
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Costantini | + 3048 word(s) | 3048 | 2021-08-11 10:46:29 | | | |
| 2 | Dean Liu | Meta information modification | 3048 | 2021-09-24 02:59:20 | | |
Video Upload Options
Marine sponges represent a fascinating phylum of marine invertebrates, hosting a wide symbiotic community together with a huge production of secondary metabolites. The sponge-associated biota may bring together a broad group of phylogenetic lineages, including archaea, bacteria, and fungi.
1. Introduction
The discovery of marine bioactive metabolites as potential drugs for the pharmaceutical, nutraceutical, and cosmeceutical industries prompted several research projects relying on the identification of novel chemical moieties with innovative biological functions [1]. Recently, the cosmeceutical field has been fast-growing, since consumers have given greater attention to creams and lotions containing natural compounds with pharmacological properties [2]. Cosmeceuticals are topical products containing some bioactive ingredients that mimic drug-like benefits by enhancing skin health-related function [2][3]. On a global scale, the cosmeceutical industry is gradually shifting to natural compounds for their biocompatible, safe, and eco-friendly properties [4]. The success of cosmeceutical productions primarily depends on safety; low costs; and the ability to maintain the active ingredient, deliver it in a biologically active form, and exert a biological effect through known mechanisms [5]. To overcome these latter issues, particularly related to unsuitable chemical properties, some encapsulation and nano-formulation methods were developed to greatly improve drug delivery and effectiveness [6][7][8][9][10][11].
Recently, much attention has been paid to marine anti-oxidants, including mycosporines and mycosporine-like amino acids (MAAs), carotenoids and other compounds exhibiting multiple roles within cosmeceutical field [12][13]. Some examples are pigments (e.g., carotenoids), extremely abundant in the marine environment since they are produced by all autotrophic organisms (e.g., bacteria, archaea, algae and fungi). Carotenoids include carotenes (e.g., lycopene and α- and β-carotene) and xanthophylls (e.g., astaxanthin, fucoxanthin and lutein), which showed anti-oxidant activities [14] protecting skin from Reactive Oxygen Species (ROS) that are normally released within the cells after the natural oxidation induced by UV radiation and skin aging [15]. Since synthetic compounds may exert toxic effects for human health and wellness [16], natural anti-oxidants were investigated for their potential use in cosmetics [17][18]. Anti-microbial and anti-fouling agents that protect against skin disease-related pathogens, such as Staphylococcus epidermis , Staphylococcus aureus , Pseudomonas aeruginosa and Candida albicans , were also described from various sources and considered useful tools for the formulation of cosmetic products and dermatological treatments [19][20][21][22][23][24]. Moreover, bioactive compounds with anti-tyrosinase activity found several applications in the cosmetic industry, since tyrosinase represents a key enzyme involved in melanin biosynthesis, and the block of its enzymatic activity might be used for skin whitening treatments, whose deployment is extremely popular in some countries [25]. Surfactants and emulsifiers, with both hydrophilic and hydrophobic groups, could also be used in the cosmetic field [26][27]. Several protein-polysaccharide complexes, glycolipids and lipopeptides isolated from marine microorganisms were studied for the production of biosurfactants and bioemulsifiers [28]. For instance, chitosan, due to its high water-binding capacity, was proposed as a skin moisturizer and delivery agent in cosmeceutical preparations of anti-aging products [29].
Recognized producers of marine cosmeceuticals are cyanobacteria, along with micro- and macro-algae [10][30][31][32][33], with several compounds under clinical trials or already approved for the market [34][35]. As mentioned before, sponge-associated microbiota produce a plethora of bioactive compounds with beneficial properties for human health [36]. Despite the great biotechnological relevance, so far, only a few studies have reviewed the potential applications of sponge symbiont metabolites in the cosmetic field focusing on specific sponge species [37] or grouping several taxa of marine organisms [38].
2. Sponge Symbionts in Cosmeceutical Field
Some works, together with the anti-oxidant capabilities, evaluated the growth inhibition activity of specific pathogens commonly involved in skin infections. For instance, a chlorinated quinolone, Ageloline A, isolated from Streptomyces sp. SBT345, a bacterial symbiont of the Mediterranean sponge Agelas oroides , was investigated for its radical scavenging and anti-microbial properties. This compound exhibited anti-oxidant potential on a human leukemic cell line (HL-60) and was further able to reduce oxidative stress and genomic damage induced by 4-nitroquinoline-1-oxide (NQO). Moreover, Ageloline A inhibited the growth of Chlamydia trachomatis in a dose-dependent manner with an IC 50 value of 2.14 μg/mL [39]. Anti-microbial activities against E. coli MTCC-1687, P. aeruginosa MTCC-1688, B. subtilis MTCC-441 and S. aureus MTCC-737 were also observed from a GSA10 strain associated to the sponge Halichondria glabrata (West coast of Mumbai, India). In addition, anti-oxidant properties were detected using DPPH scavenging and Total Radical-trapping Anti-oxidant Parameter (TRAP) assay. In particular, through TRAP assay, the GSA10 acted as peroxyl scavengers, and the percentage of inhibition was proportional to the GSA10 concentrations [40]. In a recent work, the crude methanolic extract and the fractions of Bacillus 2011SOCCUF3 strain isolated from the sponge S. officinalis (Cortiou and Riou, France) exhibited anti-oxidant and anti-microbial activities. In particular, DPPH assay showed a dose-dependent scavenging activity, with a percentage inhibition of 38.9-49.1% (10–50 mg/mL), and agar-well diffusion method revealed a high inhibitory effect against C. albicans at a concentration range of 2.5–20 mg/mL [41].
Concerning the anti-oxidant activity, two extracellular polysaccharides, ENP1 and ENP2, were isolated from the fermentation fluid of the sponge-derived marine fungus Epicoccum nigrum (Hainan, China). Both molecules exhibited, in vitro, a slight anti-oxidant capacity by hydroxyl, superoxide, and DPPH assay, with low half maximal effective concentration (EC 50) values (280–1570 µg/mL). However, ENP2 was found to be the most active, since a higher radical scavenging activity was measured at all concentrations tested [42]. The same properties were detected from an aromatic polyketide isolated from Aspergillus versicolor , a fungus cultured in laboratory conditions after rinsing some tissues excised from the Korean sponge Petrosia sp. (Jeju Island in South Korea). By comparing it to standard anti-oxidants, this compound displayed anti-oxidant properties at increasing concentrations (5–100 µg/mL) by DPPH assay and inhibition of lipid peroxidation, higher than butylated hydroxytoluene (BHT) [43]. Bioassay-guided fractionations led to the isolation of other anti-oxidant compounds from the sponge-derived fungus Penicillium citrinum SpI080624G1f01 (Ishigaki Island, Japan). The DPPH radical scavenging activity of a sorbicillinoid derivative, named JBIR-124, was found particularly interesting, with an IC 50 value of 13 µg/mL [44]. In a similar work, DPPH combined to Thiobarbituric acid (TBARS) and NO assay, showed significant anti-oxidative and anti-inflammatory activities of the crude extracts obtained from three fungi, Chaetomium globosum , Gymnascella dankaliensis and Nigrospora oryzae . These fungal strains were isolated from the sponge Hippospongia communis , collected between the West coast of Alexandria and the borders of Libya. In particular, C. globosum and G. dankaliensis displayed a significant inhibition of lipid peroxidation (93%) and DPPH scavenging activity (59%), respectively, whereas N. oryzae was the most effective in inhibiting NO species [45]. Extensive chemical analyses also allowed the identification of more than twenty Spiro-Phthalides and Isocoumarins from the fungus Setosphaeria sp. SCSIO41009 associated to the sponge Callyspongia sp. retrieved from Chinese waters. Among them, 7-O-demethylmonocerin evidenced a strong scavenging activity on DPPH radicals, with IC 50 value (11.2 µg/mL) comparable to ascorbic acid [46]. From the ethyl acetate extract of the Chinese strain Aspergillus europaeus WZXY-SX-4-1, isolated from the marine sponge Xestospongia testudinaria , six polyketide derivatives were separated through chromatographic method. Bioactivity screening showed that three Benzophenones exhibited the most potent scavenging activity against DPPH radicals (IC 50 = 1.7–5.4 µg/mL) as compared to the positive control (trolox) [47]. A fungus species identified as Aspergillus unguis RSPG_204 was isolated from the sponge Agelas sp. (Hurghada coast, Red Sea, Egypt). The mycelia extract and culture supernatants exhibited significant superoxide anion scavenging activity, while extremely low anti-tyrosinase capabilities were detected. Interestingly, the biological activity was corroborated by chemical analyses revealing several bioactive compounds in the supernatants of static cultures and mycelial extract [48]. Recently, a marine fungus of the same genus ( Aspergillus terreus ), living as symbiont of the marine sponge Phakellia fusca , was found to produce four butenolide derivatives. DPPH assay revealed moderate anti-oxidant properties (IC 50 = ~14–36 µg/mL) with promising application in the cosmeceutical field [49].
The bioactivity of twenty-two fungi associated to several sponge species ( Agelas citrina , Stelligera rigida , Oscarella lobularis , Celtodoryx girardae , Madracis miriabilis , Cliona celata and Spongosorites difficilis ) collected from the Red Sea was also tested for their anti-microbial and anti-oxidant capacities. An anti-microbial assay was carried out upon agar plates containing the bacterial pathogens S. aureus , P. aeroginosa and C. albicans . Among fungi under analysis, the most promising anti-microbial activities against all pathogens tested were ascribed to Aspergillus oryzae and Cladosporium cladosporioides . Regarding the anti-oxidant properties, the fresh mycelium was found more effective than the culture filtrate extract, with Aspergillus fumigatus reporting the highest percentage of DPPH scavenging activity (59.7%) [50]. A different study conducted on the sponge Amphimedon sp. (Yongxin Island, China) brought to the isolation of the fungus Peyronellaea glomerata . Chromatographic separation of the ethyl acetate extract revealed five Isocoumarins, Peyroisocumarins A-D and Isocitreoisocoumarinol, plus thirteen analogs. The anti-bacterial assays were applied to different organisms, including S. aureus and E. coli . In particular, Alternariol slightly inhibited the growth of S. aureus (MIC = 16 µM). On the other hand, through Antioxidant Response Element (ARE)-driven luciferase reporters, a significant regulation of the nuclear factor E2-related factor 2 (Nrf2), a transcription factor that responds to oxidative stress, was observed in Peyroisocumarins A and B with chlorination at side chain. Hence, these two compounds were suggested by the authors as potential leads for anti-oxidant agents [51].
Several biological activities were also found in the extracts obtained from the sponge-derived Aspergillus sydowii strain W4-2 and an unidentified fungus named FS1 (Red Sea, Egypt). The supernatant of crude extracts obtained from fungal static cultures showed a high DPPH free radical scavenging activity, plus a moderate tyrosinase inhibitory capacity of A. sydowii . Moreover, FS1 demonstrated anti-bacterial properties against S. aureus , C. albicans and P. aeruginosa [52]. Interestingly, another A. sidowii strain isolated from the Indonesian sponge KN-15-3 also demonstrated significant anti-bacterial activity on Multi-Drug Resistant S. aureus and E. coli bacteria [53]. Contrary to the results reported by El-Hady and collaborators [48][52], the ethyl acetate extract of fungal strains ( Penicillium sp., Aspergillus niger and Trichophyton megninii ) isolated from another Indonesian sponge ( Haliclona fascigera ) displayed considerable anti-tyrosinase activity. In particular, all fungi inhibited tyrosinase functionality, and only one strain of the genus Penicillium was found extremely active (IC 50 = 26 µg/mL). Overall, these species were pointed out as potential sources of tyrosinase inhibitors and skin-whitening agents [54].
3. Sponges
The ethylacetate extracts from the sponges Rhabdastrella globostellata and Spirastrella inconstans (Gulf of Mannar) were investigated for their anti-oxidant activity in vivo at different concentrations (2, 4, 6, 8 and 10 mg/kg). The oral administration in rats of the sponge extracts increased the hepatic activity of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) enzymes [55]. Moreover, the dichloromethane and methanol extracts of the sponges Fascaplysinopsis reticulata , Callyspongia siphonella , Niphates furcata , Callyspongia sp. , Callyspongia clavata and Pseudosaberites clavatus harvested from the North coast of the Persian Gulf were also investigated for their free radical scavenging capabilities by DPPH and Hydroxyl Radical Scavenging assays. In particular, the methanol extract of the sponge P. clavatus displayed the best anti-oxidant activity on DPPH (IC 50 = 234 μg/mL), while both extracts of N. furcata and F. reticulate were clearly inhibited OH radicals (~70–80%) [56]. Similarly, the anti-oxidant activity was evaluated in the total extracts of six sponges collected from Indonesia using DPPH assay. The authors found that Aaptos suberitoides induced the highest activity (IC 50 < 3 × 10 4 μg/mL), while F. reticulata , Acanthella sp., Petrosia contignata and Xestospongia exigua exerted only a slight anti-oxidant effect with IC 50 values less than 1 × 10 5 µg/mL [57]. The methanol extracts of eleven sponge species collected from six geographical sites in Turkey were evaluated for their anti-oxidant properties through DPPH, NO and superoxide radical scavenging activities. The DPPH and superoxide assays revealed a significant dose-dependent radical scavenging activity, with the sponge Dysidea avara being the most promising among all specimens under analysis (DPPH, IC 50 = 92.8 µg/mL; O 2− , 34.1 µg/mL). Concerning NO radicals, a moderate activity was recorded, with the sole methanolic extract of Ciocalypta carbolloi displaying anti-oxidant capacities (700.7 µg/mL) that were higher when compared to the control (quercetin). Interestingly, the authors noticed that the biological activity of sponge extracts was clearly correlated to the location, since the samples collected from Kemer revealed the highest anti-oxidative properties [58]. On the contrary, Botic et al. [59] observed an anti-oxidative capability of different Antarctic sponges of the genus Latrunculia through photochemiluminescence assay. The significant variation among samples was explained by a probable changing in the symbiotic community, which was not influenced by the geographical site but rather was species specific [59]. Puupehenol, a meroterpenoid isolated from the organic extract of the Hawaiian Deep-Water sponge Dactylospongia sp. was found to be both an anti-oxidant and anti-microbial compound. In fact, a significant radical scavenging activity, detected by Ferric Reducing Antioxidant Power (FRAP) Assay, and a moderate growth inhibition of the Gram-positive bacteria Staphylococcus aureus was detected [60]. Anti-oxidant activity was also demonstrated in the crude extract of the sponge Ircinia spinulosa collected from the Atlantic Moroccan coast. DPPH assay revealed considerable free radical scavenging capabilities (25.25%) of the crude extract, together with a content of polyphenols, flavonoids and tannins [61]. Tannins and flavonoids were also found in the sponges A. suberitoides , Dactylospongia elegans , Stylissa massa and Haliclona sp. (Red Sea, Egypt). The anti-oxidant activity, evaluated by the phosphomolybdenum method, revealed that, among the samples analyzed, the hexane extract of D. elegans and the ethyl acetate extract of A. suberitoides induced the highest anti-oxidant properties in comparison to the ascorbic acid [62]. Recently, DPPH and 2.2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid assays also displayed a significant anti-oxidant activity (93% and 99%, respectively) of H. aff. erectus (Red Sea, Egypt) extract at 1 mg, plus a considerable content of carotenoids (1.976 mg/g) [63]. Moreover, 141 extracts of other sponge samples collected from Mauritius were investigated by DPPH and FRAP assays. The two sponges Axinella donnani and Pseudosuberites sp. were found the most promising, with significant radical scavenging activities, measured as 92.15% (DPPH) and 10.57 Fe 2+ /g of extract (FRAP), respectively [64]. The anti-oxidant capacity was also analyzed in the protein extract and the ammonium sulfate fractions of the sponge Niphates sp. collected from the sponge reefs of Spermonde waters (South Sulawesi). Among the samples investigated, the DPPH radical scavenging was mostly observed in the crude extract (IC 50 = 5.05 µg/mL), probably due to a higher glutathione content enhancing the anti-oxidant potentialities [65].
Topic formulations with skin whitening properties have also found huge applications in the cosmetic industry. Concerning sponge-derived compounds, experiments of immunofluorescence on murine melanoma B16 cells treated with the anti-tumour compound Geoditin A, isolated from the sponge Geodia japonica (South China Sea), revealed anti-melanogenic and skin whitening properties. Increasing concentrations (0.6, 1.25 and 5 μg/mL) of Geoditin A induced a dose-dependent reduction of melanin within the cytosol and Golgi apparatus, and, similarly, a depletion of tyrosinase was observed into the endoplasmic reticulum (ER). The decrease of tyrosinase activity after Geoditin A treatment was corroborated through the detection on immunoblotting of melanogenic proteins [66]. Similarly, Gagunin D (GD) (see Figure 1 ), a diterpenoid isolated from the sponge Phorbas sp. (Gagu-Do, Korea), was revealed as a potent anti-melanogenic compound [67]. In particular, treatments of GD at increasing concentrations significantly reduced the production of melanin (IC 50 = 5.7 µg/mL) in Melan-a cells, with higher effects in comparison to the commercial skin whitening agent arbutin. This result was also confirmed by Real time qPCR on melanogenesis-related genes revealing a significant down-regulation of PAX3 , SOX10 , MITF , tyrosinase , TRP-1 and TRP-2 . Moreover, GD exposure at 10 µM on UVB irradiated human skin models demonstrated a considerable reduction of melanin biosynthesis [67].
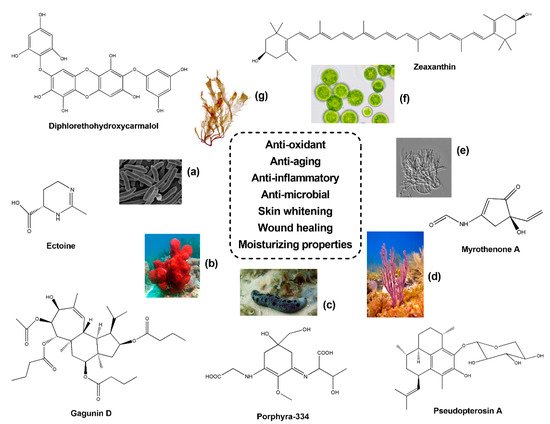
The methanol, ethanol and hexane extracts obtained from Acanthella cavernosa , a sponge collected from Bali (Indonesia), were rather explored for anti-microbial and anti-biofilm properties against Propionibacterium acnes [68], a common pathogen inducing the inflammatory events connected to acne issues. In particular, the ethanol extract displayed MIC and Minimum Bactericidal Concentration (MBC) values of 125 and 250 μg/mL, respectively, and a considerable inhibition of P. acnes biofilm at 250 μg/mL (45%). These results, combined to a slight anti-oxidant activity, suggested a possible application of these sponge extracts as cosmetic ingredients for preventing acne infections [68]. The anti-microbial activity of seven sponge extracts was also evaluated through agar well diffusion on S. epidermis , S. aureus and P. aeruginosa [69], three microbes that normally constitute skin microflora. Five sponge samples retrieved from Indian waters revealed anti-microbial activity, with Neopetrosia exigua extract being the most promising against target organisms plus C. albicans showing significant anti-fungal properties. Moreover, all methanol extracts exerted anti-oxidant effects by DPPH assay, particularly Hyrtios erecta (IC 50 = 32.5 μg/mL), followed by N. exigua (IC 50 = 36.6 μg/mL) and X. testudinaria (IC 50 = 46.7 μg/mL) species [69]. In a recent work, bioassay-guided fractionations from the CH 2Cl 2-MeOH extract of the sponge Haliclona sp. collected in the Indian Ocean led to the identification of several long-chain highly oxygenated polyacetylenes, named Osirisynes A, B, E, G, H and I. These latter compounds were all tested for catalase and sirtuin 1 activation and CDK7, proteasome, Fyn kinase, tyrosinase, and elastase inhibition, which are considered suitable targets for studying aging-related diseases. In particular, Osirisyne B was found the most effective, with a significant blockage of Fyn kinase (IC 50 = 14.7 µg/mL), CDK7 kinase (IC 50 = 7.3 µg/mL), and proteasome (IC 50 = 0.2 µg/mL) [70].
Marine natural compounds with wound healing properties were also identified as suitable sources for cosmetic manufacturing. Fibroblasts normally produce several compounds in the extracellular matrix, such as glycosaminoglycans (GAGs) or collagen, with the specific capability to adsorb the excessive exudate within tissue wounds and promote skin repair [71]. Advances in chemical extraction methods allowed the isolation of collagen and other similar substances from marine invertebrates, including sponges [72]. One of the first studies observed that collagen, extracted from the sponge Condrosia reniformis (Aegean Sea), slightly influenced skin pH and hydration, revealing promising results [73]. Then, in a different research, four sponge samples, Spongia lamella , Spongia officinalis , Hippospongia communis and Sarcotragus spinosulus collected from Sardinian beaches (Western Mediterranean Sea), were also found to produce considerable quantities of natural GAGs with good water adsorbing capabilities [74]. Moreover, a recent work evaluated the anti-oxidant, photoprotective and wound healing properties of collagen hydrolysate (MHC) fractions from the Mediterranean sponge C. reniformis [75]. DPPH and Nitro Blue Tetrazolium (NBT)/riboflavin assays displayed a high ROS and superoxide anion scavenging activity at 50 µg/mL and 100 µg/mL compared to controls. In addition, the exposure to MHC fractions increased the mRNA levels of collagen 1A ( Col1A ) in L929 murine fibroblasts and enhanced cell growth in UV flashed L929 (~8–40%) and HaCaT (~14–32%) cells, depending on the UV dose. “Scratch” tests showed good skin repair properties, particularly on keratinocytes, where cell proliferation near the wound edges was clearly visible at 6 h and 24 h of treatment, with ~22% of wound extension [75].
References
- Shinde, P.; Banerjee, P.; Mandhare, A. Marine natural products as source of new drugs: A patent review (2015–2018). Expert Opin. Ther. Pat. 2019, 29, 283–309.
- Draelos, Z.D. Cosmeceuticals: What’s real, what’s not. Dermatol. Clin. 2019, 37, 107–115.
- Ahsan, H. The biomolecules of beauty: Biochemical pharmacology and immunotoxicology of cosmeceuticals. J. Immunoass. Immunochem. 2019, 40, 91–108.
- Alves, T.F.R.; Morsink, M.; Batain, F.; Chaud, M.V.; Almeida, T.; Fernandes, D.A.; Silva, C.F.; Souto, E.B. Synthetic polymers in cosmetic formulations. Cosmetics 2020, 7, 75.
- Sharad, J. Cosmeceuticals. In Advances in Integrative Dermatology; França, K., Lotti, T., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 393–411.
- Khezri, K.; Saeedi, M.; Maleki Dizaj, S. Application of nanoparticles in percutaneous delivery of active ingredients in cosmetic preparations. Biomed. Pharmacother. 2018, 106, 1499–1505.
- Bilal, M.; Iqbal, H.M.N. New insights on unique features and role of nanostructured materials in cosmetics. Cosmetics 2020, 7, 24.
- Ben Haddada, M.; Gerometta, E.; Chawech, R.; Sorres, J.; Bialecki, A.; Pesnel, S.; Spadavecchia, J.; Morel, A.L. Assessment of antioxidant and dermoprotective activities of gold nanoparticles as safe cosmetic ingredient. Colloids Surf. B Biointerfaces 2020, 189, 110855.
- Kesavan Pillai, S.; Kleyi, P.; de Beer, M.; Mudaly, P. Layered double hydroxides: An advanced encapsulation and delivery system for cosmetic ingredients-an overview. Appl. Clay Sci. 2020, 199, 105868.
- Poulose, N.; Sajayan, A.; Ravindran, A.; Sreechithra, T.V.; Vardhan, V.; Selvin, J.; Kiran, G.S. Photoprotective effect of nanomelanin-seaweed concentrate in formulated cosmetic cream: With improved antioxidant and wound healing properties. J. Photochem. Photobiol. B Biol. 2020, 205, 111816.
- Genç, Y.; Bardakci, H.; Yücel, Ç.; Karatoprak, G.Ş.; Akkol, E.K.; Barak, T.H.; Sobarzo-Sánchez, E. Oxidative stress and marine carotenoids: Application by using nanoformulations. Mar. Drugs 2020, 18, 423.
- Vílchez, C.; Forján, E.; Cuaresma, M.; Bédmar, F.; Garbayo, I.; Vega, J.M. Marine carotenoids: Biological functions and commercial applications. Mar. Drugs 2011, 9, 319–333.
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-like amino acids and their derivatives as natural antioxidants. Antioxidants 2015, 4, 603–646.
- Birben, E.; Sahiner, U.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. Science 1997, 22, 161–168.
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from marine organisms: Biological functions and industrial applications. Antioxidants 2017, 6, 96.
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174.
- Sansone, C.; Brunet, C. Marine algal antioxidants. Antioxidants 2020, 9, 206.
- Tripathi, V.C.; Horam, S.; Singh, A.; Lata, M.; Reddy, T.J.; Arockiaraj, J.; Pasupuleti, M. The discovery of antioxidants in marine microorganisms and their protective effects on the hepatic cells from chemical-induced oxidative stress. Free Radic. Res. 2020, 54, 150–161.
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Barreiro, M.F.; González-Paramás, A.M.; Ferreira, I.C.F.R. Development of mushroom-based cosmeceutical formulations with anti-inflammatory, anti-tyrosinase, antioxidant, and antibacterial properties. Molecules 2016, 21, 1372.
- Siavash, H.C.; Fadzilah, A.A.M. Cosmeceutical values, antimicrobial activities and antioxidant properties of cashew leaves extract. Afr. J. Biotechnol. 2011, 10, 4573–14582.
- Baldisserotto, A.; Malisardi, G.; Scalambra, E.; Andreotti, E.; Romagnoli, C.; Vicentini, C.B.; Manfredini, S.; Vertuani, S. Synthesis, antioxidant and antimicrobial activity of a new phloridzin derivative for dermo-cosmetic applications. Molecules 2012, 17, 13275–13289.
- Herman, A.; Herman, A.P.; Domagalska, B.W.; Młynarczyk, A. Essential oils and herbal extracts as antimicrobial agents in cosmetic emulsion. Indian J. Microbiol. 2013, 53, 232–237.
- Adegoke, T.; Arotupin, D.; Ekundayo, T. Antimicrobial activities of some commercial cosmetics on selected cutaneous microflora. J. Adv. Microbiol. 2017, 4, 1–9.
- Kim, J.W.; Yu, H.; Park, K.M.; Chang, P.S. Antimicrobial characterization of erythorbyl laurate for practical applications in food and cosmetics. J. Chem. 2020, 2020, 1–8.
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425.
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26.
- Adu, S.A.; Naughton, P.J.; Marchant, R.; Banat, I.M. Microbial biosurfactants in cosmetic and personal skincare pharmaceutical formulations. Pharmaceutics 2020, 12, 1099.
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine microbial-derived molecules and their potential use in cosmeceutical and cosmetic products. Mar. Drugs 2017, 15, 118.
- Kong, S.Z.; Li, J.C.; Li, S.D.; Liao, M.N.; Li, C.P.; Zheng, P.J.; Guo, M.H.; Tan, W.X.; Zheng, Z.H.; Hu, Z. Anti-aging effect of chitosan oligosaccharide on d-galactose-induced subacute aging in mice. Mar. Drugs 2018, 16, 181.
- Jahan, A.; Ahmad, I.Z.; Fatima, N.; Ansari, V.A.; Akhtar, J. Algal bioactive compounds in the cosmeceutical industry: A review. Phycologia 2017, 56, 410–422.
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy—Cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 2018, 5, 68.
- Hamidi, M.; Safarzadeh Kozani, P.; Safarzadeh Kozani, P.; Pierre, G.; Michaud, P.; Delattre, C. Marine bacteria versus microalgae: Who is the best for biotechnological production of bioactive compounds with antioxidant properties and other biological applications? Mar. Drugs 2020, 18, 28.
- Thiyagarasaiyar, K.; Goh, B.H.; Jeon, Y.J.; Yow, Y.Y. Algae metabolites in cosmeceutical: An overview of current applications and challenges. Mar. Drugs 2020, 18, 323.
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101.
- Calado, R.; Leal, M.C.; Gaspar, H.; Santos, S.; Marques, A.; Nunes, M.L.; Vieira, H. How to succeed in marketing marine natural products for nutraceutical, pharmaceutical and cosmeceutical markets. In Grand Challenges in Biology and Biotechnology; Rampelotto, P.H., Trincone, A., Eds.; Springer International Publishing: Manhattan, NY, USA, 2018; pp. 317–403. ISBN 9783319690759.
- Bibi, F.; Faheem, M.; Azhar, E.; Yasir, M.; Alvi, S.; Kamal, M.; Ullah, I.; Naseer, M. Bacteria from marine sponges: A source of new drugs. Curr. Drug Metab. 2016, 17.
- Hassane, C.S.; Fouillaud, M.; Le Goff, G.; Sklirou, A.D.; Boyer, J.B.; Trougakos, I.P.; Jerabek, M.; Bignon, J.; de Voogd, N.J.; Ouazzani, J.; et al. Microorganisms associated with the marine sponge Scopalina hapalia: A reservoir of bioactive molecules to slow down the aging process. Microorganisms 2020, 8, 1262.
- Alves, A.; Sousa, E.; Kijjoa, A.; Pinto, M. Marine-derived compounds with potential use as cosmeceuticals and nutricosmetics. Molecules 2020, 25, 2536.
- Cheng, C.; Othman, E.M.; Reimer, A.; Grüne, M.; Kozjak-Pavlovic, V.; Stopper, H.; Hentschel, U.; Abdelmohsen, U.R. Ageloline A, new antioxidant and antichlamydial quinolone from the marine sponge-derived bacterium Streptomyces sp. SBT345. Tetrahedron Lett. 2016, 57, 2786–2789.
- Phadale, R.; Kumar, M.S. Characterization of an antimicrobial and antioxidant compound from a marine bacterium GSA10 associated with the sponge Halichondria glabrata. J. Microbiol. Biotechnol. Food Sci. 2018, 7, 651–658.
- Odekina, P.A.; Agbo, M.O.; Omeje, E.O. Antimicrobial and antioxidant activities of novel marine bacteria (Bacillus 2011SOCCUF3) isolated from marine sponge (Spongia officinalis). Pharm. Sci. 2020, 26, 82–87.
- Sun, H.H.; Mao, W.J.; Jiao, J.Y.; Xu, J.C.; Li, H.Y.; Chen, Y.; Qi, X.H.; Chen, Y.L.; Xu, J.; Zhao, C.Q.; et al. Structural characterization of extracellular polysaccharides produced by the marine fungus Epicoccum nigrum JJY-40 and their antioxidant activities. Mar. Biotechnol. 2011, 13, 1048–1055.
- Li, J.L.; Lee, Y.M.; Hong, J.; Bae, K.S.; Choi, J.S.; Jung, J.H. A new antioxidant from the marine sponge-derived fungus Aspergillus versicolor. Nat. Prod. Sci. 2011, 17, 14–18.
- Kawahara, T.; Takagi, M.; Shin-Ya, K. JBIR-124: A novel antioxidative agent from a marine sponge-derived fungus Penicillium citrinum SpI080624G1f01. J. Antibiot. (Tokyo) 2012, 65, 45–47.
- Abdel-Monem, N.; Abdel-Azeem, A.M.; El Ashry, E.S.H.; Ghareeb, D.A.; Nabil-Adam, A. Assessment of secondary metabolites from marine-derived fungi as antioxidant. Open J. Med. Chem. 2013, 3, 60–73.
- Pang, X.; Lin, X.; Yang, J.; Zhou, X.; Yang, B.; Wang, J.; Liu, Y. Spiro-phthalides and Isocoumarins isolated from the marine-sponge-derived fungus Setosphaeria sp. SCSIO41009. J. Nat. Prod. 2018, 81, 1860–1868.
- Du, X.; Liu, D.; Huang, J.; Zhang, C.; Proksch, P.; Lin, W. Polyketide derivatives from the sponge associated fungus Aspergillus europaeus with antioxidant and NO inhibitory activities. Fitoterapia 2018, 130, 190–197.
- El-Hady, F.K.A.; Abdel-aziz, M.S.; Shaker, K.H.; El-shahid, Z.A.; Ibrahim, L.S. Antioxidant, acetylcholinesterase and α-glucosidase potentials of metabolites from the marine fungus. Int. J. Pharm. Sci. Rev. Res. 2015, 30, 272–278.
- Sun, Y.; Liu, J.; Li, L.; Gong, C.; Wang, S.; Yang, F.; Hua, H.; Lin, H. New butenolide derivatives from the marine sponge-derived fungus Aspergillus terreus. Bioorganic Med. Chem. Lett. 2018, 28, 315–318.
- Sayed, M.A.; El-rahman, T.M.A.A.; El-diwany, A.I.; Product, M.; Sayed, S.M. Biodiversity and bioactivity of red sea sponge associated endophytic fungi. Int. J. Adv. Res. Eng. Appl. Sci. 2016, 5, 1–15.
- Zhao, Y.; Liu, D.; Proksch, P.; Yu, S.; Lin, W. Isocoumarin derivatives from the sponge-associated fungus Peyronellaea glomerata with antioxidant activities. Chem. Biodivers. 2016, 13, 1186–1193.
- El-Hady, F.K.A.; Abdel-Aziz, M.S.; Shaker, K.H.; El-Shahid, Z.A. Tyrosinase, acetylcholinesterase inhibitory potential, antioxidant and antimicrobial activities of sponge derived fungi with correlation to their GC/MS analysis. Int. J. Pharm. Sci. Rev. Res. 2014, 26, 338–345.
- Trianto, A.; Widyaningsih, S.; Radjasa, O.; Pribadi, R. Symbiotic fungus of marine sponge Axinella sp. producing antibacterial agent. Conf. Ser. Earth Environ. Sci. 2017, 55, 012005.
- Handayani, D.; Andalas, U.; Sandrawati, N.; Andalas, U.; Syafni, N.; Putra, D.P. Tyrosinase inhibitory activity of ethyl acetate extracts from marine sponge-derived fungi Haliclona fascigera. Biosci. Res. 2019, 16, 2369–2373.
- Chairman, K.; Singh, A.J.A.R.; Alagumuthu, G. Cytotoxic and antioxidant activity of selected marine sponges. Asian Pac. J. Trop. Dis. 2012, 2, 234–238.
- Seradj, H.; Moein, M.; Eskandari, M.; Maaref, F. Antioxidant activity of six marine sponges collected from the Persian gulf. Iran. J. Pharm. Sci. 2012, 8, 249–255.
- Abdillah, S.; Nurhayati, A.P.D.; Nurhatika, S.; Setiawan, E.; Heffen, W.L. Cytotoxic and antioxidant activities of marine sponge diversity at Pecaron Bay Pasir Putih Situbondo East Java, Indonesia. J. Pharm. Res. 2013, 6, 685–689.
- Aktas, N.; Genc, Y.; Gozcelioglu, B.; Konuklugil, B.; Sebnem Harput, U. Radical scavenging effect of different marine sponges from mediterranean coasts. Rec. Nat. Prod. 2013, 7, 96–104.
- Botić, T.; Cör, D.; Anesi, A.; Guella, G.; Sepčić, K.; Janussen, D.; Kersken, D.; Knez, Ž. Fatty acid composition and antioxidant activity of Antarctic marine sponges of the genus Latrunculia. Polar Biol. 2015, 38, 1605–1612.
- Hagiwara, K.; Garcia Hernandez, J.E.; Harper, M.K.; Carroll, A.; Motti, C.A.; Awaya, J.; Nguyen, H.Y.; Wright, A.D. Puupehenol, a potent antioxidant antimicrobial meroterpenoid from a Hawaiian deep-water Dactylospongia sp. sponge. J. Nat. Prod. 2015, 78, 325–329.
- Rhandour, Z.; Tarbaoui, M.; Oumam, M.; Elamraoui, B.; Bennamara, A.; Abourriche, A. Determination of polyphenols, tannins, flavonoids and antioxidant activity in extracts of two genus Ircinia marine sponges of Atlantic Morrocan Coast. Front. Mar. Sci. 2016, 3.
- Rivera, A.P.; Uy, M.M. In vitro antioxidant and cytotoxic activities of some marine sponges collected off misamis oriental coast, Philippines. E-J. Chem. 2012, 9, 354–358.
- Nabil-Adam, A.; Shreadah, M.A.; El Moneam, N.M.A.; El-Assar, S.A. Various in vitro bioactivities of secondary metabolites isolated from the sponge Hyrtios aff. erectus from the red sea coast of Egypt. Turk. J. Pharm. Sci. 2020, 17, 127–135.
- Oogarah, P.N.; Ramanjooloo, A.; Rovisham, J.; Doorga, S.; Meyepa, C.; Wilhelmus, R.; Van Soest, M.; Edgard, D.; Marie, P. Assessing antioxidant activity and phenolic content of marine sponges from mauritius waters. Int. J. Pharmacogn. Phytochem. Res. 2020, 12, 123–131.
- Warsidah, M.; Sofiana, M.S.J.; Safitri, I.; Sapar, A.; Aritonang, A.B.; Muttalib, Y.S.; Fadly, D. Protein isolation from sponge Niphates sp. as an antibacterial and antioxidant. Syst. Rev. Pharm. 2020, 11, 518–521.
- Cheung, F.W.K.; Guo, J.; Ling, Y.H.; Che, C.T.; Liu, W.K. Anti-melanogenic property of geoditin A in murine B16 melanoma cells. Mar. Drugs 2012, 10, 465–476.
- Lee, H.Y.; Jang, E.J.; Bae, S.Y.; Jeon, J.E.; Park, H.J.; Shin, J.; Lee, S.K. Anti-melanogenic activity of Gagunin D, a highly oxygenated diterpenoid from the marine sponge Phorbas sp., via modulating tyrosinase expression and degradation. Mar. Drugs 2016, 14, 212.
- Yanti, C.; Vendy, V.; Hwang, J.K. In vitro anti-acne activity of marine sponge Acanthella cavernosa extracts. Int. J. Biol. Pharm. Res. 2015, 6, 388–392.
- Chander, M.P.; Vijayachari, P. Antimicrobial and anti-oxidant potentials of marine sponges of South Andaman, India. Bangladesh J. Pharmacol. 2018, 13, 13–15.
- Campos, P.E.; Herbette, G.; Chendo, C.; Clerc, P.; Tintillier, F.; Voogd, N.J.D.; Papanagnou, E.D.; Trougakos, I.P.; Jerabek, M.; Bignon, J.; et al. Osirisynes G-I, new long-chain highly oxygenated polyacetylenes from the Mayotte marine sponge Haliclona sp. Mar. Drugs 2020, 18, 350.
- Ghatak, S.; Maytin, E.V.; MacK, J.A.; Hascall, V.C.; Atanelishvili, I.; Moreno Rodriguez, R.; Markwald, R.R.; Misra, S. Roles of proteoglycans and glycosaminoglycans in wound healing and fibrosis. Int. J. Cell Biol. 2015, 2015, 1–20.
- Pallela, R.; Ehrlich, H.; Bhatnagar, I. Marine sponges: Chemico-biological and biomedical applications. In Marine Sponges: Chemicobiological and Biomedical Applications; Pallela, R., Ehrlich, H., Eds.; Springer: New Delhi, India, 2016; pp. 1–381. ISBN 9788132227946.
- Swatschek, D.; Schatton, W.; Kellermann, J.; Müller, W.E.G.; Kreuter, J. Marine sponge collagen: Isolation, characterization and effects on the skin parameters surface-pH, moisture and sebum. Eur. J. Pharm. Biopharm. 2002, 53, 107–113.
- Langasco, R.; Cadeddu, B.; Formato, M.; Lepedda, A.J.; Cossu, M.; Giunchedi, P.; Pronzato, R.; Rassu, G.; Manconi, R.; Gavini, E. Natural collagenic skeleton of marine sponges in pharmaceutics: Innovative biomaterial for topical drug delivery. Mater. Sci. Eng. C 2017, 70, 710–720.
- Pozzolini, M.; Millo, E.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Arkel, M.; Scarfì, S. Elicited ROS scavenging activity, photoprotective, and wound-healing properties of collagen-derived peptides from the marine sponge Chondrosia reniformis. Mar. Drugs 2018, 16, 465.




