
Video Upload Options
Cardiovascular disease (CVD), which involves the onset and exacerbation of various conditions including dyslipidemia, activation of the renin–angiotensin system, vascular endothelial cell damage, and oxidative stress, is a leading cause of high mortality rates and accounts for one-third of deaths worldwide. Accordingly, as dietary changes in daily life are thought to greatly reduce the prevalence of CVD, numerous studies have been conducted to examine the potential use of foods and their bioactive components for preventing and treating CVD. In particular, seaweeds contain unique bioactive metabolites that are not found in terrestrial plants because of the harsh environment in which they survive, leading to in vitro and in vivo studies of their prevention and treatment effects.
1. Introduction
2. Marine Natural Product on Hyperlipidemia
| Seaweeds | Experimental Models | Effects (% or mmol/L) | Ref. |
|---|---|---|---|
| Himanthalia elongate, B | Hypercholesterolaemic wistar rats : 21% in diets for four weeks |
↓: TG by 28% ↑: HDL-C by 20% |
[24] |
| Gigartina pistillata, R | Hypercholesterolaemic wistar rats : 23% in diets for four weeks |
↓: TG by 30%, TC by 18%, LDL-C by 16% | [24] |
| Derbesia tenuissima, G | High-Fat Fed Rats : 5% in diets for eight weeks |
↓: TG by 38% and TC by 17% | [25] |
| Gracilaria changii, R | High-cholesterol/high-fat Sprague Dawley rats : 5% or 10% in diets for eight weeks |
5% ↓: TC by 39.19%, LDL-C by 36.36%, TG by 25.45% 10% ↓: TC by 40.34%, LDL-C by 35.95%, TG by 30.91% |
[26] |
| Ecklonia cava, B | STZ-diabetic mice : 5% in diets for four weeks |
↓: TG by 72%, TC by 53%, and LDL-C by 78% | [27] |
| Ecklonia stolonifera, B | 3T3-L1 preadipocyte cells : Phloroglucinol, Eckol, Dieckol, Dioxinodehydroeckol, Phlorofucofuroeckol A, 12.5 to 100 µM, eight days |
↓: lipid accumulation. ↓: level of adipocyte marker genes |
[28] |
| Rhizoclonium implexum, G | A: Adult Albino rats (Sprauge-Dawley) T: Triton-induced hyperlipidaemic rats H: High-fat diet-induced hyperlipidaemic rats : 10 mg/200 g/day for 12 days, OA |
A: ↓: TC by 14.4%, TG by 26.4%, LDL-C by 25.5% ↑: HDL-C by 3.1% |
[29] |
| Dictyota Indica, B | A:↓: TC by 13.5%, TG by 24.6%, LDL-C by 25.4% ↑: HDL-C by 3.1% |
||
| Padina pavonia, B | A: ↓: TC by 26.5%, TG by 37%, LDL-C by 54.3% ↑: HDL-C by 23.5% |
||
| Stoechospermum marginatum, B | A: ↓: TC by 21.7%, TG by 40.2%, LDL-C by 30% ↑: HDL-C by 6.2% |
||
| Stokeyia indica, B | A: ↓: TC by 22.6%, TG by 17.2%, LDL-C by 40.9% ↑: HDL-C by 0.7% |
||
| Jolyna laminarioides, B | A: ↓: TC by 10%, TG by 49%, LDL-C by 28.7% ↑: HDL-C by 23.5% |
||
| T: ↓: TC by 41.2%, TG by 25.2%, LDL-C by 92.4% ↑: HDL-C by 60.6% |
|||
| H: ↓: TC by 19.8%, TG by 31.6%, LDL-C by 34.5% ↑: HDL-C by 33.1% |
|||
| Sargassum binderi, B | A: ↓: TC by 20.5%, TG by 4.2%, LDL-C by 28.0%, HDL-C by 17.4% | ||
| T: ↓: TC by 37.6%, TG by 52.2%, LDL-C by 51.1% ↑: HDL-C by 8.6% |
|||
| H: ↓: TC by 2.5%, TG by 33%, LDL-C by 2.9% ↑: HDL-C by 30% |
|||
| Melanothamnus afaqhusainii, R | A: ↓: TC by 10.3%, TG by 36.1%, LDL-C by 17.5% ↑: HDL-C by 5% |
||
| T: ↓: TC by 35.2%, TG by 43.2%, LDL-C by 71.4% ↑: HDL-C by 57.3% |
|||
| H: ↓: TC by 14.2%, TG by 25.1%, LDL-C by 5.4% ↑: HDL-C by 16.8% |
|||
| Fucoidan from Sargassum henslowianum (B) | High-fat diet albino mice of BALB/c strain : 100 mg/kg/day for four weeks, OA |
↓: TC by 21.09%, TG by 6.35%, LDL-C by 18.74% | [30] |
| Carrageenans | Ischemic Heart Disease (IHD) patients : 250 mg/day for 20 days, OA |
↓: TC by 16.5%, LDL-C by 33.5% | [31] |
| Kappaphycus alvarezii, R | High-cholesterol diet Male Sprague–Dawley rats : 300 mg/kg/day for eight weeks, OA |
↓: TC by 1.91±0.62%, TG by 0.65±0.05, LDL-C by 1.65±0.08 (mmol/L) ↑: HDL-C by 1.74±0.08 (mmol/L) |
[32] |
| Sargassum polycystum, B | ↓: TC by 1.91±0.62%, TG by 0.65±0.05, LDL-C by 1.65±0.08 (mmol/L) ↑: HDL-C by 1.74±0.08 (mmol/L) |
||
| Ulva fasciata, G | High-cholesterol diet rats : 175 mg/kg/day for four weeks, OA |
↓: TC by 46.43%, TG by 69.03%, LDL-C by 81.04% ↑: HDL-C by 668.31% |
[33] |
| Ulva lactuca, G | Hypercholesterolemic diet rats : 250 mg/kg/day for four weeks, OA |
↑: HDL-C by 180% | [34] |
| Monostroma nitidum, G | lipid-loaded hepatocytes (HepG2 cell line) : 200 µg/mL for one day |
↓: Cellular cholesterol by 36%, TG by 31%, | [35] |
| Fucoidan | Hyperlipidemic diet mice : 10 to 50 mg/kg/day for four weeks, OA |
↓: TC, TG and LDL-C ↑: HDL-C |
[36] |
| Fucoxanthin | Hyperlipidemic diet mice : 21% in diets for six weeks, OA |
↓: Liver TG synthesis, adipocyte fatty acid synthesis, and cholesterol-regulating enzyme activity ↑: Plasma HDL-C ↑: Fecal TG level |
[37] |
3. Marine Natural Products Affect Endothelial Dysfunction
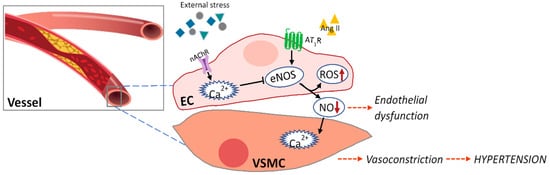
| Component | Experimental Model | Effects | Ref |
|---|---|---|---|
| Astaxanthin | ISO-induced myocardial infarction and cardiac hypertrophy model in rats : 25 mg/kg/day for two weeks, OA |
↓: ROS generation in heart tissue ↓: Oxidative damage ↑: Antioxidant enzyme activity |
[42] |
| STZ-induced diabetes in male rats : 10 mg/kg/d, OA |
↓: Blunted endothelium-dependent vasodilator responses to Ach ↓: Aorta-induced oxidative stress and LOX-1 levels ↑: eNOS levels |
[43] | |
| Dieckol | High glucose stimulation in cultured vascular endothelial cells. : 10 or 50 μg/mL |
↓: ROS production ↓: iNOS, COX-2, and NF-κB levels |
[44] |
| Eckol and its derivates | Cultured vascular endothelial cells/mice : 50∼200 μg/mL |
Protects the vascular barrier | [45] |
| DPHC from Ishige okamurae | Cultured vascular endothelial (EA.hy926) cells)/Tg(flk:EGFP) Transgenic Zebrafish : 100 μM/0.6 μM |
↑: Ach receptor and VEGF receptor 2 ↑: NO production ↑: Ca2+ release ↑: Endothelium vasodilation |
[46] |
| Sulfated polysaccharides from Padina tetrastromatica | ISO induced myocardial infarction in rats : 50 mg/kg/day for 12 days, OA |
↓: hyperlipidemia ↓: Endothelial dysfunction ↓: Inflammatory reactions |
[47] |
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N. Heart disease and stroke statistics—2021 update: A report from the American Heart Association. Circulation 2021, 143, e254–e743.
- Wong, N.D.; Lopez, V.; Tang, S.; Williams, G.R. Prevalence, treatment, and control of combined hypertension and hypercholesterolemia in the United States. Am. J. Cardiol. 2006, 98, 204–208.
- Zou, Z.; Cini, K.; Dong, B.; Ma, Y.; Ma, J.; Burgner, D.P.; Patton, G.C. Time trends in cardiovascular disease mortality across the BRICS: An age-period-cohort analysis of key nations with emerging economies using the global burden of disease study 2017. Circulation 2020, 141, 790–799.
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581.
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818.
- Figueroa, J.F.; Wadhera, R.K.; Lee, D.; Yeh, R.W.; Sommers, B.D. Community-level factors associated with racial and ethnic disparities in COVID-19 rates in Massachusetts: Study examines community-level factors associated with racial and ethnic disparities in COVID-19 rates in Massachusetts. Health Aff. 2020, 39, 1984–1992.
- Rate, C.-F.; Onder, G.; Rezza, G.; Brusaferro, S. Characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020, 23, 1775–1776.
- Bonow, R.O.; O’Gara, P.T.; Yancy, C.W. Cardiology and COVID-19. JAMA 2020, 324, 1131–1132.
- Colling, M.E.; Kanthi, Y. COVID–19-associated coagulopathy: An exploration of mechanisms. Vasc. Med. 2020, 25, 471–478.
- Moran, A.E.; Roth, G.A.; Narula, J.; Mensah, G.A. 1990–2010 global cardiovascular disease atlas. Glob. Heart 2014, 9, 3–16.
- Lopez, E.O.; Ballard, B.D.; Jan, A. Cardiovascular Disease. StatPearls, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535419/ (accessed on 5 September 2021).
- Eaton, C.B.; Feldman, H.A.; Assaf, A.R.; McPhillips, J.B.; Hume, A.L.; Lasater, T.M.; Levinson, P.; Carleton, R.A. Prevalence of hypertension, dyslipidemia, and dyslipidemic hypertension. J. Fam. Pract. 1994, 38, 17–24.
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA 2003, 289, 2560–2571.
- Yamagata, K. Prevention of cardiovascular disease through modulation of endothelial cell function by dietary seaweed intake. Phytomed. Plus 2021, 2, 100026.
- Murray, M.; Dordevic, A.L.; Ryan, L.; Bonham, M.P. An emerging trend in functional foods for the prevention of cardiovascular disease and diabetes: Marine algal polyphenols. Crit. Rev. Food Sci. Nutr. 2018, 58, 1342–1358.
- Parmenter, B.H.; Croft, K.D.; Hodgson, J.M.; Dalgaard, F.; Bondonno, C.P.; Lewis, J.R.; Cassidy, A.; Scalbert, A.; Bondonno, N.P. An overview and update on the epidemiology of flavonoid intake and cardiovascular disease risk. Food Funct. 2020, 11, 6777–6806.
- Cardoso, S.M.; Pereira, O.R.; Seca, A.M.; Pinto, D.C.; Silva, A. Seaweeds as preventive agents for cardiovascular diseases: From nutrients to functional foods. Mar. Drugs 2015, 13, 6838–6865.
- Sabirin, F.; Soo, K.K.; Ziau, H.S.; Kuen, L.S. Antihypertensive effects of edible brown seaweeds in rats. Int. J. Adv. Appl. Sci. 2016, 3, 103–109.
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216.
- Kim, Y.-G.; Cho, Y.-R.; Park, G.-M.; Won, K.-B.; Ann, S.H.; Yang, D.H.; Kang, J.-W.; Lim, T.-H.; Kim, H.-K.; Choe, J. High-density lipoprotein cholesterol and the risk of obstructive coronary artery disease beyond low-density lipoprotein cholesterol in non-diabetic individuals. Eur. J. Prevent. Cardiol. 2020, 27, 706–714.
- Navar-Boggan, A.M.; Peterson, E.D.; D’Agostino Sr, R.B.; Neely, B.; Sniderman, A.D.; Pencina, M.J. Hyperlipidemia in early adulthood increases long-term risk of coronary heart disease. Circulation 2015, 131, 451–458.
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389.
- Jin, Q.; Yu, H.; Li, P. The evaluation and utilization of marine-derived bioactive compounds with anti-obesity effect. Curr. Med. Chem. 2018, 25, 861–878.
- Villanueva, M.-J.; Morcillo, M.; Tenorio, M.-D.; Mateos-Aparicio, I.; Andrés, V.; Redondo-Cuenca, A. Health-promoting effects in the gut and influence on lipid metabolism of Himanthalia elongata and Gigartina pistillata in hypercholesterolaemic Wistar rats. Eur. Food Res. Technol. 2014, 238, 409–416.
- Kumar, S.A.; Magnusson, M.; Ward, L.C.; Paul, N.A.; Brown, L. Seaweed supplements normalise metabolic, cardiovascular and liver responses in high-carbohydrate, high-fat fed rats. Mar. Drugs 2015, 13, 788–805.
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Antioxidant and hypolipidaemic properties of red seaweed, Gracilaria changii. J. Appl. Phycol. 2014, 26, 987–997.
- Kim, M.J.; Kim, H.K. Insulinotrophic and hypolipidemic effects of Ecklonia cava in streptozotocin-induced diabetic mice. Asian Pac. J. Trop. Med. 2012, 5, 374–379.
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Ali, M.Y.; Choi, J.S. Phlorotannins isolated from the edible brown alga Ecklonia stolonifera exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBPα and PPARγ. Fitoterapia 2014, 92, 260–269.
- Ruqqia, K.; Sultana, V.; Ara, J.; Ehteshamul-Haque, S.; Athar, M. Hypolipidaemic potential of seaweeds in normal, triton-induced and high-fat diet-induced hyperlipidaemic rats. J. Appl. Phycol. 2015, 27, 571–579.
- Cuong, H.D.; Thuy, T.T.; Huong, T.T.; Ly, B.M.; Van, T.T. Structure and hypolipidaemic activity of fucoidan extracted from brown seaweed Sargassum henslowianum. Nat. Prod. Res. 2015, 29, 411–415.
- Sokolova, E.V.; Bogdanovich, L.N.; Ivanova, T.B.; Byankina, A.O.; Kryzhanovskiy, S.P.; Yermak, I.M. Effect of carrageenan food supplement on patients with cardiovascular disease results in normalization of lipid profile and moderate modulation of immunity system markers. PharmaNutrition 2014, 2, 33–37.
- Dousip, A.; Matanjun, P.; Sulaiman, M.R.; Tan, T.S.; Ooi, Y.B.H.; Lim, T.P. Effect of seaweed mixture intake on plasma lipid and antioxidant profile of hyperholesterolaemic rats. J. Appl. Phycol. 2014, 26, 999–1008.
- Borai, I.H.; Ezz, M.K.; Rizk, M.Z.; Matloub, A.; Aly, H.; El, A.; Farrag, R.; Fouad, G.I. Hypolipidemic and anti-atherogenic effect of sulphated polysaccharides from the green alga Ulva fasciata. Int. J. Pharm. Sci. Rev. Res. 2015, 31, 1–12.
- Hassan, S.; El-Twab, S.A.; Hetta, M.; Mahmoud, B. Improvement of lipid profile and antioxidant of hypercholesterolemic albino rats by polysaccharides extracted from the green alga Ulva lactuca Linnaeus. Saudi J. Biol. Sci. 2011, 18, 333–340.
- Hoang, M.H.; Kim, J.-Y.; Lee, J.H.; You, S.; Lee, S.-J. Antioxidative, hypolipidemic, and anti-inflammatory activities of sulfated polysaccharides from Monostroma nitidum. Food Sci. Biotechnol. 2015, 24, 199–205.
- Park, J.; Yeom, M.; Hahm, D.-H. Fucoidan improves serum lipid levels and atherosclerosis through hepatic SREBP-2-mediated regulation. J. Pharmacol. Sci. 2016, 131, 84–92.
- Woo, M.-N.; Jeon, S.-M.; Kim, H.-J.; Lee, M.-K.; Shin, S.-K.; Shin, Y.C.; Park, Y.-B.; Choi, M.-S. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem.-Biol. Interact. 2010, 186, 316–322.
- Jiménez-Escrig, A.; Sánchez-Muniz, F.J. Dietary fibre from edible seaweeds: Chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutr. Res. 2000, 20, 585–598.
- Patil, N.P.; Le, V.; Sligar, A.D.; Mei, L.; Chavarria, D.; Yang, E.Y.; Baker, A.B. Algal polysaccharides as therapeutic agents for atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 153.
- Bhakuni, D.S.; Silva, M. Biodynamic substances from marine flora. Bot. Mar. 1974, 17, 40–51.
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636.
- Alam, M.N.; Hossain, M.M.; Rahman, M.M.; Subhan, N.; Mamun, M.A.A.; Ulla, A.; Reza, H.M.; Alam, M.A. Astaxanthin prevented oxidative stress in heart and kidneys of isoproterenol-administered aged rats. J. Diet. Suppl. 2018, 15, 42–54.
- Zhao, Z.-W.; Cai, W.; Lin, Y.-L.; Lin, Q.-F.; Jiang, Q.; Lin, Z.; Chen, L.-L. Ameliorative effect of astaxanthin on endothelial dysfunction in streptozotocin-induced diabetes in male rats. Arzneimittelforschung 2011, 61, 239–246.
- Lee, S.-H.; Han, J.-S.; Heo, S.-J.; Hwang, J.-Y.; Jeon, Y.-J. Protective effects of dieckol isolated from Ecklonia cava against high glucose-induced oxidative stress in human umbilical vein endothelial cells. Toxicol. In Vitro 2010, 24, 375–381.
- Kim, T.H.; Lee, T.; Ku, S.-K.; Bae, J.-S. Vascular barrier protective effects of eckol and its derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 3710–3712.
- Lu, Y.A.; Jiang, Y.; Yang, H.W.; Hwang, J.; Jeon, Y.J.; Ryu, B. Diphlorethohydroxycarmalol Isolated from Ishige okamurae Exerts Vasodilatory Effects via Calcium Signaling and PI3K/Akt/eNOS Pathway. Int. J. Mol. Sci. 2021, 22, 1610.
- Lekshmi, V.S.; Kurup, G.M. Sulfated polysaccharides from the edible marine algae Padina tetrastromatica protects heart by ameliorating hyperlipidemia, endothelial dysfunction and inflammation in isoproterenol induced experimental myocardial infarction. J. Funct. Foods 2019, 54, 22–31.




