2021-09-24 03:22:15 | | |
Video Upload Options
Drought stress (DS) negatively affects plant morphological, physiological, and biochemical processes, which decrease photosynthesis, impair cell elongation and division, and reduce cell turgor pressure. Drought stress also inhibits nutrient uptake and affects gene expression, yield, and quality of crop plants. Metabolites play an essential role in plant growth and development.
1. Introduction
Drought stress (DS) negatively affects plant morphological, physiological, and biochemical processes, which decrease photosynthesis [1], impair cell elongation and division [2], and reduce cell turgor pressure [3]. Drought stress also inhibits nutrient uptake and affects gene expression, yield, and quality of crop plants [4][5]. Metabolites play an essential role in plant growth and development. Under stress conditions, metabolites are involved in cell signaling, energy storage, membrane formation and scaffolding, and whole-plant resource allocation [6]. Various abiotic stresses, including drought, disturb plant metabolism through metabolic enzyme inhibition, substrate shortage, excess demand for specific compounds, or a combination of these and many other factors. Thus, the metabolic network must be reconfigured to maintain essential metabolism, and acclimate by adopting a new steady-state in light of the prevailing stress conditions [7]. The induction of primary or secondary metabolites under drought stress can regulate the turgidity and stiffness of cells and tissues, redox homoeostasis, ion transport, and enzyme activity [8][9]. These metabolites play an important role in connecting plant genotypes and phenotypes [10][11].
Metabolomics is an effective tool for garnering comprehensive information on metabolite profiling and metabolic network analysis. It also imparts knowledge about identified and unidentified metabolites. Several reports have contributed to the recent understanding of metabolite regulation in many plant species in response to different environmental stresses, including drought, salt, heat, cold, and light stress [7][12][13]. Metabolite profiling approaches have been widely used to characterize the molecular responses to DS in plants and evaluate metabolite levels in a particular metabolite class or pathway [14][15]. It includes various analytical approaches for identifying different classes of metabolites through gas chromatography-mass spectrometry (GC-MS), liquid chromatography-mass spectrometry (LC-MS), nuclear magnetic resonance (NMR), high-performance liquid chromatography (HPLC), and capillary electrophoresis-mass spectrometry (CE-MS) in various plant species under DS [16][17][18] (Table 1).
Table 1. Key metabolites involved in various plant species under drought stress.
| Plant Species | Methods of Analysis | Tissue | Key Metabolites Involved in Drought Tolerance | References |
|---|---|---|---|---|
| Monocots | ||||
| Avena sativa | GC | Leaves | Lipids: Monoacylglycerols (MAGs), diacylglycerols (DAGs), and triacylglycerols (TAGs) and free fatty acids (FFAs) | [19] |
| FA: Palmitic acid, linolenic acid | ||||
| Brachypodium distachyon | GC/MS | Leaves | CH: Glucose, glycerol, mannobiose, maltose, sucrose, galactose | [20] |
| AA: Norvaline | ||||
| Hordeum vulgare | HPLC-DAD-MSn | Leaves | SM: Flavone glycosides, chlorogenic acids, caffeoyl-hexose, sinapoyl-hexoses, feruloyl-hexose, hydroxycinnamic acids | [21] |
| H. vulgare | GC-MS | Awns, kernels | CH: Galactinol, mannitol | [22] |
| OM: Isocitric acid, α-ketoglutaric acid | ||||
| H. vulgare | GC-MS | Grain | CH: Raffinose, mannitol, myoinositol, putrescine, | [23] |
| AA: Pyroglutamic acid | ||||
| H. vulgare | GC-MS-EI | Fifth leaf, palea | AA: Proline, glutamine, threonine, glycine, aspartate, serine, aromatic amino acids | [24] |
| Oryza sativa | GC/EI-TOF-MS | Leaves | AA: Glutamate, arginine, proline | [25] |
| PA: Spermidine, putrescine, spermine | ||||
| OM: GABA | ||||
| O. sativa | GC/MS | Leaf blades | AA: Serine, asparagine, threonine | [26] |
| Triticum aestivum | GC-TOF-MS | Shoots | CH: Sucrose, mannose, fructose | [13] |
| AA: Proline | ||||
| OM: Malic acid | ||||
| T. aestivum | GC/MS | Flag leaves | AA: Glutamine, methionine, lysine, asparagines, serine | [27] |
| T. aestivum | GC-MS | Roots, leaves | AA: Valine, tryptophan | [28] |
| OM: Malic acid, fumaric acid, citric acid, | ||||
| Seven Triticeae species | GC-MS | Roots, leaves | CH: Sucrose, trehalose, mannitol, maltose | [29] |
| AA: Proline, glutamate, alanine, glycine, asparagines, methionine, threonine, phenylalanine, homocysteine, serine, valine, tyrosine | ||||
| OM: Succinate, citrate, aspartate, gluconate, glutathione | ||||
| Zea mays | GC/MS | Leaf blades | AA: Glycine, myoinositol | [30] |
| Z. mays | 1H-NMR | Leaves | AA: Alanine | [31] |
| Lipids: Triacylglyceride | ||||
| OM: Malate, glutamate, formate | ||||
| Dicots | ||||
| African eggplant | GC-MS | Leaves | CH: Fructose, sucrose | [32] |
| AA: Proline, glutamate | ||||
| OM: Tricarboxylic cycle metabolite | ||||
| Arachis hypogaea | GC-MS | Nodules | CH: Trehalose | [33] |
| AA: Proline | ||||
| OM: GABA | ||||
| A. hypogaea | GC-MS | Leaves, roots | CH: Glucose D-ribose, D-mannitol, D-xylopyranose, xylonic acid, α-D-glucopyranose, 2-deoxyribose, L-manopyranose, myo-inositol, galactosoxime, D-fructose, D-turanose, malic acid, succinic acid, 2 butenedoic acids, 2-deoxyribose, myo-inositol | [34] |
| FA: Stearic acid, pentadecanoic acid, 8,11-octadecadienoic acid, palmitic acid, pentadecanoic acid | ||||
| Craterostigma plantagineum |
HPLC | Leaves | PAs: Putrescine, spermine, spermidine | [35] |
| Cicer arietinum | UPLC-HRMS | Leaves | AA: l-proline, l-arginine, l-histidine, l-isoleucine, tryptophan | [36] |
| OM: Allantoin | ||||
| Glycine max | 1H-NMR, 1H-1H TOCSY | Leaves, nodules | CH: Myoinositol, pinitol | [37] |
| AA: Glutamine | ||||
| OM: GABA, allantoin | ||||
| G. max | NMR | Leaves, roots | CH: Sucrose | [38] |
| AA: Alanine | ||||
| OM: Succinate, citrate, acetate | ||||
| G. max | GC-MS | Leaves | SM: 5-methoxytryptamine, 4-hydroxycinnamic acid, ferulic acid, salicylic acid | [39] |
| OM: Fluorine | ||||
| Lentils | GC/EI-TOF-MS | Cotyledons, radicles, shoots | PAs: Putrescine, cadaverine | [40] |
| CH: Erythronic acid | ||||
| OM: Isocitric acid, nicotinic acid | ||||
| Nicotiana tabacum | GC/MS, LC/MS | Leaves, roots | CH: Mannitol, trehalose, myoinositol, galactinol | [40] |
| OM: GABA | ||||
| Nigella sativa | GC | Seeds(10 black cumin genotypes) | FA: Stearic acid, palmitic acid, oleic acid, linoleic acid, linolenic acid, myristic acid, arachidic acid | [41] |
| Portulaca oleracea | GC | Leaves | FA: Palmitic acid, linolenic acid, linoleic acid, oleic acid, stearic acid, arachidic acid, behenic acid | [42] |
| Vigna unguiculata | GC-TOF | Seeds | CH: Galactinol | [43] |
| AA: Proline | ||||
| SM: Quercetin | ||||
| V. unguiculata | GC-TOF | Leaves | CH: Rhamnose, raffinose | [44] |
| Vitis vinifera | SPME-GC-MS | Leaves | SM: Quercetin-3-O-glucoside, kaempferol-3-O-glucoside | [45] |
| OM: Citric acid, 2-methyl-butanal phenylacetaldehyde |
AA, amino acid; CH: carbohydrate; EI, electrospray ionization; FA, fatty acid; GABA, γ-aminobutyric acid; GC-MS, gas chromatography-mass spectrometry; HPLC-DAD-MS, high-performance liquid chromatography coupled with diode-array detection and multiple-stage mass spectrometry; LC-MS, liquid chromatography-mass spectrometry; 1H-NMR, nuclear magnetic resonance; OM, other metabolites; PAs, Polyamines; SM, secondary metabolites; SPME-GC-MS, solid phase micro extraction-gas chromatography mass spectrometry; TOCSY, total correlation spectroscopy; TOF, time-of-flight; UPLC-HRMS, ultra-performance liquid chromatography-high-resolution mass spectrometry.
2. Metabolomics and Its Application in Drought Tolerance of Plants
Environmental stresses, such as drought, salinity, and high temperatures, can trigger hyper-accumulation of a vast array of metabolites in plants [46][47]. Plant secondary metabolites (SMs) are derivatives of primary metabolites (PMs) produced by plants to fight a variety of unfavorable physiological changes induced due to stressors [48][49]. Drought is one of the most significant environmental stresses on agricultural production worldwide [50]. In plants, DS adaptation is a complicated biological process that involves dynamic trends in metabolite composition and gene expression [51]. Plant tolerance to DS is typically determined by their ability to maintain an appropriate level of primary and secondary metabolic processes and defense responses [47]. Metabolomic analysis can investigate and recognize key differences between DS-tolerant and DS-sensitive plant species/genotypes and connect links between genotypic and phenotypic changes in plants during DS [52]. Two main methods (non-targeted and targeted) are used to understand metabolic reprogramming in plants under abiotic stress [53][54][55]. Non-targeted metabolomics provides an overview of the most abundant metabolites in plants under various environmental stresses. Targeted metabolomics detects, estimates, and analyzes known metabolites in plants under various environmental stresses [56][57]. Therefore, metabolomics studies can reveal the important role for metabolic reprogramming, including regulation and accumulation of PM and SM levels in plants under DS and biotechnological applications for DS management of agricultural crop plants [58][59].
Drought stress directly affects plant metabolism, resulting in profound changes in biosynthesis and transport of PMs and SMs [60][61]. Primary metabolites are important for the proper development of plant cells and directly implicated in plant growth processes, photosynthesis, and respiration [47][62]. They include sugars, polyols, amino acids (AAs), and lipids that allow plants to acclimatize and recover from DS [63] ( Figure 1 ; Table 1 ).
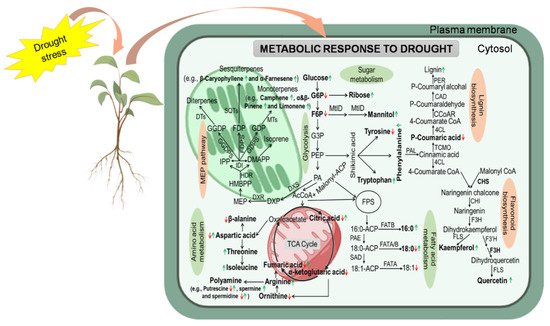
Lipids are cellular macromolecules with structural, energy storage, and signalling roles in plant biological systems [64]. Lipids act as signaling mediators [65][66] to mitigate the negative impacts of environmental stressors [67][68]. Plant lipids principally include glycerolipids (e.g., phospholipids, galactolipids, sphingolipids, triacylglycerols) and extracellular lipids (e.g., suberin, cutin, and waxes). Sanchez-Martin et al. [19] profiled different classes of lipids, including polar lipids (PLs), monoacylglycerols (MAGs), diacylglycerols (DAGs), and triacylglycerols (TAGs) and free fatty acids (FFAs) in drought-tolerant (cv. Patones) and drought-sensitive (cv. Flega) oat cultivars differing in their response to drought stress. Saturated FAs, particularly palmitic acid in the DAG and TAG fractions, increased in drought-sensitive cv. Flega. In contrast, drought-tolerant cv. Patones was characterized by the early induction of signaling-related fatty acids and lipids, such as linolenic acid and DAGs [19]. Moradi et al. [69] examined lipid profiling in drought-tolerant and drought-sensitive thyme plants under prolonged drought stress and found that lipid components decreased in sensitive plants but increased in tolerant plants. They proposed that combining lipid profiling with physiological parameters represented a promising tool for investigating the mechanisms of plant response to DS at the non-polar metabolome level [69]. The composition of lipid components changes under DS. The lipid contents in A . thaliana leaves decreased progressively in response to DS. However, the lipid content of highly dehydrated leaves quickly increased after rehydration [70].
Drought elevated the levels of major lipid components, indicating enhanced lipid biosynthesis and/or reduced lipid degradation [69]. Stress-induced changes in the lipid profile cause membrane lipid remodeling and activation of plant defense mechanisms against biotic and abiotic stresses, including drought [71][72].
3. Metabolomic and Molecular Responses to Drought
Metabolic regulation is the key mechanism implicated in the safeguarding of cell osmotic potential during abiotic stress. The metabolite profiling approach has been widely used to characterize molecular responses of plants under abiotic stress [73]. Apart from its importance for cell function, water is an important component of plants due to its undeviating involvement in metabolite transportation and essential nutrients to various plant parts. Inaccessibility of sufficient water or higher transpiration rates enhances DS and changes metabolite production [74]. Drought tolerance strategies of plants comprise numerous biological mechanisms at the cell, organ, and whole-plant levels when stimulated at different phases of plant growth. Drought stress affects plants at several levels, including the molecular level [75], increasing the accumulation of drought-related proteins and metabolites [76]. Several molecular pathway cascades, including perception of water deficiency, activation of signaling network, and transcriptional, metabolic, and regulatory element responses improve plant resistance to DS [77]. Molecular mechanisms of the drought response are strongly governed by regulatory elements, such as transcription factors (TFs) and protein kinases. Transcription factor families, such as MYB, NAC, bZIP, AP2/ERF, and AREB/ABF, regulate stomatal movement and the expression of drought-responsive genes upstream or downstream of a metabolic pathway [78][79].
The molecular response to drought stress is a multi-genic trait controlled by many genes. Several genes related to DS at the transcriptional level have been investigated in microarray and real-time polymerase chain reaction (RT-PCR) studies [73][75][76][77][78][79][80][81][82]. Functional validation revealed that these genes protect against dehydration stress through stress perception, signal transduction, and transcriptional regulatory networks responses to drought tolerance [83][84]. Therefore, understanding molecular responses to drought tolerance can provide insights for enhancing drought tolerance in sensitive plant species. Significant efforts have been made to explore the molecular mechanisms used by plants to cope with DS. Plants respond to DS by reprogramming their transcriptional, proteomic, and metabolic pathways to protect cells from stress-mediated damage [84][85][86]. Primary metabolites, such as glucose, sucrose, and trehalose, function as signal molecules to regulate gene expression involved in plant growth and the stress response [87]. Drought stress elevates ROS production in diverse cellular compartments, particularly chloroplasts and mitochondria, which is controlled by an adaptable and cooperative antioxidant system that balances intracellular ROS levels and sets the redox status of plant cells [88].
4. Genetic Engineering of Metabolic Genes to Improve Drought Tolerance in Plants
The genetic engineering approach is widely used to enhance plant tolerance to various environmental stresses, including drought, by engineering candidate genes for crop improvement [61][89][90][91]. Drought stress can seriously impact plant growth, photosynthesis, water relations, yield, pigment content, and membrane integrity [92]. Plants have evolved various interconnected signaling networks to regulate drought-responsive genes to produce various classes of proteins, including transcription factors, enzymes, molecular chaperones, and other functional proteins, for drought tolerance [93]. Developing drought-tolerant plants using the genetic engineering approach requires identifying key genetic determinants underlying DS tolerance in plants and introducing metabolic genes into crops for expression. Drought-responsive genes are involved in signaling cascades, transcriptional regulation (e.g., transcription factors and protein kinase/phosphatase), and functional proteins that protect cell membranes [94]. Other proteins, such as antioxidants, osmotin, late embryogenesis abundant proteins, and proteins associated with the uptake and transport of water and ions, such as aquaporins and sugar transporters, also respond to DS. Drought tolerance is a complex trait involving the activation of signaling mechanisms and differentially expressed molecular responses [95].
Numerous drought-responsive genes have been isolated from various sources, including plants; their characterization for enhancing drought tolerance by developing transgenic plants with increased level of metabolites shown in Table 2 . The schematic representation of the proposed model for the application of metabolic genes involved in drought tolerance in plants is shown in Figure 2 . It shows the specific responses of metabolic genes under DS in transgenic plants. It also depicts the accumulation of various key metabolites such as glycine betaine, proline, polyamines, trehalose, mannitol, lipids, flavonoids, and other important metabolites in transgenic plants under DS. The accumulation of these metabolites leads to various cellular responses, such as ROS detoxification, modulation of antioxidant activities, increased relative water contents (RWC), decreased electrolytic leakage (EL) and malondialdehyde (MDA) contents, and structural adaptation of membranes, resulting in morphological changes that improve growth and drought tolerance in plants ( Figure 2 ). Targeted metabolites can be enhanced by overexpression of single or multiple genes that produce either direct desired molecule/metabolites or enzymes implicated in the production of the targeted metabolites. The upregulation of these genes resulted in biosynthesis of the metabolite responsible for osmolyte synthesis [96]. The C1A cysteine protease (CysProt) family is one the most abundant proteins, also known as papain-like CysProt). The upregulation of C1A CysProt genes is important for protein breakdown during stress responses by reorganizing metabolism, remodeling cell protein compounds, degrading damaged or unnecessary proteins, and remobilizing nutrients [56][97][98]. Gomez-Sanchez et al. [99] reported that drought stimulated the entire C1A CysProt family and upregulates HvPap-1 and HvPap - 19 genes in H . vulgare leaves. Transgenic Arabidopsis overexpressing the T. aestivum cysteine protease ( TaCP ) gene showed enhanced drought tolerance and cysteine protease (CP) activity under water-stressed conditions compared to wild-type (WT) plants [100].
Table 2. Application of metabolic genes for generating transgenic crops with improved drought tolerance.
| Gene | Locus ID | Source | Transgenic Plants | Metabolite Accumulation | Stress Tolerance | References |
|---|---|---|---|---|---|---|
| Arginine decarboxylase (AtADC) | BT000682 | Arabidopsis thaliana | A. thaliana | Increased putrescine | Drought | [101] |
| Arginine decarboxylase (DsADC) | AJ251819 | Datura stramonium | Oryza sativa | Increased putrescine and spermidine | Drought | [102] |
| Arginine decarboxylase (PtADC) | HQ008237 | Poncirus trifoliata | A. thaliana | Enhanced putrescine | High osmoticum, dehydration, long-term drought, cold | [103] |
| Betaine aldehyde dehydrogenase (AnBADH) | KJ841914 | Ammopiptanthus nanus | A. thaliana | Increased glycine betaine | Drought, salt | [104] |
| Chalcone synthase (NtCHS) | LOC107801774 | Nicotiana tabacum | N. tabacum | Increased flavanoids (rutin, quercetin, naringenin) | Drought | [105] |
| Choline monooxygenase (BvCMO) | AB221007 | Beta vulgaris | N. tabacum | Increased glycine betaine | Drought, salt | [106] |
| Choline oxidase (AgcodA) | AY589052 | Arthrobacter globiformis | Solanum tuberosum | Increased glycine betaine | Water stress | [107] |
| Choline oxidase (codA) | AY304485 | A. globiformis | S. tuberosum | Increased glycine betaine | Drought, salt, oxidative | [108] |
| Cysteine protease (TaCP) | AY841792 | Triticum aestivum | A. thaliana | Increased Cysteine protease activity | Drought | [100] |
| Dehydrin (OesDHN) | KR349290 | Olea europaea | A. thaliana | Increased proline | Drought | [109] |
| Dehydrin (TdDhn-5) | AY619566 | T. durum | A. thaliana | Increased proline | Drought, salt | [110] |
| Dehydrin (ShDHN) | AK319970 | Solanum habrochaites | Solanum lycopersicum | Increased proline | Drought, salt, osmotic stress | [111] |
| Dehydrin (PmLEAs) | XM_016796383 | Prunus mume | N. tabacum | Increased proline | Drought, cold | [112] |
| Flavanone 3-hydroxylase(RsF3H) | JQ043380 | Reaumuria soongorica | R. soongorica | Increased flavonoids and anthocyanin | Drought, UV-B radiation | [113] |
| Mannitol dehydrogenase (CaMTD) | LOC101510334 | Cicer arietinum | C. arietinum | Increased flavonoids | Drought | [114] |
| Mannitol-1-phosphate dehydrogenase (EcmtlD) | EFF7369098 | Escherichia coli | T. aestivum | Increased mannitol | Drought | [115] |
| Ornithine δ-aminotransferase (Atδ-OAT) | NM_123987 | A. thaliana | O. sativa | Increased proline | Drought | [116] |
| Ornithine δ-aminotransferase (OsOAT) | LOC Os03g44150 | O. sativa | O. sativa | Increased proline | Drought | [117] |
| Spermidine synthase (CfSPDS) | BD142348 | Cucurbita ficifolia | A. thaliana | Increased spermidine synthase activity and spermidine content | Drought, chilling, freezing, salinity, hyperosmosis | [118] |
| Trehalose-6-phosphate synthase (EcTPS; otsA) and Trehalose-6-phosphate phosphatase (EcTPP; otsB) |
NC_000913 | E. coli | O. sativa | Increased trehalose | Drought, salt, cold | [119] |
| Trehalose-6-phosphate synthase1 (OsTPS1) | HM050424 | O. sativa | O. sativa | Increased trehalose and proline | Drought, salt, and cold | [120] |
| Trehalose-6-phosphatesynthase1 (ScTPS1) and trehalose-6-phosphate synthase2 (ScTPS2) | NC_001134 | Saccharomyces cerevisiae | N. tabacum | Enhanced trehalose | Drought | [121] |
| Wax synthase/acyl-CoA:diacylglycerol acyltransferase (AtWSD1) | AT5G37300 | A. thaliana | A. thaliana and Camelina sativa | Increased deposition of epicuticular wax crystals and leaf and stem wax loading | Drought | [122] |
| WRI4-like gene (CeWRI4) | MW039149 | Cyperus esculentus | A. thaliana | Increased cuticular wax biosynthesis and deposition | Drought | [123] |
| Δ1-pyrroline-5-carboxylate synthetase (VaP5CS) | VIRPYRR | Vigna aconitifolia | N. tabacum | Increased proline | Drought | [124] |
| Δ1-pyrroline-5-carboxylate synthetase genes (OsP5CS) | D49714 | O. sativa | P. hybrida | Increased proline | Drought | [125] |
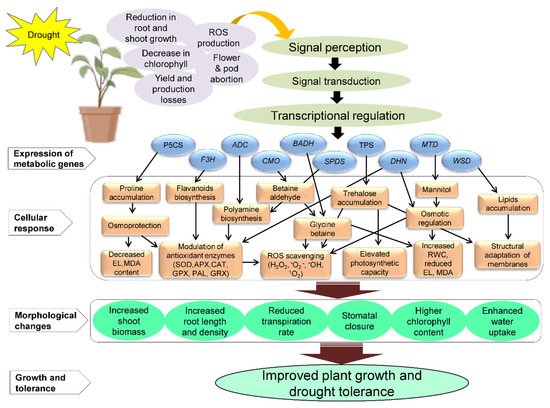
Flavonoids are important SMs that play significant roles in maintaining the cellular redox balance of plant cells. Chalcone synthase (CHS) is the key enzyme in the flavonoid biosynthesis pathway and is modulated under DS. Transgenic N. tabacum plants overexpressing the NtCHS gene showed enhanced drought tolerance and oxidative stress responses under DS, relative to control plants [105]. Transgenic Brassica napus overexpressing the BR biosynthesis gene AtDWARF4 from Arabidopsis had improved drought tolerance [126]. Overexpressing Gossypium hirsutum Gh4CL7 gene enhanced drought stress tolerance in Arabidopsis [127]. Drought tolerance is conferred in N. tabacum plants through the overexpression of sweet potato cinnamate 4-hydroxylase ( IbC4H ), promoting phenolic compound accumulation and increasing the expression of stress-responsive genes [128]. The role of metabolic gene expression for providing drought tolerance in plants is summarized in Table 2 . Overall, we conclude that induction of metabolite biosynthesis provides a defensive level of drought protection and enhances growth tolerance in drought-stressed plants.
References
- McKay, J.K.; Richards, J.H.; Mitchell-Olds, T. Genetics of drought adaptation in Arabidopsis thaliana: I. Pleiotropy contributes to genetic correlations among ecological traits. Mol. Ecol. 2003, 12, 1137–1151.
- Bal, W.; Kozlowski, H.; Robbins, R.; Pettit, L.D. Improving drought tolerance by exogenous application of glycine betaine and salicylic acid in sunflower. J. Agron. Crop. Sci. 2010, 194, 193–199.
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinauer Associates Inc. Publishers: Sunderland, MA, USA, 2006.
- Cattivelli, L.; Rizza, F.; Badeck, F.W.; Mazzucotelli, E.; Mastrangelo, A.M.; Francia, E.; Marè, C.; Tondelli, A.; Stanca, A.M. Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Field Crop. Res. 2008, 105, 1–14.
- Prasad, P.V.V.; Staggenborg, S.; Ristic, Z. Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants. Adv. Agric. Syst. Model. Ser. 2008, 1, 301–355.
- Wen, W.; Li, K.; Alseekh, S.; Omranian, N.; Zhao, L.; Zhou, Y.; Xiao, Y.; Jin, M.; Yang, N.; Liu, H.; et al. Genetic determinants of the network of primary metabolism and their relationships to plant performance in a maize recombinant inbred line population. Plant Cell 2015, 27, 1839–1856.
- Obata, T.; Fernie, A.R. The use of metabolomics to dissect plant responses to abiotic stresses. Cell. Mol. Life Sci. 2012, 69, 3225–3243.
- Seki, M.; Umezawa, T.; Urano, K.; Shinozaki, K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007, 10, 296–302.
- Rodziewicz, P.; Swarcewicz, B.; Chmielewska, K.; Wojakowska, A.; Stobiechi, M. Influence of abiotic stresses on plant hormone and metabolome changes. Acta Physiol. Plant 2014, 36, 1–19.
- Fiehn, O.; Kopka, J.; Dormann, P.; Altmann, T.; Trethewey, R.N.; Willmitzer, L. Metabolite profiling for plant functional genomics. Nat. Biotechnol. 2000, 18, 1157–1161.
- Suhre, K.; Gieger, C. Genetic variation in metabolic phenotypes: Study designs and applications. Nat. Rev. Genet. 2012, 13, 759–769.
- Wu, X.; Cai, K.; Zhang, G.; Zeng, F. Metabolite profiling of barley grains subjected to water stress: To explain the genotypic difference in drought induced impacts on malting quality. Front. Plant Sci. 2017, 8, 1547.
- Guo, R.; Shi, L.; Jiao, Y.; Li, M.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Metabolic responses to drought stress in the tissues of drought-tolerant and drought-sensitive wheat genotype seedlings. AoB Plants 2018, 10, ply016.
- Weckwerth, W.; Loureiro, M.E.; Wenzel, K.; Fiehn, O. Differential metabolic networks unravel the effects of silent plant phenotypes. Proc. Natl. Acad. Sci. USA 2004, 101, 7809–7814.
- Kusano, M.; Fukushima, A.; Arita, M.; Jonsson, P.; Moritz, T.; Kobayashi, M.; Hayashi, N.; Tohge, T.; Saito, K. Unbiased characterization of genotype-dependent metabolic regulations by metabolomic approach in Arabidopsis thaliana. BMC Syst. Biol. 2007, 1, 53.
- Ruan, C.J.; Ja, T.D.S. Metabolomics: Creating new potentials for unraveling the mechanisms in response to salt and drought stress and for the biotechnological improvement of xero-halophytes. Crit. Rev. Biotechnol. 2011, 31, 153–169.
- Meng, J.; Zhang, X.; Wu, H.; Bu, J.; Shi, C.; Deng, C.; Mao, Y. Morphine-induced conditioned place preference in mice: Metabolomic profiling of brain tissue to find “molecular switch” of drug abuse by gas chromatography/mass spectrometry. Anal. Chim. Acta 2012, 710, 125–130.
- Barding, G.A.; Béni, S.; Fukao, T.; Bailey-Serres, J.; Larive, C.K. Comparison of GC-MS and NMR for metabolite profiling of Rice subjected to submergence stress. J. Proteome Res. 2013, 12, 898–909.
- Sanchez-Martin, J.; Canales, F.J.; Tweed, J.K.S.; Lee, M.R.F.; Rubiales, D.; Gómez-Cadenas, A.; Arbona, V.; Mur, L.A.J.; Prats, E. Fatty acid profile changes during gradual soil water depletion in oats suggests a role for jasmonates in coping with drought. Front. Plant Sci. 2018, 9, 1077.
- Shi, H.; Ye, T.; Song, B.; Qi, X.; Chan, Z. Comparative physiological and metabolomic responses of four Brachypodium distachyon varieties contrasting in drought stress resistance. Acta Physiol. Plant 2015, 37, 122.
- Piasecka, A.; Sawikowska, A.; Kuczyńska, A.; Ogrodowicz, P.; Mikołajczak, K.; Krystkowiak, K.; Gudys, K.; Guzy-Wróbelska, J.; Krajewski, P.; Kachlicki, P. Drought-related secondary metabolites of barley (Hordeum vulgare L.) leaves and their metabolomic quantitative trait loci. Plant J. 2017, 89, 898–913.
- Wenzel, A.; Frank, T.; Reichenberger, G.; Herz, M.; Engel, K.H. Impact of induced drought stress on the metabolite profiles of barely grain. Metabolomics 2015, 11, 454–467.
- Lanzinger, A.; Frank, T.; Reichenberger, G.; Herz, M.; Engel, K.H. Metabolite profiling of barley grain subjected to induced drought stress: Responses of free amino acids in differently adapted cultivars. J. Agric. Food Chem. 2015, 63, 4252–4261.
- Hein, J.A.; Sherrard, M.E.; Manfredi, K.P.; Abebe, T. The fifth leaf and spike organs of barley (Hordeum vulgare L.) display different physiological and metabolic responses to drought stress. BMC Plant Biol. 2016, 16, 248.
- Do, P.T.; Degenkolbe, T.; Erban, A.; Heyer, A.G.; Kopka, J.; Köhl, K.I.; Hincha, D.K.; Zuther, E. Dissecting rice polyamine metabolism under controlled long-term drought stress. PLoS ONE 2013, 8, e60325.
- Degenkolbe, T.; Do, P.T.; Kopka, J.; Zuther, E.; Hincha, D.K.; Kohl, K.I. Identification of drought tolerance markers in a diverse population of rice cultivars by expression and metabolite profiling. PLoS ONE 2013, 8, e63637.
- Yadav, A.K.; Carroll, A.J.; Estavillo, G.M.; Rebetzke, G.J.; Pogson, B.J. Wheat drought tolerance in the field is predicted by amino acid responses to glasshouse-imposed drought. J. Exp. Bot. 2019, 70, 4931–4948.
- Kang, Z.; Babar, M.A.; Khan, N.; Guo, J.; Khan, J.; Islam, S.; Shrestha, S.; Shahi, D. Comparative metabolomic profiling in the roots and leaves in contrasting genotypes reveals complex mechanisms involved in post-anthesis drought tolerance in wheat. PLoS ONE 2019, 14, e0213502.
- Ullah, N.; Yüce, M.; NeslihanÖztürkGökçe, Z.; Budak, H. Comparative metabolite profiling of drought stress in roots and leaves of seven Triticeae species. BMC Genom. 2017, 18, 1–12.
- Obata, T.; Witt, S.; Lisec, J.; Palacios-Rojas, N.; Florez-Sarasa, I.; Yousfi, S.; Araus, J.L.; Cairns, J.E.; Fernie, A.R. Metabolite profiles of maize leaves in drought, heat, and combined stress field trials reveal the relationship between metabolism and grain yield. Plant Physiol. 2015, 169, 2665–2683.
- Sun, C.; Gao, X.; Fu, J.; Zhou, J.; Wu, X. Metabolic response of maize (Zea mays L.) plants to combined drought and salt stress. Plant Soil. 2015, 388, 99–117.
- Mibei, E.; Owino, W.; Ambuko, J.; Giovannoni, J.; Onyango, A. Metabolomic analyses to evaluate the effect of drought stress on selected African eggplant accessions. J. Sci. Food Agric. 2018, 98, 205–216.
- Furlan, A.L.; Bianucci, E.; Castro, S.; Dietz, K.J. Metabolic features involved in drought stress tolerance mechanisms in peanut nodules and their contribution to biological nitrogen fixation. Plant Sci. 2017, 263, 12–22.
- Gundaraniya, S.A.; Ambalam, P.S.; Tomar, R.S. Metabolomic profiling of drought-tolerant and susceptible peanut (Arachis hypogaea L.) genotypes in response to drought Stress. ACS Omega 2020, 5, 31209–31219.
- Alcazar, R.; Bitrián, M.; Bartels, D.; Koncz, C.; Altabella, T.; Tiburcio, A.F. Polyamine metabolic canalization in response to drought stress in Arabidopsis and the resurrection plant Craterostigma plantagineum. Plant Signal. Behav. 2011, 6, 243–250.
- Khan, N.; Bano, A.; Rahman, M.A.; Rathinasabapathi, B. UPLC-HRMS-based untargeted metabolic profiling reveals changes in chickpea (Cicer arietinum) metabolome following long-term drought stress. Plant Cell Environ. 2019, 42, 115–132.
- Silvente, S.; Sobolev, A.P.; Lara, M. Metabolite adjustments in drought tolerant and sensitive soybean genotypes in response to water stress. PLoS ONE 2012, 7, e38554.
- Coutinho, I.; Henning, L.; Döpp, S.; Nepomuceno, A.; Moraes, L.; Marcolino-Gomes, J.; Richter, C.; Schwalbe, H.; Colnago, L. Flooded soybean metabolomic analysis reveals important primary and secondary metabolites involved in the hypoxia stress response and tolerance. Environ. Exp. Bot. 2018, 153, 176–187.
- Wang, X.; Guo, R.; Li, M.; Liu, Y.; Zhao, M.; Fu, H.; Liu, X.; Wang, S.; Shi, L. Metabolomics reveals the drought-tolerance mechanism in wild soybean (Glycine soja). Acta Physiol. Plant 2019, 41, 1–11.
- Muscolo, A.; Junker, A.; Klukas, C.; Weigelt-Fischer, K.; Riewe, D.; Altmann, T. Phenotypic and metabolic responses to drought and salinity of four contrasting lentil accessions. J. Exp. Bot. 2015, 66, 5467–5480.
- Bayati, P.; Karimmojeni, H.; Razmjoo, J. Changes in essential oil yield and fatty acid contents in black cumin (Nigella sativa L.) genotypes in response to drought stress. Ind. Crops Prod. 2020, 155, 112764.
- Saheri, F.; Barzin, G.; Pishkar, L.; Boojar, M.M.A.; Babaeekhou, L. Foliar spray of salicylic acid induces physiological and biochemical changes in purslane (Portulaca oleracea L.) under drought stress. Biologia 2020, 75, 2189–2200.
- Goufo, P.; Moutinho-Pereira, J.M.; Jorge, T.F.; Correia, C.M.; Oliveira, M.R.; Rosa, E.A.S.; António, C.; Trindade, H. Cowpea (Vigna unguiculata L. Walp.) Metabolomics: Osmoprotection as a physiological strategy for drought stress resistance and improved yield. Front. Plant Sci. 2017, 8, 586.
- Gomes, A.M.; Rodrigues, A.P.; António, C.; Rodrigues, A.M.; Leitão, A.E.; Batista-Santos, P.; Nhantumbo, N.; Massinga, R.; Ribeiro-Barros, A.I.; Ramalho, J.C. Drought response of cowpea (Vigna unguiculata (L.) Walp.) landraces at leaf physiological and metabolite profile levels. Environ. Exp. Bot. 2020, 175, 104060.
- Griesser, M.; Weingart, G.; Schoedl-Hummel, K.; Neumann, N.; Becker, M.; Varmuza, K.; Liebner, F.; Schuhmacher, R.; Forneck, A. Severe drought stress is affecting selected primary metabolites, polyphenols, and volatile metabolites in grapevine leaves (Vitis vinifera cv. Pinot noir). Plant Physiol. Biochem. 2015, 88, 17–26.
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608.
- Patel, M.K.; Kumar, M.; Li, W.; Luo, Y.; Burritt, D.J.; Alkan, N.; Tran, L.-S.P. Enhancing salt tolerance of plants: From metabolic reprogramming to exogenous chemical treatments and molecular approaches. Cells 2020, 9, 2492.
- Kinghorn, A.D. The discovery of drugs from higher plants. Biotechnic 1994, 26, 81–108.
- Ramakrishna, A.; Ravishankar, G.A. Influences of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731.
- Kumar, M.; Yusuf, M.A.; Nigam, M.; Kumar, M. An update on genetic modification of chickpea for increased yield and stress tolerance. Mol. Biotechnol. 2018, 60, 651–663.
- Urano, K.; Maruyama, K.; Ogata, Y.; Morishita, Y.; Takeda, M.; Sakurai, N.; Suzuki, H.; Saito, K.; Shibata, D.; Kobayashi, M.; et al. Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J. 2009, 57, 1065–1078.
- Llanes, A.; Andrade, A.; Alemano, S.; Luna, V. Metabolomic approach to understand plant adaptations to water and salt stress. In Plant Metabolites and Regulation under Environmental Stress; Ahmad, P., Ahanger, M.A., Singh, V.P., Tripathi, D.K., Alam, P., Alyemeni, M.N., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 133–144.
- Mishra, A.; Patel, M.K.; Jha, B. Non-targeted metabolomics and scavenging activity of reactive oxygen species reveal the potential of Salicornia brachiata as a functional food. J. Funct. Foods 2015, 13, 21–31.
- Patel, M.K.; Mishra, A.; Jha, B. Untargeted metabolomics of halophytes. In Marine Omics: Principles and Applications; Kim, S., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 309–325.
- Pandey, S.; Patel, M.K.; Mishra, A.; Jha, B. Physio-biochemical composition and untargeted metabolomics of cumin (Cuminum cyminum L.) make it promising functional food and help in mitigating salinity stress. PLoS ONE 2015, 10, e0144469.
- Roberts, L.D.; Souza, A.L.; Gerszten, R.E.; Clish, C.B. Targeted metabolomics. Curr. Protoc. Mol. Biol. 2012, 98, 30.
- Van Meulebroek, L.; Hanssens, J.; Steppe, K.; Vanhaecke, L. Metabolic fingerprinting to assess the impact of salinity on carotenoid content in developing tomato fruits. Int. J. Mol. Sci. 2016, 17, 821.
- Zagorchev, L.; Seal, C.E.; Kranner, I.; Odjakova, M. A central role for thiols in plant tolerance to abiotic stress. Int. J. Mol. Sci. 2013, 14, 7405–7432.
- Cusido, R.M.; Onrubia, M.; Sabater-Jara, A.B.; Moyano, E.; Bonfill, M.; Goossens, A.; Palazon, J. A rational approach to improving the biotechnological production of taxanes in plant cell cultures of Taxus spp. Biotechnol. Adv. 2014, 32, 1157–1167.
- Di Ferdinando, M.; Brunetti, C.; Agati, G.; Tattini, M. Multiple functions of polyphenols in plants inhabiting unfavourable Mediterranean areas. Environ. Exp. Bot. 2014, 103, 107–116.
- Ma, X.J.; Yu, T.F.; Li, X.H.; Cao, X.Y.; Ma, J.; Chen, J.; Zhou, Y.B.; Chen, M.; Ma, Y.Z.; Zhang, J.H.; et al. Overexpression of GmNFYA5 confers drought tolerance to transgenic Arabidopsis and soybean plants. BMC Plant Biol. 2020, 20, 123.
- Kumar, R.; Bohra, A.; Pandey, A.K.; Pandey, M.K.; Kumar, A. Metabolomics for plant improvement: Status and prospects. Front. Plant Sci. 2017, 8, 1302.
- Fraire-Velázquez, S.L.; Balderas-Hernández, V.E. Abiotic stress in plants and metabolic responses. In Abiotic Stress—Plant Responses and Applications in Agriculture; Vahdati, K., Leslie, C., Eds.; Intech: Rijeka, Croatia, 2013; pp. 25–46.
- Svenningsson, H.; Liljenberg, C. Membrane lipid changes in root cells of rape (brassica napus) as a function of water-deficit stress. Physiol. Plant 1986, 68, 53–58.
- Quartacci, M.F.; Pinzino, C.; Sgherri, C.L.; Navari-Izzo, F. Lipid composition and protein dynamics in thylakoids of two wheat cultivars differently sensitive to drought. Plant Physiol. 1995, 108, 191–197.
- Hubac, C.; Guerrier, D.; Ferran, J.; Tremolieres, A. Change of leaf lipid composition during water stress in two genotypes of Lupines albus resistant or susceptible to drought. Plant Physiol. Biochem. 1989, 27, 737–744.
- Bahl, J.; Francke, B.; Monéger, R. Lipid composition of envelopes, prolamellar bodies and other plastid membranes in etiolated, green and greening wheat leaves. Planta 1976, 129, 193–201.
- Eastman, P.; Rashid, A.; Camm, E. Changes of the photosystem 2 activity and thylakoid proteins in spruce seedlings during water stress. Photosynthetica 1998, 34, 201–210.
- Moradi, P.; Mahdavi, A.; Khoshkam, M.; Iriti, M. Lipidomics unravels the role of leaf lipids in thyme plant response to drought stress. Int. J. Mol. Sci. 2017, 18, 2067.
- Gigon, A.; Matos, A.; Laffray, D.; Zully-Fodil, Y.; Phan-Thi, A.T. Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (Ecotype Columbia). Ann. Bot. 2004, 94, 345–351.
- Okazaki, Y.; Saito, K. Roles of lipids as signaling molecules and mitigators during stress response in plants. Plant J. 2014, 79, 584–596.
- Moellering, E.R.; Muthan, B.; Benning, C. Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 2010, 330, 226–228.
- Fabregas, N.; Fernie, A.R. The metabolic response to drought. J. Exp. Bot. 2019, 70, 1077–1085.
- Jogawat, A.; Yadav, B.; Lakra, N.; Singh, A.K.; Narayan, O.P. Crosstalk between phytohormones and secondary metabolites in the drought stress tolerance of crop plants: A review. Physiol. Plant 2021, 172, 1106–1132.
- Anjum, S.A.; Xie, X.; Wang, L. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032.
- Ramachandra-Reddy, A.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202.
- Liu, J.; Chen, N.; Chen, F.; Cai, B.; Dal Santo, S.; Tornielli, G.B.; Pezzotti, M.; Cheng, Z.M. Genome-wide analysis and expression profile of the bZIP transcription factor gene family in grapevine (Vitis vinifera). BMC Genom. 2014, 15, 281.
- Kim, T.H.; Bohmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591.
- Joshi, R.; Wani, S.H.; Singh, B.; Bohra, A.; Dar, Z.A.; Lone, A.A.; Pareek, A.; Singla-Pareek, S.L. Transcription factors and plants response to drought stress: Current understanding and future directions. Front. Plant Sci. 2016, 7, 1029.
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227.
- Talame, V.; Ozturk, N.Z.; Bohnert, H.J.; Tuberosa, R. Barley transcript profiles under dehydration shock and drought stress treatments: A comparative analysis. J. Exp. Bot. 2007, 58, 229–240.
- Guo, P.; Baum, M.; Grando, S.; Ceccarelli, S.; Bai, G.; Li, R.; Korff, M.V.; Varshney, R.K.; Graner, A.; Valkoun, J. Differentially expressed genes between drought-tolerant and drought-sensitive barley genotypes in response to drought stress during the reproductive stage. J. Exp. Bot. 2009, 60, 3531–3544.
- Wang, F.Z.; Wang, Q.B.; Kwon, S.Y.; Kwak, S.S.; Su, W.A. Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. J. Plant Physiol. 2005, 162, 465–472.
- Umezawa, T.; Fujita, M.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Engineering drought tolerance in plants: Discovering and tailoring genes to unlock the future. Curr. Opin. Biotechnol. 2006, 17, 113–122.
- Skirycz, A.; Vandenbroucke, K.; Clauw, P.; Maleux, K.; De Meyer, B.; Dhondt, S.; Pucci, A.; Gonzalez, N.; Hoeberichts, F.; Tognetti, V.B.; et al. Survival and growth of Arabidopsis plants given limited water are not equal. Nat. Biotechnol. 2011, 29, 212–214.
- Claeys, H.; Inzé, D. The agony of choice: How plants balance growth and survival under water-limiting conditions. Plant Physiol. 2013, 162, 1768–1779.
- Maruyama, K.; Urano, K.; Yoshiwara, K.; Morishita, Y.; Sakurai, N.; Suzuki, H.; Kojima, M.; Sakakibara, H.; Shibata, D.; Saito, K.; et al. Integrated analysis of the effects of cold and dehydration on rice metabolites, phytohormones, and gene transcripts. Plant Physiol. 2014, 164, 1759–1771.
- Kaur, G.; Asthir, B. Molecular responses to drought stress in plants. Biol. Plant 2017, 61, 201–209.
- Yang, S.; Vanderbeld, B.; Wan, J.; Huang, Y. Narrowing down the targets: Towards successful genetic engineering of drought-tolerant crops. Mol. Plant 2010, 3, 469–490.
- He, C.; Zhang, W.; Gao, Q.; Yang, A.; Hu, X.; Zhang, J. Enhancement of drought resistance and biomass by increasing the amount of glycine betaine in wheat seedlings. Euphytica 2011, 177, 151–167.
- Kumar, M.; Yusuf, M.A.; Yadav, P.; Narayan, S.; Kumar, M. Overexpression of chickpea defensin gene confers tolerance to water-deficit stress in Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 290.
- Praba, M.L.; Cairns, J.E.; Babu, R.C.; Lafitte, H.R. Identification of physiological traits underlying cultivar differences in drought tolerance in rice and wheat. J. Agron. Crop. Sci. 2009, 195, 30–46.
- Hu, H.; Xiong, L. Genetic engineering and breeding of drought-resistant crops. Annu. Rev. Plant Biol. 2014, 65, 715–741.
- Nakashima, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 2014, 5, 170.
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.; Ghafoor, A.; Du, X. Insights into drought stress signaling in plants and the molecular genetic basis of cotton drought tolerance. Cells 2019, 9, 105.
- Sinha, R.; Bala, M.; Kumar, M.; Sharma, T.R.; Singh, A.K. Methods for screening legume crops for abiotic stress tolerance through physiological and biochemical approaches. In Legume Genomics: Methods and Protocols, Methods in Molecular Biology; Jain, M., Garg, R., Eds.; Springer: New York, NY, USA, 2020; pp. 277–303.
- Diaz-Mendoza, M.; Velasco-Arroyo, B.; González-Melendi, P.; Martínez, M.; Díaz, I. C1A cysteine protease–cystatin interactions in leaf senescence. J. Exp. Bot. 2014, 65, 3825–3833.
- Wang, W.; Zhou, X.M.; Xiong, H.X.; Mao, W.Y.; Zhao, P.; Sun, M.X. Papain-like and legumain-like proteases in rice: Genome-wide identification, comprehensive gene feature characterization and expression analysis. BMC Plant Biol. 2018, 18, 87.
- Gomez-Sanchez, A.; González-Melendi, P.; Santamaria, M.E.; Arbona, V.; Lopez-Gonzalvez, A.; Garcia, A.; Hensel, G.; Kumlehn, J.; Martínez, M.; Díaz, I. Repression of drought-induced cysteine-protease genes alters barley leaf structure and responses to abiotic and biotic stresses. J. Exp. Bot. 2019, 70, 2143–2155.
- Zhang, Q.W.; Wang, C.X.; Li, X.Y.; Guo, Z.A.; Jing, R.L.; Zhao, J.; Chang, X.P. Isolation and characterization of a gene encoding a polyethylene glycol-induced cysteine protease in common wheat. J. Biosci. 2010, 35, 379–388.
- Alcazar, R.; Planas, J.; Saxena, T.; Zarza, X.; Bortolotti, C.; Cuevas, J.; Bitrián, M.; Tiburcio, A.F.; Altabella, T. Putrescine accumulation confers drought tolerance in transgenic Arabidopsis plants over-expressing the homologous Arginine decarboxylase 2 gene. Plant Physiol. Biochem. 2010, 48, 547–552.
- Capell, T.; Bassie, L.; Christou, P. Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc. Natl. Acad. Sci. USA 2004, 101, 9909–9914.
- Wang, J.; Sun, P.P.; Chen, C.L.; Wang, Y.; Fu, X.Z.; Liu, J.H. An arginine decarboxylase gene PtADC from Poncirus trifoliata confers abiotic stress tolerance and promotes primary root growth in Arabidopsis. J. Exp. Bot. 2011, 62, 2899–2914.
- Yu, H.Q.; Zhou, X.Y.; Wang, Y.G.; Zhou, S.F.; Fu, F.L.; Li, W.C. A betaine aldehyde dehydrogenase gene from Ammopiptanthus nanus enhances tolerance of Arabidopsis to high salt and drought stresses. Plant Growth Regul. 2017, 83, 265–276.
- Zhao, M.; Jin, L.; Hu, B.; Yao, H.; Gao, Y.; Wang, R.; Li, F.; Guo, J.; Li, K.; Zhao, M.; et al. Overexpression of chalcone synthase gene improves flavonoid accumulation and drought tolerance in tobacco. Durh. NC Res. Sq. 2019, 1–11.
- Zhang, J.; Tan, W.; Yang, X.H.; Zhang, H.X. Plastid-expressed choline monooxygenase gene improves salt and drought tolerance through accumulation of glycine betaine in tobacco. Plant Cell Rep. 2008, 27, 1113–1124.
- Cheng, Y.J.; Deng, X.P.; Kwak, S.S.; Chen, W.; Eneji, A.E. Enhanced tolerance of transgenic potato plants expressing choline oxidase in chloroplasts against water stress. Bot Stud. 2013, 54, 1–9.
- Ahmad, R.; Kim, M.D.; Back, K.H.; Kim, H.S.; Lee, H.S.; Kwon, S.Y.; Murata, N.; Chung, W.I.; Kwak, S.S. Stress-induced expression of choline oxidase in potato plant chloroplasts confers enhanced tolerance to oxidative, salt, and drought stresses. Plant Cell Rep. 2008, 27, 687–698.
- Chiappetta, A.; Muto, A.; Bruno, L.; Woloszynska, M.; Van Lijsebettens, M.; Bitonti, M.B. A dehydrin gene isolated from feral olive enhances drought tolerance in Arabidopsis transgenic plants. Front. Plant Sci. 2015, 6, 392.
- Brini, F.; Hanin, M.; Lumbreras, V.; Amara, I.; Khoudi, H.; Hassairi, A.; Pagès, M.; Masmoudi, K. Overexpression of wheat dehydrin DHN-5 enhances tolerance to salt and osmotic stress in Arabidopsis thaliana. Plant Cell Rep. 2007, 26, 2017–2026.
- Liu, H.; Yu, C.; Li, H.; Ouyang, B.; Wang, T.; Zhang, J.; Wang, X.; Ye, Z. Overexpression of ShDHN, a dehydrin gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses in tomato. Plant Sci. 2015, 231, 198–211.
- Bao, F.; Du, D.; An, Y.; Yang, W.; Wang, J.; Cheng, T.; Zhang, Q. Overexpression of Prunus mume dehydrin genes in tobacco enhances tolerance to cold and drought. Front. Plant Sci. 2017, 8, 151.
- Liu, M.; Li, X.; Liu, Y.; Cao, B. Regulation of flavanone 3-hydroxylase gene involved in the flavonoid biosynthesis pathway in response to UV-B radiation and drought stress in the desert plant, Reaumuria soongorica. Plant Physiol. Biochem. 2013, 73, 161–167.
- Kumar, M. Gene Mining and Application for Development of Drought Tolerant Transgenic Chickpea (Cicer arietinum L) lines. Ph.D. Thesis, Integral University, Lucknow, India, 2019. Available online: http://hdl.handle.net/10603/272521 (accessed on 24 August 2021).
- Abebe, T.; Guenzi, A.C.; Martin, B.; Cushman, J.C. Tolerance of mannitol-accumulating transgenic wheat to water stress and salinity. Plant Physiol. 2003, 131, 1748–1755.
- Wu, L.; Fan, Z.; Guo, L.; Li, Y.; Zhang, W.; Qu, L.J.; Chen, Z. Overexpression of an Arabidopsis δ-OAT gene enhances salt and drought tolerance in transgenic rice. Chin. Sci. Bull. 2003, 48, 2594–2600.
- You, J.; Hu, H.; Xiong, L. An ornithine δ-aminotransferase gene OsOAT confers drought and oxidative stress tolerance in rice. Plant Sci. 2012, 197, 59–69.
- Kasukabe, Y.; He, L.; Nada, K.; Misawa, S.; Ihara, I.; Tachibana, S. Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol. 2004, 45, 712–722.
- Garg, A.K.; Kim, J.K.; Owens, T.G.; Ranwala, A.P.; Do Choi, Y.; Kochian, L.V.; Wu, R.J. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc. Natl. Acad. Sci. USA 2002, 99, 15898–15903.
- Li, H.W.; Zang, B.S.; Deng, X.W.; Wang, X.P. Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta 2011, 234, 1007–1018.
- Karim, S.; Aronsson, H.; Ericson, H.; Pirhonen, M.; Leyman, B.; Welin, B.; Mäntylä, E.; Palva, E.T.; Van Dijck, P.; Holmström, K.O. Improved drought tolerance without undesired side effects in transgenic plants producing trehalose. Plant Mol. Biol. 2007, 64, 371–386.
- Abdullah, H.M.; Rodriguez, J.; Salacup, J.M.; Castañeda, I.S.; Schnell, D.J.; Pareek, A.; Dhankher, O.P. Increased cuticle waxes by overexpression of WSD1 improves osmotic stress tolerance in Arabidopsis thaliana and Camelina sativa. Int. J. Mol. Sci. 2021, 22, 5173.
- Cheng, C.; Hu, S.; Han, Y.; Xia, D.; Huang, B.L.; Wu, W.; Hussain, J.; Zhang, X.; Huang, B. Yellow nutsedge WRI4-like gene improves drought tolerance in Arabidopsis thaliana by promoting cuticular wax biosynthesis. BMC Plant Biol. 2020, 20, 498.
- Borgo, L.; Marur, C.J.; Vieira, L.G.E. Effects of high proline accumulation on chloroplast and mitochondrial ultrastructure and on osmotic adjustment in tobacco plants. Acta Sci. Agron. 2015, 37, 191–199.
- Yamada, M.; Morishita, H.; Urano, K.; Shiozaki, N.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Yoshiba, Y. Effects of free proline accumulation in petunias under drought stress. J. Exp. Bot. 2005, 56, 1975–1981.
- Sahni, S.; Prasad, B.D.; Liu, Q.; Grbic, V.; Sharpe, A.; Singh, S.P.; Krishna, P. Overexpression of the brassinosteroid biosynthetic gene DWF4 in Brassica napus simultaneously increases seed yield and stress tolerance. Sci. Rep. 2016, 6, 28298.
- Sun, S.C.; Xiong, X.P.; Zhang, X.L.; Feng, H.J.; Zhu, Q.H.; Sun, J.; Li, Y.J. Characterization of the Gh4CL gene family reveals a role of Gh4CL7 in drought tolerance. BMC Plant Biol. 2020, 20, 1–15.
- Wang, A.; Zhu, M.; Luo, Y.; Liu, Y.; Li, R.; Kou, M.; Wang, X.; Zhang, Y.; Meng, X.; Zheng, Y. A sweet potato cinnamate 4-hydroxylase gene, IbC4H, increases phenolics content and enhances drought tolerance in tobacco. Acta Physiol. Plant 2017, 39, 276.




