
Video Upload Options
Capsule-based dry powder inhalers (cDPIs) are widely utilized in the delivery of pharmaceutical powders to the lungs. In these systems, the fundamental nature of the interactions between the drug/formulation powder, the capsules, the inhaler device, and the patient must be fully elucidated in order to develop robust manufacturing procedures and provide reproducible lung deposition of the drug payload.
1. Introduction
Dry powder inhalers (DPIs) are widely utilized for the treatment of multiple lung diseases including asthma [1], chronic obstructive pulmonary disorder (COPD) [2], cystic fibrosis (CF) [3], and CF-related Pseudomonas aeruginosa infections [4], virus-related lung infections [5] and systemic diseases like diabetes [6]. While various dose metering systems have been developed for DPIs, including blisters or reservoir-based devices [7], capsule-based DPIs (cDPIs) remain an important system for the therapeutic delivery of inhaled powders, with half of all DPIs on the market using this dose metering mechanism [8]. cDPIs have been shown to provide accurate and consistent drug delivery [9] with multiple patient feedback mechanisms (e.g., visual, auditory) to assure that the dose was delivered [10].
The successful delivery of therapeutics from cDPI delivery systems involves a complex interplay of factors associated with the powder formulation, the formulation-capsule, and the device-capsule interactions. Consistency and predictability of the delivered dose to the patient are of great importance in product development and manufacturing of new chemical entities (NCE) or generic products. Demonstrating bioavailability and/or bioequivalence of DPI products remains an important product attribute for drug approval by the regulatory authorities [11]. Apart from pharmacologic reasons, failures in the development of NCE or generic inhaled therapies can stem from either a lack of understanding about aspects of the drug/formulation powder [12], device [13][14][15], and/or the mechanisms by which they interact. Several reviews have been published on DPI formulation design [16] or engineering strategy [17] as well as how the design [18] and characteristics [19] of DPI devices affect powder aerosolization performance revealing the multifactorial challenge of DPI products. This review seeks to expand these previously published analyses by providing insights into the critical material attributes (CMA) and critical process parameters (CPP) and hence critical quality criteria (CQA) to be considered for cDPI development and manufacture ( Figure 1 ). Especially it relates to the inclusion of the capsule and device component as well as its metering system in order to provide further guidance on a QbD approach for the development of NCE or generic cDPI products.
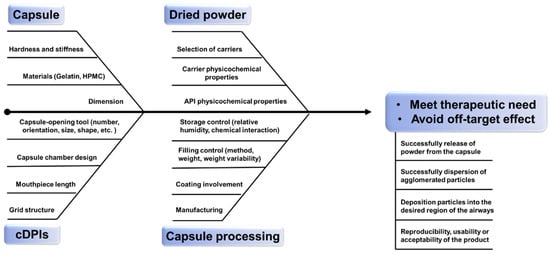
2. Overview of Quality by Design Approach for cDPIs
As defined by the International Council for Harmonization’s Q8R2 guideline, QbD is a “systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management” (ICH Q8R2) [20]. A QbD approach is intended to generate sufficient product and process understanding that the robustness of the manufacturing process and the reproducibility of the clinical performance of the final drug product is assured. The basis of a typical QbD approach involves a clearly defined quality target product profile (QTPP), followed by a risk assessment in order to identify potential CQAs, CMAs, and CPPs of the process or product. The QTPP provides a comprehensive summary of all the required targets that will ensure the quality, safety, and efficacy of a specific product for the patient. The CQAs are the properties or characteristics of the product that should be within an appropriate limit, range, or distribution to ensure the desired product quality and performance (ICH Q8) [21]. The CMAs of the input materials and their properties can be identified, optimized, and controlled to ensure the desired quality of output materials [21]. A process parameter whose variability has an impact on a CQA and therefore should be monitored or controlled is termed as CPPs (ICH Q8) [21]. In addition, a number of design of experiments (DoEs) are performed to further delineate the design space and associated control strategies [22].
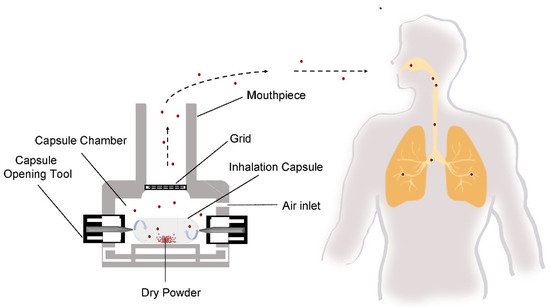
The delivery of drugs to the lungs via cDPI carries a unique set of quality and performance criteria that are unique to the pulmonary route of delivery. Depending upon the therapeutic indication, cDPIs may be utilized for the delivery of a variety of formulation systems including a binary mixture of large carrier particles and micronized drug particles, a carrier-free, high-dose (>5–10 mg) drug formulations [23], or engineered particle using process technologies like spray drying [24]. Successful delivery of the drug to the lungs via cDPI involves the completion of several steps ( Figure 2 ), including (1) capsule opening or piercing by the device, (2) release of powder from the capsule, (3) entrainment in the airflow, (4) dispersion of deaggregation of particles into primary particles or separation of carrier particles and drug particles, and (5) deposition of the drug particles into the desired region of the airways. The reproducibility of each of these steps and subsequently the reproducibly of the delivered dose and therapeutic effect is dependent upon patient-associated factors, such as inspiratory force or correct actuation of the device, as well as product factors like the initial raw material properties of the formulation components (e.g., particle shape, size, surface properties, crystallinity, moisture content of the excipients and drug) and capsule, the processing approach (e.g., milling, blending, spray drying, capsule filling), and the packaging and storage of the cDPI product.
Essentially, the QTPP of cDPIs requires that the therapeutic need of the patient be met while avoiding off-target drug effects or toxicities. It is well known that lung deposition of therapeutic particles is challenging and depends upon many factors including drug formulation factors, device, patient parameters, and also the disease state. The requirement for systemic or targeted/topical therapeutic effect must also be considered with regard for the desired location of drug deposition. In case cDPIs are being used to achieve systemic therapeutic effects, it is likely to require deposition in the large-surface area, capillarity-rich region of the alveoli [25]. In fixed-dose combination therapy cases, where a synergistic effect is required the deposition efficiency and location of each drug in the formulation are even more important [26].
The approach to the pharmaceutical development described by the ICH Q8 (R2) guideline states that: “In all cases, the product should be designed to meet patients’ needs and the intended product performance.” Consequently, in addition to pharmacologic-based adverse drug reactions or toxicities, any other factor that reduces the usability or acceptability of the product by the patient should be considered [27]. For example, detachment of capsule pieces and subsequent inhalation by the patient may result in fragment deposition in the throat [28]. Variability in aerosol performance may result in unintended off-target effects of potent drugs, as noted in the case of inhaled insulin [29] or gene therapy agents [30]. Likewise, an unexpected increase in oropharyngeal deposition of drugs that are intended for lung-targeted therapeutic effects may result in the occurrence of systemic side effects [31].
3. Critical Material Attributes (CMAs) in cDPIs
A study from Telko et al. used a statistical experimental design to look specifically at capsule material type effects on powder charging, which is thought to correlate with aerosol performance by affecting powder detachment from surfaces of capsule walls [32]. The authors determined the choice of the capsule (gelatin vs. HPMC) has a large effect on the polarity of the charge but only a minor effect on the magnitude of the charge from the powders. They found the use of HPMC-based capsules led to a higher triboelectric charging of the powder than gelatin-based capsules. However, it has been observed in other studies that a higher potential for triboelectrification was observed in gelatin capsules when compared to HPMC capsules [33], and therefore this effect should be further evaluated and might depend much more on the moisture content of the shells and the formulation itself.
Capsule dimensions are based on their respective fill capacities, which in turn are based on the tapped density of the dry powder [34]. Capsule dimension is represented by a numerical size assignment, with larger numerical sizes representing a smaller internal capsule volume that ranges numerically from sizes 000 (the largest size) to 5 (the smallest size) [35]. While capsule size 3 is the standard cDPI capsules, larger and smaller capsule sizes are being evaluated for high-dose drugs or potent APIs. It should be noted that even though the capsule sizes are standardized, capsule dimensions can vary between suppliers. Especially, the close length of the capsule might be important for the piercing performance as well as the movements within the capsule chamber. In this respect, it needs to be assured that the capsules are closed properly and provide sufficient closing strength preventing reopening when pierced at the hemispherical ends. When considering general capsule motion from within a fixed capsule chamber, the smaller capsules may be thought to have more free space and thus can move freely in a turbulent fluid flow. Additionally, there is a long distance for the capsule to travel to impact the chamber walls, which may reduce the number of possible collisions in a given time compared to larger capsules that fit more squarely inside the device chamber. This was illustrated in a study performed by Coates et al., in which the overall levels of turbulence within the device were found to diminish with the increase in capsule size [36]. The higher frequency of collisions noted with the larger capsule sizes resulted in increased device powder retention after inhalation. However, while smaller capsules may demonstrate the release of dry powder from the device, this must be balanced with the reduced carrying capacity of the capsule, which may require an increased number of doses loading and inhalation maneuvers by the patient for a therapeutic effect to be achieved.
The weight ranges of capsules must be narrower for inhalation than standard powder filling due to the low fill weights and potential rejections on the high-speed encapsulators [37]. And the microbial limits on inhalation capsules must be lower due to the drug product inside being delivered directly to the lungs.
Altogether, the capsule-related CMAs lead to the finding that capsule dimension should balance powder payload and aerosol performance, and the moisture contents of the inhalation capsule need to be controlled within a narrow range.
4. Critical Process Parameters (CPPs) in cDPIs
Differently, Desmond et al., coated the interior surface of the capsule with magnesium stearate (MgSt), which can form stronger Langmuir type films on surfaces potentially sequester the direct contact between formulation powder and capsule [38][39][40][41]. They used two capsule-based DPIs, the Rotahaler ® , and the Aerolizer ® , and found that the retained powder in the capsule largely decreased with a concentration of 0.3 g/mL MgSt in Aerolizer ® and 0.05 g/mL in Rotahaler ® [42]. The enhancement effect on powder emptying was also verified by Srinivas et al., who found that capsules coated with MgSt had a decrease in capsule drug retention compared to uncoated [43]. Interestingly, the same effect was found if using excipient enhanced growth (EEG) [44][45] particles or L-leucine, indicating that any substance with a lubricant nature may reduce capsule retention [43]. Lastly, it was thought theoretically that the particle size distribution of the lubricant used in carrier-based formulations would affect detachment and de-agglomeration and cause fluctuations in capsule retention, however, this did not show in the experiment [43].
It is well known that ambient factors can cause the denaturation of gelatin capsules. This is mostly due to the nature of gelatin, which can have decreases rigidity from undergoing gamma radiation [46], increases brittleness from incompatible solvents [47][48], hygroscopic fill [49], and is incompatible with reducing sugars, plasticizers, and preservatives [50]. In addition, having the proper relative humidity (RH) for storage is of great significance. RH can potentially change the original stiffness or hardness of the capsule [51]. Too low in RH gives rise to capsule brittleness [51], but too high of an RH brings about capsule stickiness [52][53]. Several studies have suggested that there was a correlation between the ambient moisture content and the piercing profile of the capsule in the device. Most notably, a higher penetration force was needed to puncture capsules stored in lower moisture environments for both HPMC and gelatin capsules [54]. Furthermore, Coulman’s study showed a higher percentage of gelatin capsules with a ‘regular’ shaped puncture after removing the angular pin from punctured capsules if stored in desiccators with Mg (NO 3) 2 to create higher moisture contents. But no significant differences have been found between different HPMC capsules [55]. Also, RH within different capsules can play a role in the fluidization and dispersion of powders from within capsules. The formation of liquid bridges between formulation particles as well as the capsule wall results from high RH. These liquid bridges create a binding capillary force that can lead to increased powder retention [56][57][58].
At the aspect of some preloaded capsules, chemical interaction may occur during storage if without consideration of incompatibility between capsule shells material and formulation components. An example is a highly moisture-sensitive drug, salicylic acid, which will degrade at high ambient humidity. One study found that the amount of salicylic acid degradation of salicylic acid was higher when stored in gelatin capsules vs. HPMC capsules at the initial time point (only slightly higher in gelatin than HPMC) and at 3, 6, 12, and 18 months when stored at 25 °C at 60% relative humidity [59]. One explanation for this occurrence is the significantly higher moisture content in gelatin capsules versus HPMC hard capsules. Therefore, in cases where the drug is extremely sensitive to moisture, HPMC-based capsules may provide a better option for storage [33]. Also, when loading drugs that have a high sensitivity to oxidation careful attention needs to be made for capsule selection as HPMC capsules are known to be more permeable to oxygen than gelatin capsules. When comparing two films of HPMC and gelatin, each 100 µm in thickness, the observed oxygen permeation values were 166 cm 3/m 2/day and 3.41 cm 3/m 2/day, respectively. This demonstrates a substantial difference that could ultimately affect the potency of the drug [33]. While this oxygen permeation can be a potential pitfall for the drug product, this effect can be mitigated by utilizing proper packaging and storage of the capsules before delivering the dose (e.g., the capsules can be stored in blister packaging and opened before administration).
This suggests that CPPs for inhalation capsule manufacturing involve several potential factors to be investigated like capsule coating, capsule filling method, capsule filling weight, weight variability, and storage RH and temperature control should be carefully considered in cDPIs product development.
References
- Van der Palen, J.; Klein, J.; Van Herwaarden, C.; Zielhuis, G.; Seydel, E. Multiple inhalers confuse asthma patients. Eur. Respir. J. 1999, 14, 1034–1037.
- Jarvis, S.; Ind, P.W.; Shiner, R.J. Inhaled Therapy in Elderly COPD Patients; Time for Re-Evaluation? Oxford University Press: Oxford, UK, 2007.
- Schuster, A.; Haliburn, C.; Döring, G.; Goldman, M.H.; Group, F.S. Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (Colobreathe DPI) in patients with cystic fibrosis: A randomised study. Thorax 2013, 68, 344–350.
- Ding, L.; Wang, J.; Cai, S.; Smyth, H.; Cui, Z. Pulmonary Biofilm-Based Chronic Infections and Inhaled Treatment Strategies. Int. J. Pharm. 2021, 604, 120768.
- Brunaugh, A.D.; Seo, H.; Warnken, Z.; Ding, L.; Seo, S.H.; Smyth, H.D.C. Development and Evaluation of Inhalable Composite Niclosamide-Lysozyme Particles: A Broad-Spectrum, Patient-Adaptable Treatment for Coronavirus Infections and Sequalae. PLoS ONE 2021, 16, e0246803.
- Klonoff, D.C. Afrezza Inhaled Insulin: The Fastest-Acting FDA-Approved Insulin on the Market Has Favorable Properties; SAGE: Los Angeles, CA, USA, 2014.
- Anderson, P.J. History of aerosol therapy: Liquid nebulization to MDIs to DPIs. Respir. Care 2005, 50, 1139–1150.
- Berkenfeld, K.; Lamprecht, A.; McConville, J.T. Devices for dry powder drug delivery to the lung. AAPS PharmSciTech 2015, 16, 479–490.
- Wauthoz, N.; Hennia, I.; Dejaeger, B.; Ecenarro, S.; Amighi, K. Proposed algorithm for healthcare professionals based on product characteristics and in vitro performances in different use conditions using formoterol-based marketed products for inhalation. Int. J. Pharm. 2017, 530, 415–429.
- Edwards, D. Applications of capsule dosing techniques for use in dry powder inhalers. Ther. Deliv. 2010, 1, 195–201.
- Lawrence, X.Y.; Li, B.V. FDA Bioequivalence Standards; Springer: New York, NY, USA, 2014; Volume 13.
- Weers, J.G.; Miller, D.P. Formulation design of dry powders for inhalation. J. Pharm. Sci. 2015, 104, 3259–3288.
- Donovan, M.J.; Kim, S.H.; Raman, V.; Smyth, H.D. Dry powder inhaler device influence on carrier particle performance. J. Pharm. Sci. 2012, 101, 1097–1107.
- Zhou, Q.T.; Tong, Z.; Tang, P.; Citterio, M.; Yang, R.; Chan, H.-K. Effect of device design on the aerosolization of a carrier-based dry powder inhaler—A case study on Aerolizer® Foradile®. AAPS J. 2013, 15, 511–522.
- Coates, M.S.; Chan, H.-K.; Fletcher, D.F.; Raper, J.A. Influence of air flow on the performance of a dry powder inhaler using computational and experimental analyses. Pharm. Res. 2005, 22, 1445–1453.
- Shetty, N.; Cipolla, D.; Park, H.; Zhou, Q.T. Physical stability of dry powder inhaler formulations. Expert Opin. Drug Deliv. 2020, 17, 77–96.
- de Boer, A.H.; Hagedoorn, P.; Hoppentocht, M.; Buttini, F.; Grasmeijer, F.; Frijlink, H.W. Dry powder inhalation: Past, present and future. Expert Opin. Drug Deliv. 2017, 14, 499–512.
- Levy, M.L.; Carroll, W.; Izquierdo Alonso, J.L.; Keller, C.; Lavorini, F.; Lehtimäki, L. Understanding Dry Powder Inhalers: Key Technical and Patient Preference Attributes. Adv Ther. 2019, 36, 2547–2557.
- Lavorini, F.; Pistolesi, M.; Usmani, O.S. Recent advances in capsule-based dry powder inhaler technology. Multidiscip Respir. Med. 2017, 12, 11.
- Mishra, V.; Thakur, S.; Patil, A.; Shukla, A. Quality by design (QbD) approaches in current pharmaceutical set-up. Expert Opin. Drug Deliv. 2018, 15, 737–758.
- Haleem, R.M.; Salem, M.Y.; Fatahallah, F.A.; Abdelfattah, L.E. Quality in the pharmaceutical industry–A literature review. Saudi Pharm. J. 2015, 23, 463–469.
- Buttini, F.; Rozou, S.; Rossi, A.; Zoumpliou, V.; Rekkas, D.M. The application of quality by design framework in the pharmaceutical development of dry powder inhalers. Eur. J. Pharm. Sci. 2018, 113, 64–76.
- Brunaugh, A.D.; Smyth, H.D. Formulation techniques for high dose dry powders. Int. J. Pharm. 2018, 547, 489–498.
- Seville, P.C.; Li, H.-y.; Learoyd, T.P. Spray-dried powders for pulmonary drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2007, 24, 307–360.
- Mansour, H.M.; Rhee, Y.S.; Wu, X. Nanomedicine in pulmonary delivery. Int. J. Nanomed. 2009, 4, 299–319.
- Brunaugh, A.D.; Sharma, S.; Smyth, H. Inhaled fixed-dose combination powders for the treatment of respiratory infections. Expert Opin. Drug Deliv. 2021, 18, 1–15.
- Dal Negro, R.W.; Turco, P.; Povero, M. Patients’ usability of seven most used dry-powder inhalers in COPD. Multidiscip. Respir. Med. 2019, 14, 1–9.
- Renswouw, D.C.V.; Laarhoven, A.C.V.; Haren, M.J.V.; Bouvy, M.L.; Weda, M. Storage instructions for inhalation capsules: Consequences of incorrect storage and adherence in daily practice. J. Pharm. Pract. 2010, 23, 548–552.
- Perera, A.D.; Kapitza, C.; Nosek, L.; Fishman, R.S.; Shapiro, D.A.; Heise, T.; Heinemann, L. Absorption and metabolic effect of inhaled insulin: Intrapatient variability after inhalation via the Aerodose insulin inhaler in patients with type 2 diabetes. Diabetes Care 2002, 25, 2276–2281.
- Lee, A.Y.; Cho, M.-H.; Kim, S. Recent advances in aerosol gene delivery systems using non-viral vectors for lung cancer therapy. Expert Opin. Drug Deliv. 2019, 16, 757–772.
- Wenzler, E.; Fraidenburg, D.R.; Scardina, T.; Danziger, L.H. Inhaled Antibiotics for Gram-Negative Respiratory Infections. Clin. Microbiol. Rev. 2016, 29, 581–632.
- Telko, M.J.; Kujanpää, J.; Hickey, A.J. Investigation of triboelectric charging in dry powder inhalers using electrical low pressure impactor (ELPI™). Int. J. Pharm. 2007, 336, 352–360.
- Richardson, M. Impact of Capsule Selection on Formulation Stability in Dry Powder Inhalers (DPIs). 2011. Available online: https://www.inhalationmag.com/white-papers/capsugel/ (accessed on 1 April 2011).
- Murachanian, D. An Introduction to Two-Piece Hard Capsules and Their Marketing Benefits. In Pharmaceutical Dosage Forms; CRC Press: London, UK, 2017; pp. 15–30.
- Majee, S.B.; Avlani, D.; Biswas, G. HPMC as capsule shell material: Physicochemical, pharmaceutical and biopharmaceutical properties. Int. J. Pharm. Pharm. Sci. 2017, 9, 1–6.
- Coates, M.S.; Fletcher, D.F.; Chan, H.-K.; Raper, J.A. The role of capsule on the performance of a dry powder inhaler using computational and experimental analyses. Pharm. Res. 2005, 22, 923–932.
- Murachanian, D. Two-piece hard capsules for pharmaceutical formulations. J. GXP Compliance 2010, 14, 31.
- Bolhuis, G. Film formation by magnesium stearate during mixing and its effect on tabletting. Pharm. Weekblad. 1975, 110, 317–325.
- Hussain, M.H.; York, P.; Timmins, P. A study of the formation of magnesium stearate film on sodium chloride using energy-dispersive X-ray analysis. Int. J. Pharm. 1988, 42, 89–95.
- Uzunović, A.; Vranić, E. Effect of magnesium stearate concentration on dissolution properties of ranitidine hydrochloride coated tablets. Bosn. J. Basic Med. Sci. 2007, 7, 279.
- Wang, J.; Wen, H.; Desai, D. Lubrication in tablet formulations. Eur. J. Pharm. Biopharm. 2010, 75, 1–15.
- Heng, D.; Lee, S.H.; Ng, W.K.; Chan, H.-K.; Kwek, J.W.; Tan, R.B. Novel alternatives to reduce powder retention in the dry powder inhaler during aerosolization. Int. J. Pharm. 2013, 452, 194–200.
- Behara, S.R.; Farkas, D.R.; Hindle, M.; Longest, P.W. Development of a high efficiency dry powder inhaler: Effects of capsule chamber design and inhaler surface modifications. Pharm. Res. 2014, 31, 360–372.
- Hindle, M.; Longest, P.W. Condensational growth of combination drug-excipient submicrometer particles for targeted high-efficiency pulmonary delivery: Evaluation of formulation and delivery device. J. Pharm. Pharmacol. 2012, 64, 1254–1263.
- Longest, P.W.; Tian, G.; Li, X.; Son, Y.-J.; Hindle, M. Performance of combination drug and hygroscopic excipient submicrometer particles from a softmist inhaler in a characteristic model of the airways. Ann. Biomed. Eng. 2012, 40, 2596–2610.
- Fassihi, A.; Parker, M. Influence of gamma radiation on the gel rigidity index and binding capability of gelatin. J. Pharm. Sci. 1988, 77, 876–879.
- Tochio, S.; Nagata, S.; Yamashita, S. The influence of the composition of test fluids on dissolution from HPMC capsules. AAPS PharmSci 2002, 4, W4340.
- Podczeck, F.; Jones, B.E. The in vitro dissolution of theophylline from different types of hard shell capsules. Drug Dev. Ind. Pharm. 2002, 28, 1163–1169.
- Bell, J.; Stevenson, N.; Taylor, J. A moisture transfer effect in hard gelatin capsules of sodium cromoglycate. J. Pharm. Pharmacol. 1973, 25, 96.
- Missaghi, S.; Fassihi, R. Evaluation and comparison of physicomechanical characteristics of gelatin and hypromellose capsules. Drug Dev. Ind. Pharm. 2006, 32, 829–838.
- Kontny, M.J.; Mulski, C.A. Gelatin capsule brittleness as a function of relative humidity at room temperature. Int. J. Pharm. 1989, 54, 79–85.
- Chang, R.K.; Raghavan, K.S.; Hussain, M.A. A study on gelatin capsule brittleness: Moisture tranfer between the capsule shell and its content. J. Pharm. Sci. 1998, 87, 556–558.
- Geuns, E.R.; Toren, J.S.; Barends, D.M.; Bult, A. Decrease of the stage-2 deposition in the twin impinger during storage of beclomethasone dipropionate dry powder inhalers in controlled and uncontrolled humidities. Eur. J. Pharm. Biopharm. 1997, 44, 187–194.
- Kuentz, M.; Rothenhäusler, B.; Röthlisberger, D. Time domain 1H NMR as a new method to monitor softening of gelatin and HPMC capsule shells. Drug Dev. Ind. Pharm. 2006, 32, 1165–1173.
- Chong, R.H.; Jones, B.E.; Díez, F.; Birchall, J.C.; Coulman, S.A. Evaluating the sensitivity, reproducibility and flexibility of a method to test hard shell capsules intended for use in dry powder inhalers. Int. J. Pharm. 2016, 500, 316–325.
- Young, P.M.; Price, R. The influence of humidity on the aerosolisation of micronised and SEDS produced salbutamol sulphate. Eur. J. Pharm. Sci. 2004, 22, 235–240.
- Price, R.; Young, P.; Edge, S.; Staniforth, J. The influence of relative humidity on particulate interactions in carrier-based dry powder inhaler formulations. Int. J. Pharm. 2002, 246, 47–59.
- Das, S.; Larson, I.; Young, P.; Stewart, P. Influence of storage relative humidity on the dispersion of salmeterol xinafoate powders for inhalation. J. Pharm. Sci. 2009, 98, 1015–1027.
- Nagata, S. Cellulosecapsules–Analternative To Gelatin. Biomed. Polym. Polym. Ther. 2001, 53, 53–62.





