The main representatives of jasmonate compounds include jasmonic acid and its derivatives, mainly methyl jasmonate. Extracts from plants rich in jasmonic compounds show a broad spectrum of activity, i.e., anti-cancer, anti-inflammatory and cosmetic. Studies of the biological activity of jasmonic acid and its derivatives in mammals are based on their structural similarity to prostaglandins and the compounds can be used as natural therapeutics for inflammation. Jasmonates also constitute a potential group of anti-cancer drugs that can be used alone or in combination with other known chemotherapeutic agents.
- jasmonic acid
- methyl jasmonate
- anti-inflammation
- anti-cancer
- anti-aging
1. Jasmonate Compounds in Plants
Jasmonates are lipid derivatives (cyclic derivatives of unsaturated fatty acids) that belong to the group of plant growth regulators, which do not have a complex chemical structure [1]. The best known compounds belonging to the group are jasmonic acid (JA) and its methyl ester–methyl jasmonate (MJ) [2]. Jasmonic acid was first isolated from filtrates of the fungus Lasiodiplodia theobromae [3]. Its methyl derivative, however, was the first compound from the large group of jasmonates isolated from the essential oils of Jasminum grandiflorurm [4] and Rosmarinum officinalis [5].
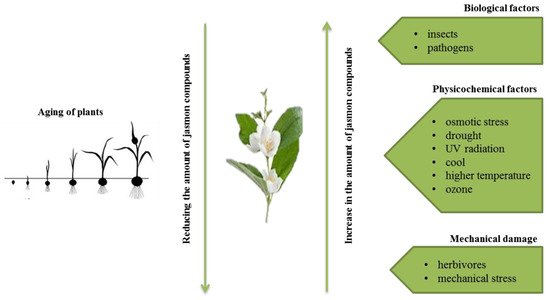
The presence of jasmon compounds has been confirmed in almost all types of tissues of higher plants, i.e., flowering plants, bryophytes, and ferns. They are present, among others, in stems (combinations with amino acids), roots, tubers, leaves (combinations with amino acids; isoleucine or valine), flowers (conjugates with phenylalanine, tryptophan, and tyrosine), fruits (conjugates with isoleucine), and flower pollen [6]. Jasmonates are also components of essential oils and give fragrance to many flowers (e.g., jasmine) and fruits (e.g., apples).
2. Chemical Structure of Jasmonate
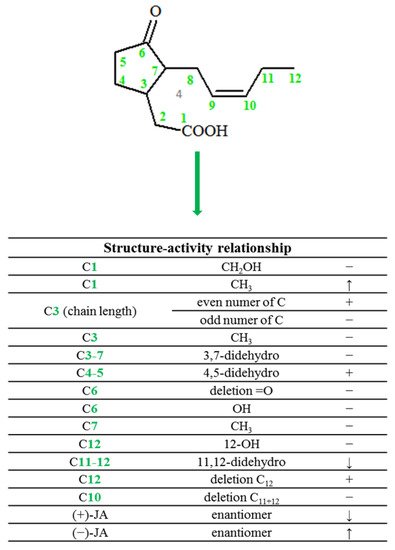
In plants, jasmonic acid exists in the following forms: (-)-JA and (+)-epi-JA. Due to the fact that cis stereoisomers are thermodynamically less stable, they epimerize at the C-7 atom to the stable trans form, which at the same time shows higher biological activity (Figure 2).
37. Biological Activity of Jasmonates and Their Derivatives
3.1. Anti-Inflammatory
7.1. Anti-Inflammatory
| Jasmonates Derivatives | Cells | Concentration/IC | 50 | References |
|---|---|---|---|---|
| Methyl jasmonate | RAW264.7 (macrophage) |
50 and 100 μM | [10] | [65] |
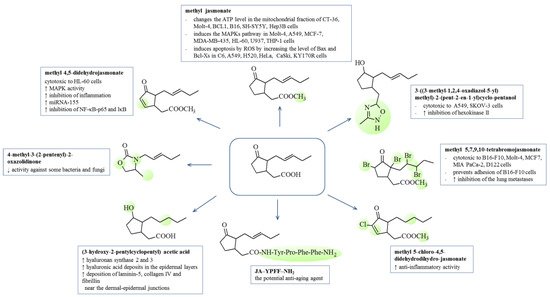
| methyl 4,5-didehydrojasmonate DHJM | RAW264.7 (macrophage) |
6.25, 12.5, 25, and 50 μM |
[14] | [68] |
| methyl 5,7,9,10-tetrabromojasmonate | melanoma cells B16-F10 |
0.042 mM | [15] | [69] |
| methyl 5-chloro-4,5-didehydrodihydro-jasmonate | RAW264.7 (macrophage) |
12.5 and 20 µM | [10] | [65] |
| t-butyl 5-chloro-4,5-didehydrodihydro-jasmonate | RAW264.7 (macrophage) |
3.12, 6.25, 12.5 and 25 μM |
[13] | [67] |
| 3-((3-methyl-1,2,4-oxadiazol-5-yl) methyl)-2-(pent-2-en-1-yl)cyclo-pentanol | A549 SKOV-3 |
4564 mM 6077 mM |
[16] | [70] |
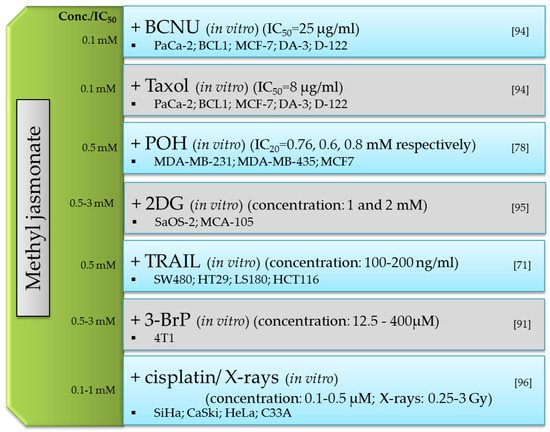
3.2. Anticancer
7.2. Anticancer
| MJ-Mechanism of Anticancer Action | Cancer Cells | MJ IC | 50 | Literature |
|---|---|---|---|---|
| lymphoma B | 2 mM | [18] | [72] | |
| bioenergy involving ATP depletion via mitochondrial disturbance |
mouse colon cancer CT-26 | 3 mM (max conc) | [19] | [73] |
| human T-lymphoblastic leucemia cell line MOLT-4 | 3 mM (max conc) | [19] | [73] | |
| mouse leucemia BCL1 | 3 mM (max conc) | [19] | [73] | |
| mouse melanoma B16 | 2.6 mM | [15] | [69] | |
| hepatocellular carcinoma HCC (LM3, BEL-7402, Hep3B, SMMC-7721) | 1.65 mM | [5] | ||
| neuroblastoma SH-SY5Y | 3 mM (max conc) | [20] | [74] | |
| liver cancer Hep3B | 0.5 mM | [21] | [75] | |
| induction of re-differentiation by activation of the MAPK kinase cascade |
human T-lymphoblastic leucemia cell line MOLT-4 | 0.5 mM | [21] | [75] |
| lung cancer A549 | 4.937 mM | [22] | [76] | |
| human breast cancer MCF-7 | 2 mM | [23] | [77] | |
| human melanocytic MDA-MB-435 | 1.9 mM | [24] | [78] | |
| leukemia HL-60 | 0.4 mM | [25] | [79] | |
| induction of apoptosis by the generation of ROS |
glioblastoma C6 | 5 mM | [26] | [80] |
| non-small cell lung cancer A549 i H520 | 2 mM and 2.5 mM | [24] | [78] | |
| cervical carcinoma HeLa, CaSki, SiHa i C33A | 3.0 mM, 2.2 mM, 3.3 mM and 1.7 mM | [27] | [81] | |
| prostate cancer PC-3 | 5 mM | [28] | [82] |