
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Iwona Jarocka-Karpowicz | + 2469 word(s) | 2469 | 2021-08-10 11:22:54 | | | |
| 2 | Lindsay Dong | Meta information modification | 2469 | 2021-09-28 03:43:52 | | |
Video Upload Options
The main representatives of jasmonate compounds include jasmonic acid and its derivatives, mainly methyl jasmonate. Extracts from plants rich in jasmonic compounds show a broad spectrum of activity, i.e., anti-cancer, anti-inflammatory and cosmetic. Studies of the biological activity of jasmonic acid and its derivatives in mammals are based on their structural similarity to prostaglandins and the compounds can be used as natural therapeutics for inflammation. Jasmonates also constitute a potential group of anti-cancer drugs that can be used alone or in combination with other known chemotherapeutic agents.
1. Jasmonate Compounds in Plants
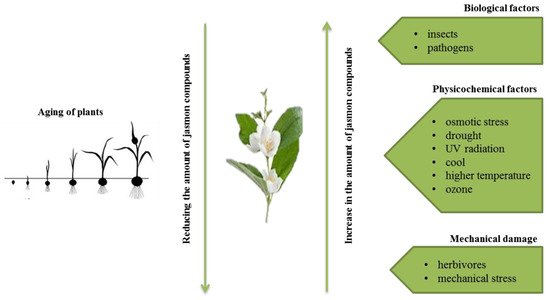
Jasmonates are lipid derivatives (cyclic derivatives of unsaturated fatty acids) that belong to the group of plant growth regulators, which do not have a complex chemical structure [1]. The best known compounds belonging to the group are jasmonic acid (JA) and its methyl ester–methyl jasmonate (MJ) [2]. Jasmonic acid was first isolated from filtrates of the fungus Lasiodiplodia theobromae [3]. Its methyl derivative, however, was the first compound from the large group of jasmonates isolated from the essential oils of Jasminum grandiflorurm [4] and Rosmarinum officinalis [5].
The presence of jasmon compounds has been confirmed in almost all types of tissues of higher plants, i.e., flowering plants, bryophytes, and ferns. They are present, among others, in stems (combinations with amino acids), roots, tubers, leaves (combinations with amino acids; isoleucine or valine), flowers (conjugates with phenylalanine, tryptophan, and tyrosine), fruits (conjugates with isoleucine), and flower pollen [6]. Jasmonates are also components of essential oils and give fragrance to many flowers (e.g., jasmine) and fruits (e.g., apples).
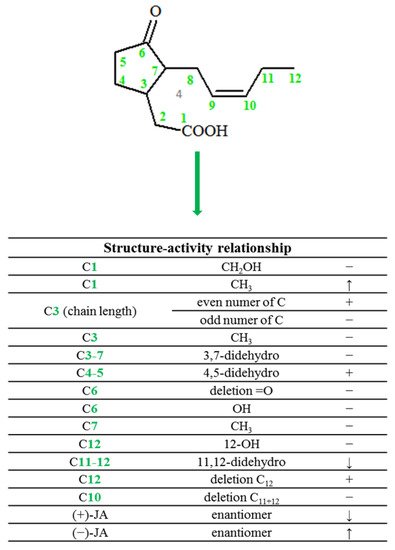
2. Chemical Structure of Jasmonate
In plants, jasmonic acid exists in the following forms: (-)-JA and (+)-epi-JA. Due to the fact that cis stereoisomers are thermodynamically less stable, they epimerize at the C-7 atom to the stable trans form, which at the same time shows higher biological activity (Figure 2).
3. Biological Activity of Jasmonates and Their Derivatives
3.1. Anti-Inflammatory
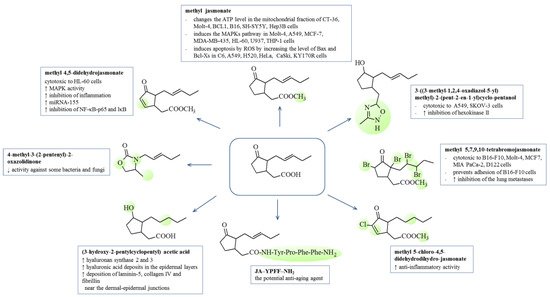
| Jasmonates Derivatives | Cells | Concentration/IC50 | References |
|---|---|---|---|
| Methyl jasmonate | RAW264.7 (macrophage) |
50 and 100 μM | [10] |
| methyl 4,5-didehydrojasmonate DHJM | RAW264.7 (macrophage) |
6.25, 12.5, 25, and 50 μM |
[14] |
| methyl 5,7,9,10-tetrabromojasmonate | melanoma cells B16-F10 |
0.042 mM | [15] |
| methyl 5-chloro-4,5-didehydrodihydro-jasmonate | RAW264.7 (macrophage) |
12.5 and 20 µM | [10] |
| t-butyl 5-chloro-4,5-didehydrodihydro-jasmonate | RAW264.7 (macrophage) |
3.12, 6.25, 12.5 and 25 μM |
[13] |
| 3-((3-methyl-1,2,4-oxadiazol-5-yl) methyl)-2-(pent-2-en-1-yl)cyclo-pentanol | A549 SKOV-3 |
4564 mM 6077 mM |
[16] |
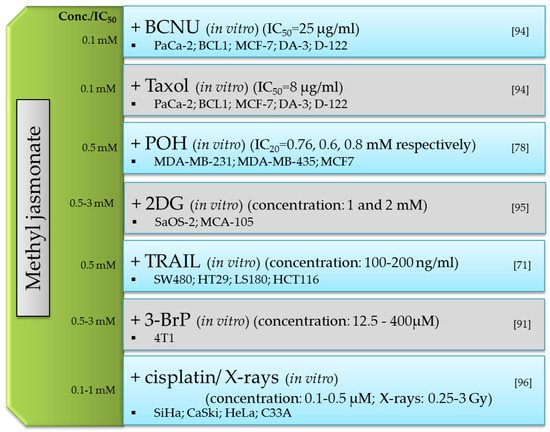
3.2. Anticancer
| MJ-Mechanism of Anticancer Action | Cancer Cells | MJ IC50 |
Literature |
|---|---|---|---|
| lymphoma B | 2 mM | [18] | |
| bioenergy involving ATP depletion via mitochondrial disturbance |
mouse colon cancer CT-26 | 3 mM (max conc) | [19] |
| human T-lymphoblastic leucemia cell line MOLT-4 | 3 mM (max conc) | [19] | |
| mouse leucemia BCL1 | 3 mM (max conc) | [19] | |
| mouse melanoma B16 | 2.6 mM | [15] | |
| hepatocellular carcinoma HCC (LM3, BEL-7402, Hep3B, SMMC-7721) | 1.65 mM | [5] | |
| neuroblastoma SH-SY5Y | 3 mM (max conc) | [20] | |
| liver cancer Hep3B | 0.5 mM | [21] | |
| induction of re-differentiation by activation of the MAPK kinase cascade |
human T-lymphoblastic leucemia cell line MOLT-4 | 0.5 mM | [21] |
| lung cancer A549 | 4.937 mM | [22] | |
| human breast cancer MCF-7 | 2 mM | [23] | |
| human melanocytic MDA-MB-435 | 1.9 mM | [24] | |
| leukemia HL-60 | 0.4 mM | [25] | |
| induction of apoptosis by the generation of ROS |
glioblastoma C6 | 5 mM | [26] |
| non-small cell lung cancer A549 i H520 | 2 mM and 2.5 mM | [24] | |
| cervical carcinoma HeLa, CaSki, SiHa i C33A | 3.0 mM, 2.2 mM, 3.3 mM and 1.7 mM | [27] | |
| prostate cancer PC-3 | 5 mM | [28] |
3.3. Cosmetic Activities
References
- Saniewski, M. The Role of Jasmonates in Ethylene Biosynthesis. In Biology and Biotechnology of the Plant Hormone Ethylene; Kanellis, A.K., Chang, C., Kende, H., Grierson, D., Eds.; NATO ASI Series; Springer: Dordrecht, The Netherlands, 1997; pp. 39–45. ISBN 978-94-011-5546-5.
- Ho, T.-T.; Murthy, H.N.; Park, S.-Y. Methyl Jasmonate Induced Oxidative Stress and Accumulation of Secondary Metabolites in Plant Cell and Organ Cultures. Int. J. Mol. Sci. 2020, 21, 716.
- Félix, C.; Salvatore, M.M.; DellaGreca, M.; Meneses, R.; Duarte, A.S.; Salvatore, F.; Naviglio, D.; Gallo, M.; Jorrín-Novo, J.V.; Alves, A.; et al. Production of Toxic Metabolites by Two Strains of Lasiodiplodia Theobromae, Isolated from a Coconut Tree and a Human Patient. Mycologia 2018, 110, 642–653.
- Demole, E.; Lederer, E.; Mercier, D. Isolement et Détermination de La Structure Du Jasmonate de Méthyle, Constituant Odorant Caractéristique de l’essence de Jasmin. Helv. Chim. Acta 1962, 45, 675–685.
- Cesari, I.M.; Carvalho, E.; Figueiredo Rodrigues, M.; Mendonça, B.D.S.; Amôedo, N.D.; Rumjanek, F.D. Methyl Jasmonate: Putative Mechanisms of Action on Cancer Cells Cycle, Metabolism, and Apoptosis. Int. J. Cell Biol. 2014, 2014, 572097.
- Wilmowicz, E.; Frankowski, K.; Sidłowska, M.; Kućko, A.; Kesy, J.; Gasiorowski, A.; Glazińska, P.; Kopcewicz, J. Jasmonate biosynthesis--the latest discoveries. Postepy Biochem. 2012, 58, 26–33.
- Ghasemi Pirbalouti, A.; Sajjadi, S.E.; Parang, K. A Review (Research and Patents) on Jasmonic Acid and Its Derivatives. Arch. Pharm. 2014, 347, 229–239.
- Wasternack, C. Jasmonates: An Update on Biosynthesis, Signal Transduction and Action in Plant Stress Response, Growth and Development. Ann. Bot. 2007, 100, 681–697.
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446.
- Dang, H.T.; Lee, H.J.; Yoo, E.S.; Hong, J.; Bao, B.; Choi, J.S.; Jung, J.H. New Jasmonate Analogues as Potential Anti-Inflammatory Agents. Bioorg. Med. Chem. 2008, 16, 10228–10235.
- Jarocka-Karpowicz, I.; Markowska, A. Jasmonate Compounds and Their Derivatives in the Regulation of the Neoplastic Processes. Molecules 2021, 26, 2901.
- Ishii, Y.; Kiyota, H.; Sakai, S.; Honma, Y. Induction of Differentiation of Human Myeloid Leukemia Cells by Jasmonates, Plant Hormones. Leukemia 2004, 18, 1413–1419.
- Dang, H.T.; Lee, Y.M.; Kang, G.J.; Yoo, E.S.; Hong, J.; Lee, S.M.; Lee, S.K.; Pyee, Y.; Chung, H.-J.; Moon, H.R.; et al. In Vitro Stability and in Vivo Anti-Inflammatory Efficacy of Synthetic Jasmonates. Bioorg. Med. Chem. 2012, 20, 4109–4116.
- Lee, H.-J.; Maeng, K.; Dang, H.-T.; Kang, G.-J.; Ryou, C.; Jung, J.H.; Kang, H.-K.; Prchal, J.T.; Yoo, E.-S.; Yoon, D. Anti-Inflammatory Effect of Methyl Dehydrojasmonate (J2) Is Mediated by the NF-ΚB Pathway. J. Mol. Med. Berl. Ger. 2011, 89, 83–90.
- Reischer, D.; Heyfets, A.; Shimony, S.; Nordenberg, J.; Kashman, Y.; Flescher, E. Effects of Natural and Novel Synthetic Jasmonates in Experimental Metastatic Melanoma. Br. J. Pharmacol. 2007, 150, 738–749.
- Sucu, B.O.; Ipek, O.S.; Kurtulus, S.O.; Yazici, B.E.; Karakas, N.; Guzel, M. Synthesis of Novel Methyl Jasmonate Derivatives and Evaluation of Their Biological Activity in Various Cancer Cell Lines. Bioorganic Chem. 2019, 91, 103146.
- Flescher, E. Jasmonates in Cancer Therapy. Cancer Lett. 2007, 245, 1–10.
- Fingrut, O.; Reischer, D.; Rotem, R.; Goldin, N.; Altboum, I.; Zan-Bar, I.; Flescher, E. Jasmonates Induce Nonapoptotic Death in High-Resistance Mutant P53-Expressing B-Lymphoma Cells. Br. J. Pharmacol. 2005, 146, 800–808.
- Goldin, N.; Arzoine, L.; Heyfets, A.; Israelson, A.; Zaslavsky, Z.; Bravman, T.; Bronner, V.; Notcovich, A.; Shoshan-Barmatz, V.; Flescher, E. Methyl Jasmonate Binds to and Detaches Mitochondria-Bound Hexokinase. Oncogene 2008, 27, 4636–4643.
- Tong, Q.-S.; Jiang, G.-S.; Zheng, L.-D.; Tang, S.-T.; Cai, J.-B.; Liu, Y.; Zeng, F.-Q.; Dong, J.-H. Methyl Jasmonate Downregulates Expression of Proliferating Cell Nuclear Antigen and Induces Apoptosis in Human Neuroblastoma Cell Lines. Anticancer. Drugs 2008, 19, 573–581.
- Rotem, R.; Heyfets, A.; Fingrut, O.; Blickstein, D.; Shaklai, M.; Flescher, E. Jasmonates: Novel Anticancer Agents Acting Directly and Selectively on Human Cancer Cell Mitochondria. Cancer Res. 2005, 65, 1984–1993.
- Kim, J.H.; Lee, S.Y.; Oh, S.Y.; Han, S.I.; Park, H.G.; Yoo, M.-A.; Kang, H.S. Methyl Jasmonate Induces Apoptosis through Induction of Bax/Bcl-XS and Activation of Caspase-3 via ROS Production in A549 Cells. Oncol. Rep. 2004, 12, 1233–1238.
- Yeruva, L.; Elegbede, J.A.; Carper, S.W. Methyl Jasmonate Decreases Membrane Fluidity and Induces Apoptosis through Tumor Necrosis Factor Receptor 1 in Breast Cancer Cells. Anticancer. Drugs 2008, 19, 766–776.
- Yeruva, L.; Hall, C.; Elegbede, J.A.; Carper, S.W. Perillyl Alcohol and Methyl Jasmonate Sensitize Cancer Cells to Cisplatin. Anticancer. Drugs 2010, 21, 1–9.
- Cohen, S.; Flescher, E. Methyl Jasmonate: A Plant Stress Hormone as an Anti-Cancer Drug. Phytochemistry 2009, 70, 1600–1609.
- Oh, S.Y.; Kim, J.H.; Park, M.J.; Kim, S.M.; Yoon, C.S.; Joo, Y.M.; Park, J.S.; Han, S.I.; Park, H.G.; Kang, H.S. Induction of Heat Shock Protein 72 in C6 Glioma Cells by Methyl Jasmonate through ROS-Dependent Heat Shock Factor 1 Activation. Int. J. Mol. Med. 2005, 16, 833–839.
- Kniazhanski, T.; Jackman, A.; Heyfets, A.; Gonen, P.; Flescher, E.; Sherman, L. Methyl Jasmonate Induces Cell Death with Mixed Characteristics of Apoptosis and Necrosis in Cervical Cancer Cells. Cancer Lett. 2008, 271, 34–46.
- Jiang, G.; Zhao, J.; Xiao, X.; Tao, D.; Gu, C.; Tong, Q.; Luo, B.; Wang, L.; Zeng, F. AN N-Terminal Smac Peptide Sensitizes Human Prostate Carcinoma Cells to Methyl Jasmonate-Induced Apoptosis. Cancer Lett. 2011, 302, 37–46.
- Bustamante, E.; Pedersen, P.L. High Aerobic Glycolysis of Rat Hepatoma Cells in Culture: Role of Mitochondrial Hexokinase. Proc. Natl. Acad. Sci. USA 1977, 74, 3735–3739.
- Raviv, Z.; Cohen, S.; Reischer-Pelech, D. The Anti-Cancer Activities of Jasmonates. Cancer Chemother. Pharmacol. 2013, 71, 275–285.
- Li, J.; Chen, K.; Wang, F.; Dai, W.; Li, S.; Feng, J.; Wu, L.; Liu, T.; Xu, S.; Xia, Y.; et al. Methyl Jasmonate Leads to Necrosis and Apoptosis in Hepatocellular Carcinoma Cells via Inhibition of Glycolysis and Represses Tumor Growth Mice. Oncotarget 2017, 8, 45965–45980.
- Francisco-Marquez, M.; Galano, A. The Reactions of Plant Hormones with Reactive Oxygen Species: Chemical Insights at a Molecular Level. J. Mol. Model. 2018, 24, 255.
- Besson, J.C.F.; de Carvalho Picoli, C.; Matioli, G.; Natali, M.R.M. Methyl Jasmonate: A Phytohormone with Potential for the Treatment of Inflammatory Bowel Diseases. J. Pharm. Pharmacol. 2018, 70, 178–190.
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2019, 10, 1614.
- Ezekwudo, D.; Shashidharamurthy, R.; Devineni, D.; Bozeman, E.; Palaniappan, R.; Selvaraj, P. Inhibition of Expression of Anti-Apoptotic Protein Bcl-2 and Induction of Cell Death in Radioresistant Human Prostate Adenocarcinoma Cell Line (PC-3) by Methyl Jasmonate. Cancer Lett. 2008, 270, 277–285.
- Zhang, M.; Su, L.; Xiao, Z.; Liu, X.; Liu, X. Methyl Jasmonate Induces Apoptosis and Pro-Apoptotic Autophagy via the ROS Pathway in Human Non-Small Cell Lung Cancer. Am. J. Cancer Res. 2016, 6, 187–199.
- Škubník, J.; Pavlíčková, V.; Ruml, T.; Rimpelová, S. Current Perspectives on Taxanes: Focus on Their Bioactivity, Delivery and Combination Therapy. Plants Basel Switz. 2021, 10, 569.
- Yousefi, S.; Darvishi, P.; Yousefi, Z.; Pourfathollah, A.A. Effect of Methyl Jasmonate and 3-Bromopyruvate Combination Therapy on Mice Bearing the 4 T1 Breast Cancer Cell Line. J. Bioenerg. Biomembr. 2020, 52, 103–111.
- Cai, S.; Xu, Y.; Cooper, R.J.; Ferkowicz, M.J.; Hartwell, J.R.; Pollok, K.E.; Kelley, M.R. Mitochondrial Targeting of Human O6-Methylguanine DNA Methyltransferase Protects against Cell Killing by Chemotherapeutic Alkylating Agents. Cancer Res. 2005, 65, 3319–3327.
- Wang, X.; Tournier, C. Regulation of Cellular Functions by the ERK5 Signalling Pathway. Cell. Signal. 2006, 18, 753–760.
- Heyfets, A.; Flescher, E. Cooperative Cytotoxicity of Methyl Jasmonate with Anti-Cancer Drugs and 2-Deoxy-D-Glucose. Cancer Lett. 2007, 250, 300–310.
- Elia, U.; Flescher, E. PI3K/Akt Pathway Activation Attenuates the Cytotoxic Effect of Methyl Jasmonate toward Sarcoma Cells. Neoplasia N. Y. 2008, 10, 1303–1313.
- Milrot, E.; Jackman, A.; Flescher, E.; Gonen, P.; Kelson, I.; Keisari, Y.; Sherman, L. Enhanced Killing of Cervical Cancer Cells by Combinations of Methyl Jasmonate with Cisplatin, X or Alpha Radiation. Invest. New Drugs 2013, 31, 333–344.
- Kapuścińska, A.; Nowak, I. Wykorzystanie Wybranych Fitohormonów w Przemyśle Kosmetycznym i Farmaceutycznym; Rośliny–przegląd Wybranych Zagadnień: Lublin, Poland, 2016; pp. 160–174. ISBN 978-83-65598-13-4.
- Scognamiglio, J.; Jones, L.; Letizia, C.S.; Api, A.M. Fragrance Material Review on Methyl Jasmonate. Food Chem. Toxicol. 2012, 50, S572–S576.
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and Cancer. Ann. Afr. Med. 2019, 18, 121–126.
- Pawełczyk, A.; Zaprutko, L. Microwave Assisted Synthesis of Unsaturated Jasmone Heterocyclic Analogues as New Fragrant Substances. Eur. J. Med. Chem. 2009, 44, 3032–3039.
- Pawełczyk, A.; Sowa-Kasprzak, K.; Michalak, J.; Kędzia, B.; Zaprutko, L. Ocena Aktywności Antybiotycznej Z-Jasmonu Oraz Jego Pochodnych Heterocyklicznych. Postępy Fitoter. 2017, 18, 171–177.




