
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Diaconeasa Zorita | + 3689 word(s) | 3689 | 2021-08-29 09:19:53 | | | |
| 2 | Conner Chen | Meta information modification | 3689 | 2021-09-23 02:59:29 | | |
Video Upload Options
From all types of cancer, cervical cancer manages to be in top four most frequent types, with a 6.5% rate of occurrence. The infectious vector that induces the disease, the high-risk Human papillomavirus (HPV), which is a sexually transmitted virus, is capable of transforming the host cell by modulating some of the principal signaling pathways responsible for cell cycle arrest, proliferation, and survival. Fortunately, like other cancer types, cervical cancer can be treated by chirurgical interventions or chemoradiotherapy, but these methods are not exactly the lucky clover of modern medicine because of the adverse effects they have. That is the reason why in the last years the emphasis has been on alternative medicine, more specifically on phytochemicals, as a substantial number of studies showed that diet contributes to cancer prevention and treatment. All these studies are trying to find new chemopreventive agents with less toxicity but high effectiveness both in vitro and in vivo. Polyphenols have great potential in cervical cancer prevention, with strong effects on gene modulation.
1. HPV: Structure, Pathogenicity and Transformation Activity
HPV is a member of the Papillomaviridae family and appears to be one of the most common viral pathogens that can lead to sexually transmitted infections worldwide [1][2].
The HPV genome is represented by a small double-stranded and highly conserved DNA with a molecular weight of 5 × 106 Daltons and contains approximately 7906 base pairs, including two coding regions (E and L) and one non-coding region called the long control region or upstream regulatory region (URR) (Figure 1) [3][4]. The E region encodes six early proteins (E1, E2, E4, E5, E6, and E7), three of them being regulatory proteins (E1, E2, and E4) and three of them being oncoproteins (E5, E6, and E7) that participate in the processes of replication and transformation of the host cells [5]. E1 and E2 are specifically involved in transcription and replication, E4 is involved in the process of virion release, E5 modulates cell proliferation, and E6 and E7 control the principal signaling pathways in the host cell [6]. The L region encodes two late proteins (L1 and L2), which are the structural proteins that form the viral capsid; L1 is responsible for the major viral capsid and L2 is responsible for the minor viral capsid [3][5][6]. The long control region contains the viral open reading frame (ORF) and the promoter and enhancer elements that modulate the viral DNA replication and transcription [5][6].
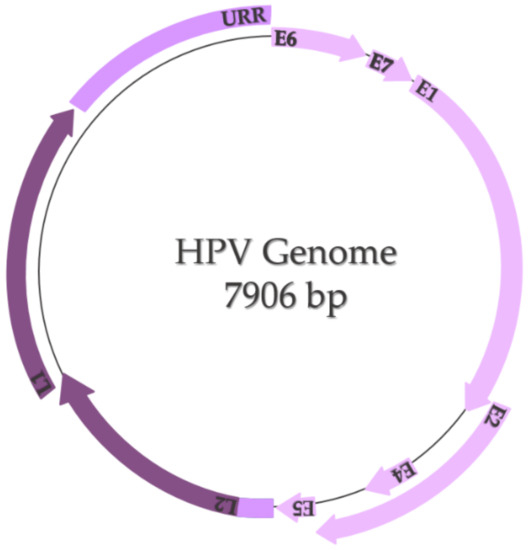
Figure 1. HPV genome (adapted after Bowden and Kyrgiou, 2020 [6]).
In nature, HPVs represent only five out of all 39 genera of the Papillomaviridae family: alpha, beta, gamma, mu, and nu papillomaviruses, the alpha-papillomavirus being the one that causes genital warts [2]. In terms of viruses’ serotype, every HPV is genetically different based on the nucleotide sequence of the gene that encodes the L1 protein; thus, the classification is based on the chronological order of the dates on which they were found. Another form of classification is related to the carcinogenic potential of the HPVs-group 1 carcinogens (carcinogenic for humans), group 2A carcinogens (probably carcinogenic for humans), and group 2B carcinogens (potentially carcinogenic for humans) [4]. Of all high-risk HPVs, the most carcinogenic are HPV16 (approximately 50% of all cervical cancers are associated with this strain) and HPV18 because they are primarily involved in squamous epithelial lesions [7].
Infection with HPV is highly associated with sexual activity, non-sexual transmission, and transmission via fomites. Once the virus enter the body, it manages to interact with squamous epithelial cells via surface receptors such as α-6 integrines or heparin sulfate proteoglycans, infect them, and get access to basal cells during any form of abrasion. Here, in the basal cell, the expression of the E1 and E2 genes is induced, which means that the rolling circle replication begins [8]. The viral genome is integrated in the host genome, leading to the loss of E2’s ORF. This aspect is very important because E2 is the transcriptional repressor of E6 and E7 oncogenes. With that being said, when the replication is over, the E2’s ORF is missing and thus the E6 and E7 genes are overexpressed, which leads to cell transformation [9]. Another possible way to prevent the E2-mediated repression is by methylation of E2 binding sites within the URR [10]. After the transformation, L1 and L2 proteins will make the capsid and the mature virus ready to be released by the E4 protein to other cells [8].
From a molecular perspective, in order for HPV to transform the host cell, it must initiate a series of genetic changes. Thus, in order to prevent or even treat cervical cancer, it is necessary to understand not only the virus’ mechanism of invasion but also the mechanism by which the virus transforms the host cell into a cancer cell. To achieve this goal, it is necessary to visualize the overall image of the cell, focusing on the cellular signaling pathways responsible for the cell cycle, cell growth, and proliferation and induction of apoptosis. More specifically, it is necessary to analyze the possible mutations in the main proto-oncogenes and tumor suppressor genes (TSG), or the possible complexes that may occur due to the presence of HPV in the host cell (Figure 2):
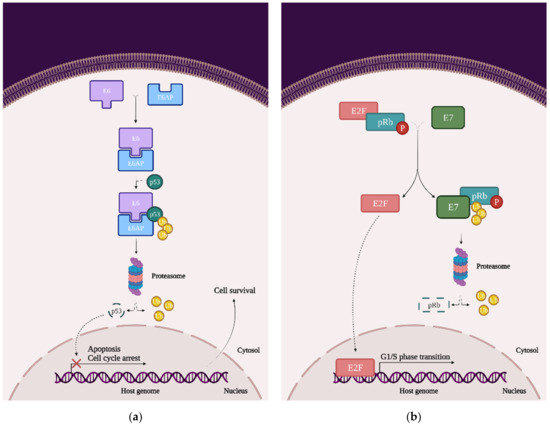
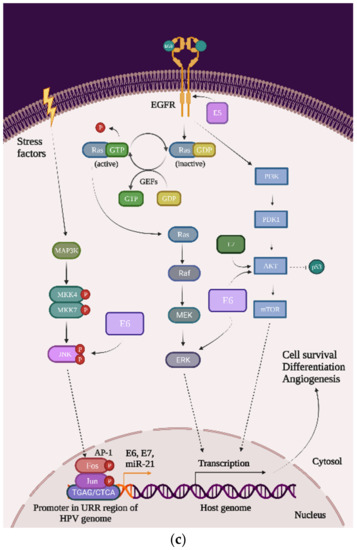
Figure 2. The impact of HPV viral proteins on the main signaling pathways responsible for cell survival, proliferation, and differentiation: (a) the effect of the E6 oncoprotein on the p53 tran-scription factor; (b) the effect of the E7 oncoprotein on the pRb tumor-suppression protein; and (c) the effects of the E5, E6, and E7 oncoproteins on EGFR phosphorylation, the PI3K/Akt/mTOR pathway, JNK, ERK, and the AP-1 complex.
-
p53: This transcription factor is involved in processes such as the cell cycle arrest, apoptosis, or induction of DNA damage response. In cervical cancer cells, HPVs are capable of inducing p53 ubiquitination via forming a complex between p53, the E6 oncoprotein, and the ubiquitin E3 ligase E6-associated protein (E6AP). This process will lead to p53 degradation by the proteasome and inevitably to chromosomal instability and avoidance of apoptosis and cell cycle arrest (Figure 2a) [11];
-
pRb and pocket proteins: The retinoblastoma protein (pRb) is a tumor-suppressor protein and, together with p107 and p130, they form “the pocket proteins” that control the cell cycle. pRb needs to bind to the E2F transcription factor in order to reduce its expression and keep the cell in a G1/S phase. In cervical cancer cells, HPV’s E7 protein binds to the pRb-E2F complex and releases the E2F. E2F will be now expressed, which means that the cell will pass the G1/S phase and the pRb will be eventually degraded by the proteasome (this mechanism of degradation requires the binding to the cullin-2 ubiquitin ligase complex) (Figure 2b) [10];
-
EGFR: The epidermal growth factor receptor (EGFR) is a transmembrane protein that contains an extracellular region that binds the ligands (such as the epidermal growth factor (EGF)), a transmembrane region, and an intracellular region, namely homodimers that have the catalytic site. Once the ligand is bound to the receptor, the EGFR homodimers autophosphorylate and activate some cellular pathways such as the mitogen-activated protein kinase (MAPK), phosphoinositide-3-kinase (PI3K), and protein kinase B (AKT). Primarily, EGFRs are involved in the signaling pathway that controls cell proliferation, differentiation, angiogenesis, and migration and survival, and the high expression of EGFR’s genes is associated with a poor prognosis in many cancer types. In cervical cancer, the HPV oncoprotein E5 increases the phosphorylation level of EGFRs, which lead to hyperproliferation (Figure 2c) [11];
-
PI3K/Akt/mTOR: This signaling cascade targets some of the most important and complex intracellular processes, which are triggered by a series of internal and external stimuli such as cell proliferation, apoptosis, energy metabolism, growth, and migration. In cervical cancer cells, both E6 and E7 oncoproteins have the ability to upregulate the expression of PI3K and Akt, which will upregulate the expression of mTOR. Once mTOR is overexpressed, it will enhance cell proliferation, which will lead to carcinogenesis (Figure 2c) [12][13];
-
MAPK/JNK: c-Jun N-terminal kinase (JNK) is a member of the subfamily Ser/Thr kinases (and is one of the three main classes of MAPK) and consists of ten isoforms encoded by three different genes, namely JNK1 (four isoforms ubiquitously expressed), JNK2 (four isoforms ubiquitously expressed), and JNK3 (two isoforms). The JNK signaling pathway can modulate oncogenic and tumor suppressive functions but it depends on the tissue in which it exercises its function. In cervical cancer cells, the E6 oncoprotein manages to increase JNK1/2 phosphorylation via the PDZ-binding motif. With that being said, when JNK1/2 is phosphorylated, c-Jun expression is activated, which induces the proliferation and expression of viral oncoproteins (Figure 2c) [11][14];
-
MAPK/ERK: The extracellular signal-regulated kinase (ERK) represents another one of the three major classes of MAPK. The ERK pathway is associated with a large variety of processes such as proliferation, senescence, angiogenesis, survival, apoptosis, and differentiation. In cervical cancer cells, the E6 oncoprotein can upregulate the expression of ERK and both the E6 and E7 oncoproteins can regulate hypoxia-inducible factor 1α (HIF-1α), interleukine-8 (IL-8), and the vascular endothelial growth factor (VEGF), which can lead to high rates of proliferation, differentiation, and angiogenesis (Figure 2c) [11][15][16][17];
-
AP-1: The activating protein-1 (AP-1) is an early transcription factor that plays an essential role in the transcription regulation of the HPV genome. Unlike normal cells, cervical cancer cells have high levels of AP-1 binding activity. AP-1 also represents a transcription factor family, with c-Fos and c-Jun as one of the crucial members. They bind to many consensus DNA-binding sequences (TGAG/CTCA) that are located in the promotor region of the genes and organize a series of gene expression processes of transformation, invasion, and metastasis. Furthermore, in cervical cancer cells, AP-1 upregulated microRNA miR-21 expression, which can contribute to an oncogenic potential. In cervical cancer cells, AP-1 binds to the HPV promoter located in the URR and thereby increases the expression of E6 and E7 oncoproteins, leading to carcinogenesis (Figure 2c) [11][18][19][20].
2. Polyphenols
Polyphenols compose one of the most diverse groups of plant metabolites [21] and, along with vitamins and enzymes, they represent a defense mechanism against oxidative stress caused by excess reactive oxygen species (ROS) [22][23][24]. These compounds are also the subject of many studies that focus on oxidative stress and its associated diseases such as cancer, diabetes, asthma, cardiovascular diseases, or even aging. All these studies aimed to find new chemopreventive agents that are less toxic than classical therapies but still effective [22][25].
In order to reveal their characteristics, most specifically their anticarcinogenic properties, scientists tested the polyphenols on multiple cell lines. The results emphasis that the phenolic compounds have a lot of health-promoting properties including antiproliferative, antineoplastic, proapoptotic, and anti-inflammatory activities [22][25][26]. These are the reasons why natural compounds have gained more attention over the last years, especially in the field of cancer. Polyphenols have great potential to act as anticancer drugs not only because of their properties but also because of their availability and toxicity statuses [27].
2.1. Polyphenols’ Classification
Although they are characterized as compounds with phenolic structural features, this group of dietary phenolics is diverse and contains sub-groups of phenolic compounds. Therefore, polyphenols are classified by their chemical structure (the number of phenol rings or the structural elements that bind these rings to one another) in four major classes: flavonoids, phenolic acids, lignans, and stilbenes [22][23][28]. Each class sums up a series of subclasses, all mentioned in Figure 3. In addition to these four classes, there are many more compounds that cannot be categorized into a specific class [29][23][28].
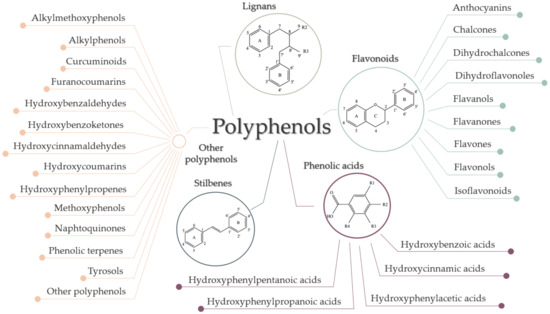
Figure 3. Classification of polyphenols.
2.2. Polyphenols’ Mechanisms of Action
Fundamentally, the primary role of polyphenols is to protect plants from photosynthetic stress, ROS, and consumption by herbivores. In addition, polyphenols represent a significant part of the human diet, namely flavonoids, and phenolic acids are the most common in our food. Numerous studies have been performed to understand the molecular mechanisms underlying their chemotherapeutic and chemopreventive properties on cervical cancer lines [30]. Following these studies, three major mechanisms of action were determined: modulation of gene expression by involving epigenetic pathways, suppression of cancer stem cells (CSCs), and modulation of the cellular redox status [31][32].
2.2.1. Modulation of Gene Expression by Involving Epigenetic Pathways
Epigenetic refers to a series of reversible heritable changes that are not encoded in the DNA but have an important role when it comes to modulating the gene expression [30]. The three main epigenetic mechanisms studied in mammalian cells are DNA methylation, post-transcriptional gene regulation by non-coding RNA (microRNAs/miRNAs), and histone modification [33].
In the normal cells, all induce chromatin remodeling, which leads to variations of cell phenotypes, but, when these mechanisms get to be aberrant, they can induce alterations in the expression of oncogenes and tumor suppressor genes. These alterations can accumulate throughout life and eventually affect the transcript stability, the complete nuclear organization of the genetic material, and lastly can initiate tumorigenesis [30][33].
Studies have shown that polyphenols are involved in epigenetic processes that influence the behavior of tumor cells and, not only that, they also are involved in the protection of normal cells by enhancing the cytotoxicity of other therapies in tumor cells [29][34].
DNA Methylation
DNA methylation is believed to be the most studied epigenetic modification in mammalian cells [35]. It occurs more specifically to regulate tumor growth and the development of carcinogenesis by activation of oncogenes, in addition to silencing TSGs [29]. Consequently, this mechanism of epigenetic machinery is responsible for X-chromosome inactivation and genomic imprinting of even the repression of repeated elements [33]. DNA methylation appears in CpG islands, which are areas of DNA in which a cytosine nucleotide is followed by a guanine nucleotide in 5′ → 3′ direction. These CpG islands are located mostly in promoter regions of the genes as well as in intergenic regions or in regions of large repetitive sequences [29][30]. The key enzymes that modulate this process are DNA methyltransferase enzymes (DNMT). They transfer a methyl group to the 5′ carbon position of cytosine to form 5-methylcytosine [36].
Natural polyphenols, such as resveratrol, genistein, quercetin, or epigallocatechin-3-gallate (EGCG), induce changes in the levels of DNMTs by the direct or indirect effect on DNMT activity (Figure 4) [29][37]. EGCG, for example, is well-known for its capacity to bind directly to the DNMTs, inactivating the enzymes [37]. Conversely, quercetin not only acts as a competitive inhibitor for various members of DNMT families and downregulates their gene expression, but it can restore the expression of TSGs by reducing the methylation of their promoters [37].

Figure 4. Potential mechanisms of action of polyphenols on epigenetic pathways.
Histone Modifications
The process of histone modification occurs because of the translational and post-translational modifications (PTMs) [36]. These PTMs occur mostly within the histones’ N-terminal tail or within their globular domain and include a variety of processes such as acetylation, biotinylation, phosphorylation, ubiquitination, SUMOylation, ADP ribosylation, proline isomerization, citrullination, butyrylation, propionylation, and glycosylation [37]. Thus, these mechanisms interrupt the chromatin organization and add new binding sites in a specific region of chromatin. The key enzymes that modulate these processes are histone acetyltransferases (HATs), histone deacetylases (HDACs), and histone methyltransferases (HMTs) [36].
Dietary polyphenols can modulate histone modification to prevent cancer by inhibiting HDAC [29][31]. Quercetin (inhibits HDAC2, HDAC4, HDAC7, and HDAC8), genistein (inhibits HDAC6 and tyrosine kinases), caffeic acid, and curcumin are the most well known for their capacity of inhibiting HDACs (Figure 4) [38].
Non-Coding RNA: MicroRNA
MicroRNAs (miRNAs) are short, non-coding single-stranded RNA fragments that regulate cellular processes through transcriptional repression and degradation of messenger RNA (mRNA) [39]. miRNA binds to the mRNA by sequence-specific base pairing with both 3’-untranslated regions of the target fragment and regions that can be realized by complete or partial complementary [40][37][39]. When miRNA binds to mRNA via imperfect complementary, translational repression will occur not only in a single fragment of mRNA, but also in tens to hundreds of different mRNAs. When miRNA binds to mRNA via perfect complementary, degradation of mRNA will occur [39]. In order to achieve this, miRNA needs a protein complex called the RNA-induced silencing complex (RISC), which is a complex that degrades the mRNA after the miRNA is fixed [37]. In vitro and in vivo studies emphasized that miRNAs are classified both as tumor suppressors and oncogenes, and their expression is downregulated or upregulated based on the tumor needs [41].
Various studies have shown that polyphenols also have a significant role in modulating mRNA function. One of the promising polyphenols in cervical cancer therapy is EGCG, a phytochemical found in green tea that has the potential to induce apoptosis in cervical cell lines. Zhu et al. tested the effect of EGCG on multiple cervical cell lines and noticed that in the CaSki cell line, EGCG upregulates miR-203, miR-125b, and mir-29, which are tumor suppressors in cervical cancer cells (Figure 6) [42][43][44][45][46].
2.2.2. Modulation of the Cellular Redox Status
Oxidative stress represents an imbalance between two processes, namely the formation and elimination of oxidative species such as superoxide anion, hydroxyl radical, and hydrogen peroxide [47]. These compounds are primarily the result of cytochrome P450 and peroxisome actions, and when they accumulate, they lead to a series of dysfunctions in the cell [48]. In normal cells, antioxidant compounds come in handy because they are capable of restoring the redox homeostasis by modulating the formation and degradation of ROS [49]. In contrast, in cancer cells, oxidative stress plays an important role in the epigenetic reprogramming of the expression of oncogenes and TSGs [31]. It is well known that cancer cells are usually under greater oxidative stress than normal cells but this property may be an advantage for the discovery of pro-oxidants that induce selective cytotoxicity in tumor cells [49].
What is fascinating about polyphenols is the fact that they can manifest their pro-oxidant properties only in tumor cells, not in normal ones, by decreasing cell viability precisely through the promotion of ROS [49][50]. A good example is curcumin; it has the potential to increase ROS levels in cervical cancer cells, which triggers endoplasmic reticulum stress (ER stress). Once ER stress is initiated, it induces ER stress-mediated apoptosis through activation of the C/EBP Homologous Protein CHOP (transcription factor involved in apoptosis) [51].
2.2.3. Suppression of Cancer Stem Cells
Cancer stem cells (CSCs) are a small subpopulation of tumor cells that play an important role in many processes such as tumorigenicity, tumorigenesis, defining tumor size, the speed of development, trans-differentiating into vascular endothelial cells or other stromal cells associated with the tumor, self-renewal, slow-cycling capacity, metastasis, and the level of regression following treatment [52][53][54]. Furthermore, in contrast with the differentiated cancer cells, CSCs are known for their low ROS levels, more efficient DNA repair responses, and promotion of glycolysis and autophagy [31]. Many studies state that the elimination of CSCs can represent a permanent cure for cancer [54]. The problem is that in cervical cancer, CSCs are associated with chemoradio-resisting properties [32][53].
Shin et al. [32] managed to prove that polyphenols might be a natural alternative to chemoradiotherapy, contributing to tumor cell destruction. More specifically, they showed that pterostilbenes are not only a promising therapy for cervical CSCs, but they also are greater inhibitors than other polyphenols such as resveratrol [32]. Their study demonstrated that pterostilbenes can:
-
induce cycle cell arrest at the S/G1 phase via the induction of p53 and p21 (both TSGs), and the reduction of cyclin E1 and cyclin B1;
-
induce apoptosis via the downregulation of Bcl-2 and Bcl-XL (antiapoptotic proteins), and ROS-mediated activation of caspase-3 and caspase-9;
-
inhibit MMP-2 and MM-9 expression (matrix metalloproteinases) [32].
2.3. A Perspective on Polyphenols’ Toxicity
So far, we have deduced that polyphenols are indeed promising agents against cervical cancer but their accelerated metabolism and reduced bioavailability are obstacles in accomplishing their activity. That is the reason why, in order to achieve the desired results, a high dose of polyphenols is needed [55]. Nevertheless, there is still a question that should be addressed: is a high dose of polyphenols equal to a high rate of absorption or a high efficiency?
It is well known that in any drug development process, a crucial part is represented by the toxicological study [56]. As for polyphenols, it seems that dose, which is linked to bioavailability in most of the cases, can be just as beneficial as it can be harmful for the body [57]. Most of the studies confirm that a higher dose of polyphenols is usually linked with toxicity, but why is this association widely recognized? The shortest and simplest answer is the notion of hormesis [58]. From a biological perspective, hormesis is an adaptive response to stress. When the cell is exposed to a lower concentration of stress-inducing agents, some signaling pathways will be activated in order to confer resistance to higher concentrations of the same agents or for other ones. From a chemical perspective, hormesis is a phenomenon that is characterized by a biphasic dose-response curve. Essentially, a chemical (in our case, a polyphenol) can act as a stimulant when given in small doses and as a toxic agent when given in high doses [59].
In this context, some of the risky doses of polyphenols that have been used in cervical cancer research, together with their harmful effects, are summarized in Table 1.
Table 1. Polyphenols’ main toxic effects based on their high administration doses.
| Compound | Dose | Model Organism | Toxicological Effects | References |
|---|---|---|---|---|
| FLAVONOIDS | ||||
| Flavonols | ||||
| Quercetin | - 2–4% above the normal dose | Mice |
|
[60][61] |
| - ≥100 µg/mL | - ↑ DNA damages | |||
| Isoflavones | ||||
| Genistein | - ≥500 ppm | Mice Humans |
|
[57][60][62] |
| Anthocyanins | ||||
| Proanthocyanidin | - ≥10 g/kg | Mice | - ↓ Growth and digestibility | [60][63] |
| - ≥100–500 µg/ml | Chick cardiomyocytes |
|
||
| Flavanols | ||||
| EGCG |
|
Mice |
|
[61][64][65] |
| PHENOLIC ACIDS | ||||
| Hydroxycinnamic acids | ||||
| Caffeic acid |
|
Mice |
|
[57] |
| Ferulic acid | - ≥500 mg/kg | Mice | - Carcinogenic to liver | [57] |
| STILBENES | ||||
| Stilbenes | ||||
| Pterostilbene | - ≥250 mg/day | Humans | - ↓ Bicarbonate, which can cause minor acid effects in blood | [66] |
| Resveratrol |
|
Humans |
|
[67] |
| OTHER POLYPHENOLS | ||||
| Curcuminoids | ||||
| Curcumin | - ≥0.5 mg/day | Humans |
|
[68] |
References
- Okunade, K.S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol. 2020, 40, 602–608.
- Calaf, G.M.; Urzúa, U.; Termini, L.; Aguayo, F. Oxidative stress in female cancers. Oncotarget 2018, 9, 23824–23842.
- Dong, Z.; Hu, R.; Du, Y.; Tan, L.; Li, L.; Du, J.; Bai, L.; Ma, Y.; Cui, H. Immunodiagnosis and Immunotherapeutics Based on Human Papillomavirus for HPV-Induced Cancers. Front. Immunol. 2021, 11, 1.
- Wang, X.; Huang, X.; Zhang, Y. Involvement of Human Papillomaviruses in Cervical Cancer. Front. Microbiol. 2018, 9, 2896.
- Chan, C.K.; Aimagambetova, G.; Ukybassova, T.; Kongrtay, K.; Azizan, A. Human Papillomavirus Infection and Cervical Cancer: Epidemiology, Screening, and Vaccination—Review of Current Perspectives. J. Oncol. 2019, 2019, 1–11.
- Bowden, S.J.; Kyrgiou, M. Human papillomavirus. Obstet. Gynaecol. Reprod. Med. 2020, 30, 109–118.
- Sammarco, M.L.; Tamburro, M.; Pulliero, A.; Izzotti, A.; Ripabelli, G. Human Papillomavirus Infections, Cervical Cancer and MicroRNAs: An Overview and Implications for Public Health. MicroRNA 2020, 9, 174–186.
- Rai, B.; Bansal, A.; Singh, M.P. Human papillomavirus-associated cancers: A growing global problem. Int. J. Appl. Basic Med. Res. 2016, 6, 84–89.
- Bechtold, V.; Beard, P.; Raj, K. Human Papillomavirus Type 16 E2 Protein Has No Effect on Transcription from Episomal Viral DNA. J. Virol. 2003, 77, 2021–2028.
- Scarth, J.A.; Patterson, M.R.; Morgan, E.L.; Macdonald, A. The human papillomavirus oncoproteins: A review of the host pathways targeted on the road to transformation. J. Gen. Virol. 2021, 102, 001540.
- Medda, A.; Duca, D.; Chiocca, S. Human Papillomavirus and Cellular Pathways: Hits and Targets. Pathogens 2021, 10, 262.
- Zhang, L.; Wu, J.; Ling, M.T.; Zhao, L.; Zhao, K.-N. The role of the PI3K/Akt/mTOR signalling pathway in human cancers induced by infection with human papillomaviruses. Mol. Cancer 2015, 14, 1–13.
- Bossler, F.; Hoppe-Seyler, K.; Hoppe-Seyler, F. PI3K/AKT/mTOR Signaling Regulates the Virus/Host Cell Crosstalk in HPV-Positive Cervical Cancer Cells. Int. J. Mol. Sci. 2019, 20, 2188.
- Morgan, E.L.; Scarth, J.A.; Patterson, M.R.; Wasson, C.W.; Hemingway, G.C.; Barba-Moreno, D.; Macdonald, A. E6-mediated activation of JNK drives EGFR signalling to promote proliferation and viral oncoprotein expression in cervical cancer. Cell Death Differ. 2021, 28, 1669–1687.
- Bonab, F.R.; Baghbanzadeh, A.; Ghaseminia, M.; Bolandi, N.; Mokhtarzadeh, A.; Amini, M.; Dadashzadeh, K.; Hajiasgharzadeh, K.; Baradaran, B.; Baghi, H.B. Molecular pathways in the development of HPV-induced cervical cancer. EXCLI J. 2021, 20, 320–337.
- Liu, F.; Lin, B.; Liu, X.; Zhang, W.; Zhang, E.; Hu, L.; Ma, Y.; Li, X.; Tang, X. ERK Signaling Pathway Is Involved in HPV-16 E6 but not E7 Oncoprotein-Induced HIF-1α Protein Accumulation in NSCLC Cells. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2016, 23, 109–118.
- Anerillas, C.; Abdelmohsen, K.; Gorospe, M. Regulation of senescence traits by MAPKs. GeroScience 2020, 42, 397–408.
- Mirzaei, H.; Khodadad, N.; Karami, C.; Pirmoradi, R.; Khanizadeh, S. The AP-1 pathway; A key regulator of cellular transformation modulated by oncogenic viruses. Rev. Med. Virol. 2019, 30, e2088.
- Prusty, B.K.; Das, B.C. Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin. Int. J. Cancer 2005, 113, 951–960.
- Díaz-González, S.D.M.; Rodríguez-Aguilar, E.D.; Meneses-Acosta, A.; Valadez-Graham, V.; Deas, J.; Gómez-Cerón, C.; Tavira-Montalván, C.A.; Arizmendi-Heras, A.; Ramírez-Bello, J.; Zurita-Ortega, M.E.; et al. Transregulation of microRNA miR-21 promoter by AP-1 transcription factor in cervical cancer cells. Cancer Cell Int. 2019, 19, 1–15.
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017, 12, 2689–2702.
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552.
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246.
- Diaconeasa, Z.; Ranga, F.; Rugina, D.; Leopold, L.; Pop, O.; Vodnar, D.; Cuibus, L.; Socaciu, C. Phenolic Content and Their Antioxidant Activity in Various Berries Cultivated in Romania. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2015, 72, 99–103.
- Frond, A.D.; Iuhas, C.I.; Stirbu, I.; Leopold, L.; Socaci, S.; Andreea, S.; Ayvaz, H.; Andreea, S.; Mihai, S.; Diaconeasa, Z.; et al. Phytochemical Characterization of Five Edible Purple-Reddish Vegetables: Anthocyanins, Flavonoids, and Phenolic Acid Derivatives. Molecules 2019, 24, 1536.
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355.
- Rampogu, S.; Ravinder, D.; Pawar, S.C.; Lee, K.W. Natural Compound Modulates the Cervical Cancer Microenvironment—A Pharmacophore Guided Molecular Modelling Approaches. J. Clin. Med. 2018, 7, 551.
- Rothwell, J.; Pérez-Jiménez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070.
- Arora, I.; Sharma, M.; Tollefsbol, T.O. Combinatorial Epigenetics Impact of Polyphenols and Phytochemicals in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2019, 20, 4567.
- Link, A.; Balaguer, F.; Goel, A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem. Pharmacol. 2010, 80, 1771–1792.
- Mileo, A.M.; Miccadei, S. Polyphenols as Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies. Oxid. Med. Cell. Longev. 2016, 2016, 1–17.
- Shin, H.J.; Han, J.M.; Choi, Y.S.; Jung, H.J. Pterostilbene Suppresses both Cancer Cells and Cancer Stem-Like Cells in Cervical Cancer with Superior Bioavailability to Resveratrol. Molecules 2020, 25, 228.
- Carlos-Reyes, Á.; Lopez-Gonzalez, J.S.; Meneses-Flores, M.; Gallardo-Rincón, D.; Ruíz-García, E.; Marchat, L.; La Vega, H.A.-D.; De La Cruz, O.N.H.; López-Camarillo, C. Dietary Compounds as Epigenetic Modulating Agents in Cancer. Front. Genet. 2019, 10, 79.
- Sayed, M.; ElHamid Mahmou, A.A. Cancer Chemoprevention by Dietary Polyphenols. In Carcinogenesis; InTech: London, UK, 2013.
- Park, L.K.; Friso, S.; Choi, S.-W. Nutritional influences on epigenetics and age-related disease. Proc. Nutr. Soc. 2011, 71, 75–83.
- Arora, I.; Sharma, M.; Sun, L.Y.; Tollefsbol, T.O. The Epigenetic Link between Polyphenols, Aging and Age-Related Diseases. Genes 2020, 11, 1094.
- Russo, G.L.; Vastolo, V.; Ciccarelli, M.; Albano, L.; Macchia, P.E.; Ungaro, P. Dietary polyphenols and chromatin remodeling. Crit. Rev. Food Sci. Nutr. 2017, 57, 2589–2599.
- De Freitas, N.L.; Deberaldini, M.G.; Gomes, D.; Pavan, A.R.; Sousa, Â.; Dos Santos, J.L.; Soares, C.P. Histone Deacetylase Inhibitors as Therapeutic Interventions on Cervical Cancer Induced by Human Papillomavirus. Front. Cell Dev. Biol. 2021, 8, 592868.
- Pedroza-Torres, A.; López-Urrutia, E.; Garcia, V.; Jacobo-Herrera, N.; Herrera, L.A.; Peralta-Zaragoza, O.; López-Camarillo, C.; De Leon, D.C.; Fernández-Retana, J.; Cerna-Cortés, J.F.; et al. MicroRNAs in Cervical Cancer: Evidences for a miRNA Profile Deregulated by HPV and Its Impact on Radio-Resistance. Molecules 2014, 19, 6263–6281.
- Park, S.-H.; Kim, M.; Lee, S.; Jung, W.; Kim, B. Therapeutic Potential of Natural Products in Treatment of Cervical Cancer: A Review. Nutrients 2021, 13, 154.
- Srivastava, S.K.; Arora, S.; Averett, C.; Singh, S.; Singh, A.P. Modulation of MicroRNAs by Phytochemicals in Cancer: Underlying Mechanisms and Translational Significance. BioMed Res. Int. 2015, 2015, 1–9.
- Zhu, Y.; Huang, Y.; Liu, M.; Yan, Q.; Zhao, W.; Yang, P.; Gao, Q.; Wei, J.; Zhao, W.; Ma, L. Epigallocatechin gallate inhibits cell growth and regulates miRNA expression in cervical carcinoma cell lines infected with different high-risk human papillomavirus subtypes. Exp. Ther. Med. 2018, 17, 1742–1748.
- Cui, F.; Li, X.; Zhu, X.; Huang, L.; Huang, Y.; Mao, C.; Yan, Q.; Zhu, J.; Zhao, W.; Shi, H. MiR-125b Inhibits Tumor Growth and Promotes Apoptosis of Cervical Cancer Cells by Targeting Phosphoinositide 3-Kinase Catalytic Subunit Delta. Cell. Physiol. Biochem. 2012, 30, 1310–1318.
- Kriegel, A.J.; Liu, Y.; Fang, Y.; Ding, X.; Liang, M. The miR-29 family: Genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol. Genom. 2012, 44, 237–244.
- Chan, Y.C.; Banerjee, J.; Choi, S.Y.; Sen, C.K. miR-210: The Master Hypoxamir. Microcirculation 2011, 19, 215–223.
- Zhu, X.; Er, K.; Mao, C.; Yan, Q.; Xu, H.; Zhang, Y.; Zhu, J.; Cui, F.; Zhao, W.; Shi, H. miR-203 Suppresses Tumor Growth and Angiogenesis by Targeting VEGFA in Cervical Cancer. Cell. Physiol. Biochem. 2013, 32, 64–73.
- Rugina, D.O.; Diaconeasa, Z.; Coman, C.; Bunea, A.; Socaciu, C.; Pintea, A. Chokeberry Anthocyanin Extract as Pancreaticβ-Cell Protectors in Two Models of Induced Oxidative Stress. Oxid. Med. Cell. Longev. 2015, 2015, 1–10.
- Silva, G.Á.F.; Nunes, R.A.L.; Morale, M.G.; Boccardo, E.; Aguayo, F.; Termini, L. Oxidative stress: Therapeutic approaches for cervical cancer treatment. Clinies 2018, 73, e548s.
- D’Angelo, S.; Martino, E.; Ilisso, C.P.; Bagarolo, M.L.; Porcelli, M.; Cacciapuoti, G. Pro-oxidant and pro-apoptotic activity of polyphenol extract from Annurca apple and its underlying mechanisms in human breast cancer cells. Int. J. Oncol. 2017, 51, 939–948.
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515.
- Kim, B.; Kim, H.S.; Jung, E.-J.; Lee, J.Y.; Tsang, B.K.; Lim, J.M.; Song, Y.S. Curcumin induces ER stress-mediated apoptosis through selective generation of reactive oxygen species in cervical cancer cells. Mol. Carcinog. 2016, 55, 918–928.
- Huang, R.; Rofstad, E.K. Cancer stem cells (CSCs), cervical CSCs and targeted therapies. Oncotarget 2017, 8, 35351–35367.
- Mendoza, G.; Rocha-Zavaleta, L.; Esparza-Ibarra, E.; Olmos, J. Cervical cancer stem cells and other leading factors associated with cervical cancer development (Review). Oncol. Lett. 2019, 18, 3423–3432.
- Chhabra, R. Cervical cancer stem cells: Opportunities and challenges. J. Cancer Res. Clin. Oncol. 2015, 141, 1889–1897.
- Yadav, N.; Parveen, S.; Banerjee, M. Potential of nano-phytochemicals in cervical cancer therapy. Clin. Chim. Acta 2020, 505, 60–72.
- Boncler, M.; Golanski, J.; Lukasiak, M.; Redzynia, M.; Dastych, J.; Watala, C. A new approach for the assessment of the toxicity of polyphenol-rich compounds with the use of high content screening analysis. PLoS ONE 2017, 12, e0180022.
- Martin, K.; Appel, C.L. Nutrition and Dietary Supplements Polyphenols as Dietary Supplements: A Double-Edged Sword. 2010. Available online: https://www.dovepress.com/ (accessed on 26 March 2021).
- Ruskovska, T.; Maksimova, V.; Milenkovic, D. Polyphenols in human nutrition: From thein vitroantioxidant capacity to the beneficial effects on cardiometabolic health and related inter-individual variability—An overview and perspective. Br. J. Nutr. 2020, 123, 241–254.
- Son, T.G.; Camandola, S.; Mattson, M.P. Hormetic Dietary Phytochemicals. NeuroMol. Med. 2008, 10, 236–246.
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 2005, 81, 326S–329S.
- Azqueta, A.; Collins, A. Polyphenols and DNA Damage: A Mixed Blessing. Nutrients 2016, 8, 785.
- Bennetau-Pelissero, C.; Breton, B.; Bennetau, B.; Corraze, G.; Le Menn, F.; Davail-Cuisset, B.; Helou, C.; Kaushik, S. Effect of Genistein-Enriched Diets on the Endocrine Process of Gametogenesis and on Reproduction Efficiency of the Rainbow Trout Oncorhynchus mykiss. Gen. Comp. Endocrinol. 2001, 121, 173–187.
- Ofosu, F.K.; Daliri, E.B.-M.; Elahi, F.; Chelliah, R.; Lee, B.-H.; Oh, D.-H. New Insights on the Use of Polyphenols as Natural Preservatives and Their Emerging Safety Concerns. Front. Sustain. Food Syst. 2020, 4, 525810.
- Lambert, J.D.; Sang, S.; Yang, C.S. Possible Controversy over Dietary Polyphenols: Benefits vs. Risks. Chem. Res. Toxicol. 2007, 20, 583–585.
- Rojo, M.; Garrosa, M.; Jiménez, P.; Girbés, T.; Garcia-Recio, V.; Cordoba-Diaz, M.; Cordoba-Diaz, D. Unexpected Toxicity of Green Tea Polyphenols in Combination with the Sambucus RIL Ebulin. Toxins 2020, 12, 542.
- Riche, D.M.; McEwen, C.L.; Riche, K.D.; Sherman, J.J.; Wofford, M.R.; Deschamp, D.; Griswold, M. Analysis of Safety from a Human Clinical Trial with Pterostilbene. J. Toxicol. 2013, 2013, 1–5.
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084.
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92.




