
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | ABDULAZIZ BASHIR KUTAWA | + 2702 word(s) | 2702 | 2021-09-16 09:01:09 | | | |
| 2 | Beatrix Zheng | + 465 word(s) | 3167 | 2021-09-17 03:33:07 | | |
Video Upload Options
Nanoparticles are materials with at least one or more dimensions at the scale of 1–100 nm. This definition has taken several materials to be named nanoparticles into account. Nanoparticles that are natural likewise occur in numerous forms, for example, oceanic salt sprays and volcanic dust.
1. Introduction
Agriculture plays a vital role by providing nourishment and serving as a source of income for many countries. It is the major source of livelihood for people in rural areas; about 86% of the rural people depend on agricultural cultivation [1]. Approximately 15–18% of crops losses occur as a result of animal pests, while weeds and microbial diseases cause 34 and 16% losses, respectively. Fungal pathogens cause about 70–80% losses in yield [1]. Approximately, there are 1.5 million species that are classified under the kingdom ‘fungi’ and these fungal pathogens are mostly parasitic and saprophytic in nature, causing different diseases in agricultural crops. Fungal pathogens may cause serious decreases in the yield of different crops worldwide each year [2][3]. Presently, disease control depends on the utilization of agrochemicals, for example, fungicides. Regardless of numerous favorable advantages, such as fast action, reliability, and high availability, fungicides can cause negative impacts on the non-target living organisms because of their toxicity and their systemic mode of action by disrupting the metabolite levels in the biosynthetic pathway of aromatic amino acids within the soil microorganisms, the development of resistance, and resurgence in the population of pests and environment [4][5]. Moreover, it is assessed and estimated that about 80–90% of sprayed fungicides are lost to the environment after or during their applications [5][6]. Accordingly, there is an urgent need to procure high-performance fungicides which are cost-effective and cause less negative impacts to the environment.
Nanotechnology has prompted the advancement of novel ideas and agriproducts having tremendous potential to address the aforementioned issues. Nanotechnology has progressed in areas of pharmacology and medicine, yet has not developed nearly as much in agricultural uses [7]. The utilization of nanotechnology in the agricultural sector is presently being investigated in the delivery of plant chemical, water, and seed control, nanobarcoding, transfer of genes, controlled release of agrochemicals, and nanosensors [8]. Many researchers have designed nanoparticles pertaining to different qualities, such as pore size, surface properties, and shape, in such a way that they would be utilized as protectants or for exact delivery through encapsulation, and adsorption of an active ingredient [8]. There is a possibility for nanotechnology in agriculture to create and give another age of fungicides and different active ingredients for fungal disease control in plants, as presented in Figure 1 .
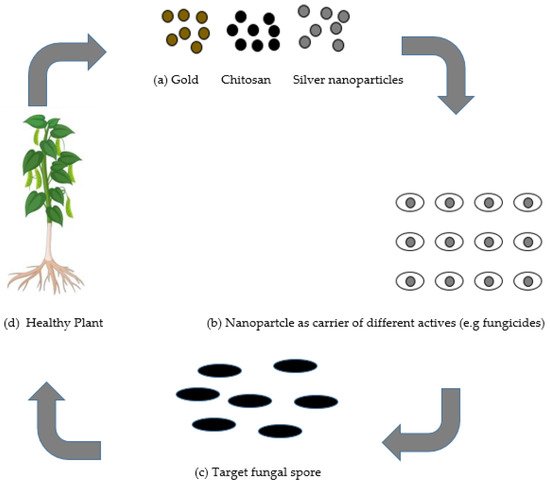
The application of nanoparticles for plants protection could be achieved using two types of mechanisms: (a) as individual nanoparticles giving protection in plants; or (b) nanoparticles as fungicides transporters of different active ingredients, for example, fertilizers, which could be applied by soaking/drenching or by spraying onto the foliar tissue, roots, or seeds. Nanoparticles serve as a carrier and may give numerous advantages such as (i) enhanced solubility of low water-soluble fungicides; (ii) enhanced shelf-life ; (iii) improved site-specific uptake in the targeted microbe; and (iv) reduced toxicity [9]. Other advantage of the nanocarrier system is the increment for the efficacy of the stability and activity of the nanofungicides under different ecological factors, essentially reducing the number as well as the quantity of applications, which accordingly diminishes harmfulness and decreases their expenses or cost.
Nanotechnology has played an important role in creating a footprint to develop several forms of formulations effectively. Up to now, nanotechnology in the area of agriculture has not reached its milestone because of the insufficient application of nanoproducts at commercial levels. Till today, only a few studies have been carried out in the fields. Therefore, there is a need for researches to shift towards the direction of testing on different crops, target fungus, and to carry out both short and long periods of field trials in order to make progress, and advances in the area of agronanotechnology. This work discusses the properties and synthesis of nanoparticles, new advancements in plant pathogenic fungal disease control by the use of nanoparticles alone as protectants, and nanoparticles as nanocarriers for fungicides. Moreover, using other nanoproducts such as agronanofungicides, Zataria multiflora , and ginger essential oils nanoformulations to control plant pathogenic fungi, as well as the biosafety and limitations of the nanoparticles applications, have been addressed. In this review, we have focused on the information that is more recent (last 5 years) and also on papers before 2016 (last 5tears), where we feel the information is not as rich. The majority of the papers discuss the synthesis, advantages, and efficacy of nanomaterials to control plant pathogenic fungi. Few papers discuss the phytotoxicity and limitations of nanomaterials applications.
2. An Overview on Nanoparticles
Nanoparticles are materials with at least one or more dimensions at the scale of 1–100 nm [10]. This definition has taken several materials to be named nanoparticles into account. Nanoparticles that are natural likewise occur in numerous forms, for example, oceanic salt sprays and volcanic dust [11]. Moreover, numerous viroid and viral particles fall in this definition of a nanoparticle. Natural nanoparticles are having different sizes and are irregular. Because of their huge surface-area-to-volume ratio and little size, they could be highly reactive and may absorb, bind, and convey mixtures of compounds, for example, DNA, small molecular drugs, proteins, probes, and RNA [12][13]. Besides the large surface area of nanoparticles, they also vary in different properties when compared with their counterparts. For example, gold is inert and is clearly a bigger structure, but could be reactive and reddish in color at the scale of nano size. Similarly, ZnO and TiO are usually found to be colorless at a nano size. Nanoparticles were found to melt at low temperatures and have high reactive potentials than their counterparts [13][14].
Nanoparticles are synthesized by various strategies and methods such as laser ablation, pyrolysis, emulsion, encapsulation, dispersion-precipitation, etc. [15][16]. New cycles and stages are developed quickly to the point that any portrayal is probably going to be obsolete soon. A lot of works have been carried out on the synthesis and preparation of nanoparticles in vivo by both microorganisms and plants [17]. The nanoparticles are categorized into several groups such as organic nanoparticles, inorganic nanoparticles, carbon base nanoparticles, and ceramic nanoparticles. The inorganic nanoparticles are further categorized into metal oxide and metal nanoparticles [18]. Likewise, carbon base nanoparticles are also further categorized into carbon nanotubes, fullerene, carbon nanofiber, carbon black nanoparticles, and graphene. These nanoparticles could also be grouped in terms of their dimension, such as two-dimension nanoparticles, three-dimension nanoparticles, and one-dimension nanoparticles [18][19]. The nanoparticles can be prepared by utilizing different approaches, e.g., bottom-up approach and top-down approach. The effects of different nanomaterials used and methods of their synthesis are listed in Table 1 .
| Nanomaterial | Preparation Method | Advantages | Disadvantages | Effect | Source(s) |
|---|---|---|---|---|---|
| Organic | |||||
| Lipid Liposomes Lipopolyplexes Solid lipid nano-particles |
Chemical: sonochemisty, reverse phase evaporation High-pressure homogenization |
It involves the use of less toxic compounds, and the delivery of DNA, xenobiotics, pesticides, essential oils, and transfection |
It requires substantial energy for effective disintegration of high-solid waste | Cytotoxicity | [20] |
| Carbon nanotubes, Nanofibers, Carbon nanospheres, activated carbon, nanodots, graphene oxide and graphene layer |
Arc-discharge, laser ablation, pyrolysis, chemical vapor deposition, and Carbonization |
Biocatalysts, sensing, neural/orthopedic implants atomic force microscope probes |
It requires the use of low pressure and noble gasses | Antimicrobial effect | [21] |
| Synthetic Dendrimers (PAMAM, PPI) Polyethylene oxide Polyethylene glycol Polylactides Polyalklycyanoacrylates |
- | Delivery of therapeutic/ diagnostic agents, pesticides delivery of DNA/RNA |
Short half-lives, and lack of targeting capability | Cytotoxic effect | [22] |
| Polymeric Natural Cellulose, Starch Gelatin, Albumin Chitin, chitosan |
Chemical: suspension, emulsion, dispersion -precipitation |
Biocompatible, biodegradable non-toxic for drug delivery delivery of DNA/RNA |
Emulsions are thermodynamically unstable and therefore must be formulated to stabilize the emulsion from the separation of the two phases | Non-toxic/non-cytotoxic | [23] |
| Inorganic | |||||
| Clay Montmorillonite layered double hydroxides |
Physical: exfoliation co-precipitation |
Delivery of pesticides, fertilizers, plant growth promoting factors |
- | inhibiting and synergistic effects | [24] |
| Metal nanoparticles AgO, TiO2, ZnO, CeO2; Fe2O3 FePd, Fe–Ni (magnetic); Silica; CdTe, CdSe (QDs) |
Physical: Arc-discharge, high energy ball milling, laser pyrolysis/ablation. Chemical: electrochemical, chemical vapor deposition sonochemistry, microemulsion sol-gel, reverse precipitation |
Photothermal therapy, imaging studies, delivery of biomolecules (proteins, peptides nucleic acids), biosensors, diagnostic procedures, implants, pesticide degradation |
It requires substantial energy for effective disintegration of high-solid waste, and the use of noble gas | Positive effect by promoting the growth of plants | [20] |
| Magnetic type | |||||
| Magnetic nanoparticle | Physical vapor deposition, mechanical attrition and chemical routes from solution | Photothermal therapy, Imaging studies, diagnostic procedures | special apparatus and formation of highly toxic gaseous as by-products | - | [18] |
| Biosynthesized type | |||||
| Biosynthesized nanoparticles (Silver and gold nanoparticles, Ag & Au NPs) | Ag+ ion reduction by culture supernatant of E. coli, gold ions reduction by Bacterial cell supernatant (Pseudomonas aeruginosa) | Delivery of pesticides and fertilizers. | Generally lower biosynthesis efficiency and lengthier production time Downstream processing of intracellular products is more complex and expensive | Antimicrobial effect | [25][26] |
| Nanocellulose and Cellulose nanocrystal | - | Degrading of biomass/bio-waste from oil palm | It has limited flexibility, low thermal stability, brittleness and low crystallization rate, which hinders its use | No cytotoxic and ecotoxic effects | [27] |
Protectant nanoparticles are a material with a range of 10–100 nm; these nanoparticles have special structures and properties that are physically, biologically, and chemically unique [2][28]. Nanoparticles alone can be used on plant foliage, roots, or seeds for defense against different pathogens, such as fungi, insects, viruses, and bacteria. Nanoparticles that are metallic, such as copper, silver, titanium dioxide, and zinc oxide, have been widely investigated for their antifungal and antibacterial characteristics [29][30][31][32].
Chitosan is also a well-known nanoparticle with suitable biological characteristics, for example, biocompatibility, non-allergenicity, antimicrobial action, and biodegradability having low-toxic effects on humans and animals [33]. It also has the ability to actuate resistance to viruses in different tissues of plants by supporting them to resist several infections brought about by the mosaic virus of snuff, peanut, alfalfa, cucumber, and potato [34][35]. Nanoparticles of chitosan have possessed a significant antifungal characteristic, for example, controlling, tomato root rot, Botrytis bunch rot (grapes), P. grisea (rice plant), and Fusarium crown [36]; however, they are less effective against bacterial pathogens [29]. Antiviral activity has been observed on tobacco necrosis virus, tobacco mosaic virus, and bean mild mosaic virus [37]. Chitosan nanoparticles are promising as they appear to have a huge potential as nanocarriers [2].
3. Prospectives of Nanoformulations in Managing Plant Pathogenic Fungi
The production of essential oils in plants is mainly for defense purposes against pathogenic microorganisms [38]. Essential oils have many benefits such as quick decomposition and with broad antifungal spectrum compared to conventional fungicides, low toxicity, and bioaccumulation. Nanoencapsulation is a nanocarrier system that is used for the encapsulation of bioactive substances [39]. It can improve the antifungal efficacy of bioactive compounds (essential oils) by the increase in cell interactions among the microorganisms and nanoparticles, because of the small size which improves the cellular uptake. Nanoencapsulation in solid lipid nanoparticles (SLNs) is an efficient technique that enhances the application of essential oils as an antifungal agent [39][40]. SLNs are novel drug delivery systems for cosmetic and pharmaceutical drug active ingredients [41]. SLNs have unique properties, such as a large surface area, high drug loading, and small size. Their sizes are in the range of 50–1000 nm. SLNs can improve the solubility of essential oil(EO) in water, protect the EO against environmental conditions such as light, oxygen, acidity, and moisture, improve the controlled release of the EO, and increase the bioavailability of entrapped bioactive [41].
The availability of phenolic compounds such as Carvacrol and Thymol are the major constituents of Zataria multiflora essential oil that inhibit the growth of Aspergillus flavus fungus. This essential oil nanoemulsion has a very strong anti-fungal activity with minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of 100 ppm, respectively [39]. Based on these results, ZEO is an appropriate and potentially natural alternative for managing A. flavus [39]. In another study, the in vitro study had also shown a sustained and controlled release of Z. multiflora essential oils (ZEO) for 40 days. The strong activity of ZEO, after being encapsulated in chitosan nanoparticles (CSNPs) under both in vivo and in vitro conditions in comparison to the unmodified ZEO, was observed on the fungus B. cinerea [40]. The in vivo study had also revealed that the encapsulated Zataria essential oils at the concentration of 1500 ppm had shown a promising activity by decreasing both the disease incidence and disease severity of Botrytis -inoculated strawberries within the 7 days of storage at a temperature of 4 °C. This was then followed by two to three more days at a temperature of 20 °C. These findings have unveiled the important role of CSNPs that served as a controlled release system for Zataria EOs in order to enhance antifungal efficacies [40].
The delivery system of Eos, such as microemulsions, nanoemulsions, liposomes, and solid lipid nanoparticles, are designed for enclosing different compounds (natural bioactive) to improve antifungal efficacy [42][43]. Nanoemulsion is the dispersal of nanoparticles comprising of two different fluids that are insoluble, specifically water and oil, one of which is dispersed by a surfactant, as presented in Figure 2 . Surfactant is needed in order to develop a formulation of nanoemulsions for interfacial layer rigidity, droplet quality under 100 nm, and droplet size reduction [44][45]. The utilization of EOs is very much designed to make explicit qualities implied for suitable uses [46][47] to manage the diseases of fungi. The decrease to the nanometric scale of the drop size could increase the zone of the substrate which then creates contact with the fungal pathogen to bring about cell death and lysis. The constituents of EOs can get to the pathways of the cell membrane due to their surface-to-volume proportions, physical characteristics, sizes, degrees of selectivity, and chemical stabilities, consequently setting the movement of EOs to arrive at their target areas [42].
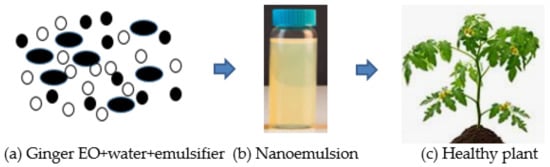
The encapsulation innovations and controlled techniques for discharge have changed the utilization of nanotechnology-based ginger EOs as an antifungal and antibacterial for the conservation of different crops [48]. It is an effective delivery framework that may only be delivered when it is required, bringing about more prominent conservation of crops and lowering the costs of crop cultivation [49]. Some industries worldwide are aiming at formulating nanofungicides for conveyance by means of nanoencapsulation into the target tissue of plants. Numerous formulations are being developed to contain nanomaterials. The materials dissociate in water in order to improve their efficiencies [50]. Accordingly, they are utilized as nanoscale particles that comprise antifungal nanoparticle suspension that could be advantageously mixed with various media such as liquids, creams, and gels [50]. Previous investigations had uncovered that diverse nanoparticles could affect different pathogens. Thus, it is important to utilize nanoscale to develop new formulations from natural products, such as Eos, for fungicides [51]. Nanoemulsions that contain citral-EOs can disturb and enter the lipid structure of the cell wall (fungi). It brings about cell membrane annihilation and protein denaturation; this is followed by conformational changes, cell death, and cytoplasmic leakage. An effective system of delivery of useful particles from EOs would work in the treatment of fungal diseases of plants [51]. Mahdavi et al. [52] uncovered that polymeric nanofibers containing ginger EOs showed the consistent and nonstop delivery of the successful compound of EOs loaded onto the nanofibers which become a remarkable tool to control plant pathogens.
4. Biosafety of Nanoparticles
This is a principal concern in the utilization of nanotechnology in the management of fungal diseases in plants [53]. A few uncertainties exist with respect to the long-term impact of utilizing nanofungicide formulations on human health and the environment [54][55][56][57]. Consequently, there is a need to assess the chance of inhaling the nanofungicide at the time of spray by the farm laborers. Shi et al. [58] studied the toxicity of chlorfenapyr (nanopesticide) on mice and expressed that the chlorfenapyr formulation at 4.84–19.36 mg kg −1 showed less toxicity than the conventional formulation on the mice. Hence, nanoformulation could lessen the impact on the environment and humans than the conventional fungicide [59][58].
Nanoformulations are seen to be safer and friendlier to the environment in disease control, yet a high level of NPs toxicity incidentally delivered to the environment could cause negative effects on other microbes and man [59]. The toxicological impacts of nanomaterials on soil microbes and plants have been generally studied. Notwithstanding, the nanotoxicity impacts of plant–soil systems of interaction are still not generally known [59]. There are numerous knowledge gaps on the agroecotoxicity of NPs; more so, there are numerous uncertain issues and new difficulties concerning the biological impacts. Mousa et al. [58] stated that there is a need to study the phytotoxic effect of seeds that are exposed to various concentrations of NPs; this involves the phytotoxicity investigation on germination, root length, and NPs uptake within the plant systems [60][61]. The application of nanosized silica-silver particles in the field helped in managing powdery mildew disease in cucurbits; about 100% disease management was obtained at 21 days after the application [62]. The NPs were discovered to be phytotoxic at a high concentration (3200 ppm) when applied in pansy and cucumber plants. Comparative investigation to convey the NPs to the target location of an infected plant was conducted by Corredor et al. [63]. The impact of NPs on various species of plants differs, and both the negative and positive impacts of this, have been discovered. The NPs may cause negative and positive impacts [64][65][66] on the root extension, which depends upon the species of plants (cucumber, soybean, corn, carrot, tomato, and cabbage). TiO 2 and ZnO manufactured nanomaterials (MNMs) affected the microbe’s community, biomass, and their diversity in the soil. Together, such reports infer that the soybean that is exposed to MNMs may be directly affected or via interaction of plant and microorganism, which includes nitrogen-fixing symbioses association that is sensitive to some metals [67][68]. Again, the phytotoxicity investigations on D-Zn-Al-LDH and H-Zn-Al-LDH were carried out on the seedlings of oil palm, and the results showed that both had the potential to reduce the phytotoxic impact when compared with their conventional counterparts [59]. To understand the potential advantages of applying NT to agriculture, the initial step to determine the transport and penetration of NPs in plants is required [69]. Since nanomaterials are brought into the soil because of human activities, they can penetrate the soil through the biosolids amended soils and atmospheric routes. The transport and penetration of NPs in the entire plant were assessed by Gonz_alez-Melendi et al. [70]. The findings indicate the potential of NPs to deliver different substances that are inhibitory to the plant fungal pathogens. Many works are required to explain the interaction between plants, phylloplane microflora, nanomaterials, soil micro-organisms, and endophytes, as well as both pathogenic and beneficial effects on the health of plants. Moreover, further investigations are required in order to develop bioindicators that would not only evaluate the effect of NPs on the environment, but also recommend different designs as well as models for the evaluation [54].
References
- Muhammad, A.; Isra, N.; Shahbaz, T.S.; Nasir, A.R.; Ehsan, H.; Muhammad, U.; Hamza, S.; Kiran, F.; Ehtsham, A.; Abdul, Q. Nanoparticles: A safe way towards fungal diseases. Arch. Phytopathol. Plant Prot. 2020, 53, 781–792.
- Worrall, E.A.; Hamid, A.; Mody, K.T.; Mitter, N.; Pappu, H.R. Nanotechnology for Plant Disease Management. Agronomy 2018, 8, 285.
- Flood, J. The importance of plant health to food security. Food Secur. 2010, 2, 215–231.
- Zaller, J.; Brühl, C.A. Editorial: Non-target Effects of Pesticides on Organisms Inhabiting Agroecosystems. Front. Environ. Sci. 2019, 7, 75.
- Stephenson, G.R. Pesticide Use and World Food Production: Risks and Benefits; ACS Publications: Washington, DC, USA, 2003.
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803.
- Sinha, K.; Ghosh, J.; Sil, P.C. 2—New pesticides: A cutting-edge view of contributions from nanotechnology for the development of sustainable agricultural pest control A2—Grumezescu, AlexandruMihai. In New Pesticides and Soil Sensors; Academic Press: Cambridge, MA, USA, 2017.
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Idris, A.S.; Hilmi, N.H.Z.; Daim, L.D.J. Chitosan-Based Agronanofungicides as a Sustainable Alternative in the Basal Stem Rot Disease Management. J. Agric. Food Chem. 2020, 68, 4305–4314.
- Balaure, P.C.; Gudovan, D.; Gudovan, I. Nanopesticides: A new paradigm in crop protection. In New Pesticides and Soil Sensors; Academic Press: London, UK, 2017; pp. 129–192.
- Sekhon, B.S. Nanotechnology in agri-food production: An overview. Nanotechnol. Sci. Appl. 2014, 7, 31–53.
- Kadar, E.; Cunliffe, M.; Fisher, A.; Stolpe, B.; Lead, J.; Shi, Z. Chemical interaction of atmospheric mineral dust-derived nanoparticles with natural seawater—EPS and sunlight-mediated changes. Sci. Total Environ. 2014, 468, 265–271.
- Waychunas, G.A. Natural nanoparticle structure, properties and reactivity from X-ray studies. Powder Diffr. J. 2009, 24, 89–93.
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed Eng. 2012, 14, 1–16.
- Wade, E.; Jason, C.W. The Future of Nanotechnology in Plant Pathology. Annu. Rev. Phytopathol. 2018, 56, 111–133.
- Astruc, D. (Ed.) Nanoparticles and Catalysis; JohnWiley & Sons: New York, NY, USA, 2008.
- Dreizin, E.L. Metal-based reactive nanomaterials. Prog. Energy Combust. Sci. 2009, 35, 141–167.
- Costa Silva, L.P.; Oliveira, J.P.; Keijok, W.J.; Silva, A.R.; Aguiar, A.R.; Guimarães, M.C.C.; Braga, F.R. Extracellular biosynthesis of silver nanoparticles using the cell-free filtrate of nematophagus fungus Duddingtonia flagans. Int. J. Nanomed. 2017, 12, 6373–6381.
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245.
- Abbasi, E.; Milani, M.; Aval, S.F.; Kouhi, M.; Akbarzadeh, A.; Nasrabadi, H.T.; Nikasa, P.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; et al. Silver nanoparticles: Synthesis methods, bio-applications and properties. Crit. Rev. Microbiol. 2014, 42, 173–180.
- Savun-Hekimoglu, B.A. Review on Sonochemistry and Its Environmental Applications. Acoustics 2020, 2, 766–775.
- Sari, A.H.; Khazali, A.; Parhizgar, S.S. Synthesis and characterization of long-CNTs by electrical arc discharge in deionized water and NaCl solution. Int. Nano Lett. 2018, 8, 19–23.
- Filipczak, N.; Yalamarty, S.S.K.; Li, X.; Parveen, F.; Torchilin, V. Developments in Treatment Methodologies Using Dendrimers for Infectious Diseases. Molecules 2021, 26, 3304.
- Mahato, R.; Narang, A. Pharmaceutical Dosage Forms and Drug Delivery, 3rd ed.; Taylor & Francis Group, LLC: New York, NY, USA, 2018.
- Liu, Y.; Tong, Z.; Prud’homme, R.K. Stabilized polymeric nanoparticles for controlled and efficient release of bifenthrin. Pest Manag. Sci. 2008, 64, 808–812.
- Simões, M.F.; Ottoni, C.A.; Antunes, A. Biogenic Metal Nanoparticles: A New Approach to Detect Life on Mars? Life 2020, 10, 28.
- Vo, T.; Nguyen, T.; Huynh, T.; Vo, T.; Nguyen, T.; Nguyen, D.; Dang, V.; Dang, C.; Nguyen, T. Biosynthesis of Silver and Gold Nanoparticles Using Aqueous Extract from Crinum latifolium Leaf and Their Applications Forward Antibacterial Effect and Wastewater Treatment. J. Nanomater. 2019, 2019, 14.
- Islam, M.N.; Rahman, F. Production and Modification of Nanofibrillated Cellulose Composites and Potential Applications. In Green Composites for Automotive Applications; Woodhead Publishing: Duxford, UK, 2019; pp. 115–141.
- Yang, W.; Peters, J.I.; Williams, R.O. Inhaled nanoparticles—A current review. Int. J. Pharm. 2008, 356, 239–247.
- Malerba, M.; Cerana, R. Chitosan effects on plant systems. Int. J. Mol. Sci. 2016, 17, 996.
- Kah, M.; Hofmann, T. Nanopesticide research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235.
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. J. Agric. Food Chem. 2012, 60, 9781–9792.
- Mishra, S.; Singh, H. Biosynthesized silver nanoparticles as a nanoweapon against phytopathogens: Exploring their scope and potential in agriculture. Appl. Microbiol. Biotechnol. 2015, 99, 1097–1107.
- Cota-Arriola, O.; Onofre Cortez-Rocha, M.; Burgos-Hernández, A.; Marina Ezquerra-Brauer, J.; Plascencia-Jatomea, M. Controlled release matrices and micro/nanoparticles of chitosan with antimicrobial potential: Development of new strategies for microbial control in agriculture. J. Sci. Food Agric. 2013, 93, 1525–1536.
- Kochkina, Z.; Pospeshny, G.; Chirkov, S. Inhibition by chitosan of productive infection of T-series bacteriophages in the Escherichia coli culture. Mikrobiologiia 1994, 64, 211–215.
- Pospieszny, H.; Chirkov, S.; Atabekov, J. Induction of antiviral resistance in plants by chitosan. Plant Sci. 1991, 79, 63–68.
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51.
- Sadeghi, R.; Rodriguez, R.J.; Yao, Y.; Kokini, J.L. Advances in nanotechnology as they pertain to food and agriculture: Benefits and risks. Annu. Rev. Food Sci. Technol. 2017, 8, 467–492.
- Pinilla, C.M.B.; Lopes, N.A.; Brandelli, A. Lipid-Based Nanostructures for the Delivery of Natural Antimicrobials. Molecules 2021, 26, 3587.
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Nanoencapsulation of Zataria multiflora essential oil preparation and characterization with enhanced antifungal activity for controlling Botrytis cinerea, the causal agent of gray mould disease. Innov. Food Sci. Emerg. Technol. 2015, 28, 73–80.
- Rahimi, V.; Hekmatimoghaddam, S.; Jebali, A.; Sadrabad, E.K.; Heydari, A.; Mohajeri, F.A. Chemical Composition and Antifungal Activity of Essential Oil of Zataria Multiflora. J. Nutr. Food Secur. 2019, 4, 1–6.
- Ilk, S.; Saglam, N.; Özgen, M. Kaempferol loaded lecithin/chitosan nanoparticles: Preparation, characterization, and their potential applications as a sustainable antifungal agent. Artif. Cells Nanomed. Biotechnol. 2017, 45, 907–916.
- Adamu, A.; Ahmad, K.; Siddiqui, Y.; Ismail, I.S.; Asib, N.; Bashir Kutawa, A.; Adzmi, F.; Ismail, M.R.; Berahim, Z. Ginger Essential Oils-Loaded Nanoemulsions: Potential Strategy to Manage Bacterial Leaf Blight Disease and Enhanced Rice Yield. Molecules 2021, 26, 3902.
- Prasad, R.; Kumar, V.; Prasad, K.S. Nanotechnology in sustainable agriculture: Present concerns and future aspects. Afr. J. Biotechnol. 2014, 13, 705–713.
- Acharya, A.; Pal, P.K. Agriculture nanotechnology: Translating research outcome to field applications by luencing environmental sustainability. NanoImpact 2020, 19, 100232.
- Echeverria, J.; de Albuquerque, R.D.D.G. Nanoemulsions of essential oils: New tool for control of vector-borne diseases and in vitro effects on some parasitic agents. Medicines 2019, 6, 42.
- Pedro, A.S.; Santo, I.E.; Silva, C.V.; Detoni, C.; Albuquerque, E. The use of nanotechnology as an approach for essential oil-based formulations with antimicrobial activity. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Médnez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; pp. 1364–1374.
- Lim, C.J.; Basri, M.; Omar, D.; Abdul Rahman, M.B.; Salleh, A.B.; Raja Abdul Rahman, R.N.Z. Physicochemical characterization and formation of glyphosate-laden nanoemulsion for herbicide formulation. Ind. Crops Prod. 2012, 36, 607–613.
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop Prot. 2012, 35, 64–70.
- Nirmala, M.J.; Nagarajan, R. Recent research trends in fabrication and applications of plant essential oil based nanoemulsions. J. Nanomed. Nanotechnol. 2017, 8, 434.
- Rai, M.; Ingle, A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012, 94, 287–293.
- Lu, W.C.; Huang, D.W.; Wang, C.C.R.; Yeh, C.H.; Tsai, J.C.; Huang, Y.T.; Li, P.H. Preparation, characterization, and antimicrobial activity of nanoemulsions incorporating citral essential oil. J. Food Drug Anal. 2018, 26, 82–89.
- Mahdavi, V.; Rafiee-Dastjerdi, H.; Asadi, A.; Razmjou, J.; Fathi Achachlouei, B. Synthesis of Zingiber officinale essential oil-loaded nanofiber and its evaluation on the potato tuber moth, Phthorimaea operculella (Lepidoptera: Gelechiidae). J. Crop Prot. 2018, 7, 39–49.
- Mujeebur, R.K.; Tanveer, F.R. Nanotechnology: Scope and Application in Plant Disease Management. Plant Pathol. J. 2014, 13, 214–231.
- Mustafa, M.A.; Ali, A.; Manickam, S.; Siddiqui, Y. Ultrasound-assisted chitosan–surfactant nanostructure assemblies: Towards maintaining postharvest quality of tomatoes. Food Bioprocess Technol. 2014, 7, 2102–2111.
- Zahid, N.; Maqbool, M.; Ali, A.; Siddiqui, Y.; Abbas Bhatti, Q. Inhibition in production of cellulolytic and pectinolytic enzymes of Colletotrichum gloeosporioides isolated from dragon fruit plants in response to submicron chitosan dispersions. Sci. Hortic. 2019, 243, 314–319.
- Ali, A.; Cheong, C.K.; Zahid, N. Composite Effect of Propolis and Gum Arabic to Control Postharvest Anthracnose and Maintain Quality of Papaya during Storage. Int. J. Agric. Biol. 2014, 16, 1117–1122.
- Ali, A.; Zahid, N.; Manickam, S.; Siddiqui, Y.; Alderson, P.G. Double Layer Coatings: A new technique for maintaining physico-chemical characteristics and antioxidant properties of dragon fruit during storage. Food Bioprocess Technol. 2014, 7, 2366–2374.
- Mousa, A.A.; Hassan, A.; Mahindra, R.; Ernest, S.; Kamel, A.A. Myconanoparticles: Synthesis and their role in phytopathogens management. Biotechnol. Biotechnol. Equip. 2015, 29, 221–236.
- Maluin, F.N.; Hussein, M.Z.; Idris, A.S. An Overview of the Oil Palm Industry: Challenges and Some Emerging Opportunities for Nanotechnology Development. Agronomy 2020, 10, 356.
- Kaushal, M. Role of microbes in plant protection using intersection of nanotechnology and biology. In Nanobiotechnology Applications in Plant Protection; Abd-Elsalam, K.A., Prasad, R., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 111–135.
- Kumari, M.; Ernest, V.; Mukherjee, A.; Chandrasekaran, N. In vivo nanotoxicity assays in plant models. Methods Mol. Biol. 2012, 926, 399–410.
- Park, H.-J.; Kim, S.H.; Kim, H.J.; Choi, S.-H. A new composition of nanosized silica-silver for control of various plant diseases plant. Pathol. J. 2006, 22, 295–302.
- Corredor, E.; Testillano, P.S.; Coronado, M.-J.; González-Melendi, P.; Fernández-Pacheco, R.; Marquina, C.; Ibarra, M.R.; De La Fuente, J.M.; Rubiales, D.; de Luque, A.P.; et al. Nanoparticle penetration and transport in living pumpkin plants: In situ subcellular identification. BMC Plant Biol. 2009, 9, 45.
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Biris, A.S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 2009, 3, 3221–3227.
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 20, 1–8.
- Ge, Y.; Schimel, J.P.; Holden, P.A. Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ. Sci. Technol. 2011, 45, 1659–1664.
- Lee, S.; Kim, S.; Kim, S.; Lee, I. Effects of soil-plant interactive system on response to exposure to ZnO nanoparticles. J. Microbiol. Biotechnol. 2012, 22, 1264–1270.
- Degrassi, G.; Bertani, I.; Devescovi, G.; Fabrizi, A.; Gatti, A.; Venturi, V. Response of plant-bacteria interaction models to nanoparticles. EQA 2012, 8, 39–50.
- Nowack, B.; Ranville, J.F.; Diamond, S.; Gallego-Urrea, J.A.; Metcalfe, C.; Rose, J.; Horne, N.; Koelmans, A.A.; Klaine, S.J. Potential scenarios for nanomaterial release and subsequent alteration in the environment. Environ. Toxicol. Chem. 2012, 31, 50–59.
- González-Melendi, P.; Fernández-Pacheco, R.; Coronado, M.J.; Corredor, E.; Testillano, P.S.; Risueño, M.C.; Marquina, C.; Ibarra, M.R.; Rubiales, D.; de Luque, A.P. Nanoparticles as smart treatment-delivery systems in plants; assessment of different techniques of microscopy for their visualization in plant tissues. Ann. Bot. 2008, 101, 187–195.




