| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Norazian Mohd Hassan | + 6591 word(s) | 6591 | 2021-07-28 06:18:01 | | | |
| 2 | Vivi Li | Meta information modification | 6591 | 2021-09-16 03:34:40 | | |
Video Upload Options
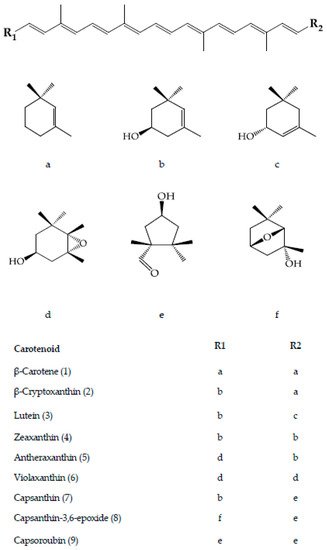
Pepper of the Capsicum species is a common ingredient in various food preparations by different cultures worldwide. The Capsicum is recognised by its five main domesticated species, namely Capsicum annuum, C. baccatum, C. chinense, C. frutescens and C. pubescens. The genetic diversity in Capsicum offers fruits in wide ranges of morphology and carotenoid profile. Carotenoids enhance the value of pepper from a nutritional standpoint, despite being commonly prized for the pharmacologically active pungent capsaicinoids. Carotenoids of pepper comprise mainly of the unique, powerful and highly stable capsanthin and capsoroubin, together with β-carotene, β-cryptoxanthin, lutein, zeaxanthin, antheraxanthin and violaxanthin. These carotenoids are present at diverse profile and varying levels, biosynthetically connected to the fruit maturity stages.
1. Introduction
2. Carotenoids of Capsicum Species
| Species | Cultivar | Colour | Carotenoid Concentration | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||||
| Red Mountain | Red | 31.8 ± 4.54 | 4.6 ± 0.71 | 2.73 ± 0.77 | 0.59 ± 0.16 | ND | 3.61 ± 0.44 | ND | ND | 43.32 ± 6.88 | ||
| Magnifico | Red | 28.33 ± 2.83 | 4.48 ± 0.78 | 2.06 ± 0.06 | 0.4 ± 0.04 | ND | ND | ND | ND | 35.99 ± 3.51 | ||
| Nagano | Red | 22.88 ± 1.65 | 4.01 ± 0.32 | 2.01 ± 0.05 | 0.31 ± 0.02 | ND | ND | ND | ND | 29.82 ± 2.01 | ||
| aC. annuum | Preludium | Red | 29.56 ± 0.67 | 2.67 ± 0.09 | 1.94 ± 0.17 | 0.33 ± 0.02 | ND | ND | ND | ND | 35.05 ± 0.90 | [21] |
| Adami Red | Red | 28.62 ± 0.99 | 2.59 ± 0.17 | 1.88 ± 0.18 | 0.32 ± 0.02 | ND | ND | ND | ND | 33.94 ± 1.38 | ||
| Raon Red | Red | 13.26 ± 5.14 | 1.74 ± 0.82 | 0.83 ± 0.17 | 0.13 ± 0.07 | ND | ND | ND | ND | 13.51 ± 1.51 | ||
| Red | Red | 21.55 ± 5.93 | 3.09 ± 0.97 | 1.13 ± 0.21 | 0.30 ± 0.09 | ND | ND | ND | ND | 29.11 ± 1.52 | ||
| RD-Glory | Red | 3.98 ± 0.47 | 0.95 ± 0.09 | 0.86 ± 0.02 | 0.47 ± 0.24 | ND | 24.05 ± 0.16 | ND | ND | 37.25 ± 0.63 | ||
| Mazzona | Orange | ND | ND | 1.23 ± 0.01 | 1.12 ± 0.04 | 28.39 ± 1.00 | 151.39 ± 4.85 | 6.19 ± 0.09 | 0.29 ± 0.00 | 190.43 ± 5.66 | ||
| Orange Glory | Orange | ND | ND | 0.98 ± 0.09 | 1.09 ± 0.08 | 15.77 ± 1.52 | 145.92 ± 13.17 | 5.52 ± 2.00 | 0.26 ± 0.04 | 171.95 ± 17.13 | ||
| Orange Star | Orange | ND | ND | 0.62 ± 0.05 | 0.64 ± 0.08 | 25.27 ± 3.21 | 140.05 ± 14.48 | 4.47 ± 1.59 | 0.22 ± 0.10 | 172.77 ± 19.71 | ||
| aC. annuum | Raon Orange | Orange | ND | ND | 0.76 ± 0.01 | 0.55 ± 0.01 | 22.24 ± 0.51 | 88.80 ± 1.06 | 0.89 ± 0.07 | ND | 115.01 ± 1.46 | [21] |
| Mini Goggal Orange | Orange | ND | ND | 0.63 ± 0.01 | 0.64 ± 0.01 | 17.32 ± 0.48 | 89.89 ± 2.89 | 1.87 ± 0.77 | ND | 111.83 ± 4.25 | ||
| Orange | Orange | ND | ND | 0.75 ± 0.03 | 0.61 ± 0.01 | 25.10 ± 0.39 | 115.53 ± 1.11 | 3.06 ± 0.32 | ND | 146.93 ± 1.93 | ||
| OE-Glory | Orange | ND | ND | 0.66 ± 0.16 | 0.48 ± 0.11 | 19.31 ± 4.00 | 85.06 ± 19.08 | 2.00 ± 0.49 | 0.28 ± 0.07 | 109.69 ± 24.32 | ||
| Jorrit | Yellow | ND | ND | 0.15 ± 0.04 | 0.03 ± 0.01 | 8.75 ± 2.29 | 0.66 ± 0.31 | 0.81 ± 0.25 | 0.14 ± 0.03 | 11.38 ± 3.14 | ||
| Coletti | Yellow | ND | ND | 0.21 ± 0.01 | 0.05 ± 0.00 | 13.83 ± 0.94 | 0.71 ± 0.06 | 1.48 ± 0.06 | 0.72 ± 0.30 | 18.2 ± 1.46 | ||
| Sven | Yellow | ND | ND | 0.15 ± 0.01 | 0.05 ± 0.01 | 13.16 ± 1.07 | 0.63 ± 0.04 | 1.06 ± 0.20 | 0.20 ± 0.01 | 16.21 ± 1.42 | ||
| aC. annuum | Atalante | Yellow | ND | ND | 0.17 ± 0.00 | 0.03 ± 0.00 | 11.97 ± 0.56 | 0.45 ± 0.09 | ND | 1.05 ± 0.57 | 15.31 ± 1.37 | [21] |
| Raon Yellow | Yellow | ND | ND | 0.30 ± 0.00 | 0.43 ± 0.04 | 21.08 ± 0.84 | 2.22 ± 0.18 | 1.35 ± 0.41 | 2.85 ± 1.34 | 29.70 ± 0.67 | ||
| Yellow | Yellow | ND | ND | 0.29 ± 0.06 | 0.29 ± 0.00 | 18.32 ± 6.79 | 1.27 ± 0.08 | ND | 0.85 ± 0.09 | 22.32 ± 7.23 | ||
| YW-Glory | Yellow | ND | ND | 0.27 ± 0.03 | 0.27 ± 0.07 | 15.17 ± 1.25 | 1.19 ± 0.52 | ND | 0.55 ± 0.21 | 18.45 ± 1.02 | ||
| b,cC. annuum | Pimenta PMO | Red | NA | NA | b 4442.72 ± 1.0 | b111.12 ± 0.19 | b 195.75 ± 0.25 | b 460.03 ± 3.13 | NA | NA | c 1064.35 ± 19.38 | [33] |
| Pimenta Amarela | Yellow | NA | NA | ND | ND | b 312.79 ± 0.12 | b 8.78 ± 0.03 | NA | NA | c91.26 ± 8.59 | ||
| b,cC. baccatum var. umbilicatum | Pimenta Chumbinho Baião | Red | NA | NA | b 454.08 ± 0.20 | b 1456.24 ± 0.80 | b 139.85 ± 0.14 | b 1291.29 ± 0.40 | NA | NA | c 580.98 ± 51.91 | [33] |
| Pimenta Biquinho | Orange | NA | NA | ND | ND | b 687.71 ± 0.66 | b 25.56 ± 0.02 | NA | NA | c 208.45 ± 12.65 | ||
| b,cC. chinense | Pimenta Curuçazinho | Yellow | NA | NA | b 33.48 ± 1.06 | ND | b 89.91 ± 0.65 | b 355.68 ± 0.35 | NA | NA | c 73.80 ± 2.93 | [33] |
| Pimenta Murupi | Yellow | NA | NA | ND | ND | b 262.96 ± 0.08 | b 20.81 ± 5.99 | NA | NA | c 79.56 ± 7.12 | ||
3. Health-Promoting Functional Attributes
| Functional Property | Type of Study | Biological/Pharmacological/ Clinical Activity | Species/Variety/ Cultivar |
Extract/Fraction/ Carotenoid |
Carotenoid Content/Purity | Dose | Effects/Identified Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|
| Hypoglycaemic | In vitro | α-Amylase inhibitory | C. chinense cv. Habanero | Lipophilic hexane fraction from ethanol extract of immature (I) and mature (M) fruits. | 62.7 ± 5.5 (I) and 362 ± 7.8 (M) mg β-carotene eq./100 mg FW | IC50, 9.88 (I) and 29.58 (M) µg/mL |
A selective α-amylase inhibitory activity. No α-glucosidase inhibitory activity. | [40] |
| C. annuum var. acuminatum small | Lipophilic hexane fraction of ethanol extract from deseeded air-dried mature fruits. | Not determined | IC50, 6.9 µg/mL |
A selective α-amylase inhibitory activity. Inactive as α-glucosidase inhibitor. | [51] | |||
| C. annuum var. cerasiferum | Lipophilic hexane fraction of ethanol extract from deseeded air-dried mature fruits. | Not determined | IC50, 20.1 µg/mL |
A selective α-amylase inhibitory activity. No α-glucosidase inhibitory activity. | [51] | |||
| C. annuum var. acuminatum big | Lipophilic fraction of ethanol extract from deseeded air-dried immature fruits. | Not determined | IC50, 8.7 µg/mL |
A selective α-amylase inhibitory activity. Inactive as α-glucosidase inhibitor. | [52] | |||
| C. annuum cv. Fiesta, Acuminatum, Orange Thai and Cayenne Golden | Lipophilic hexane fraction of ethanol extract from deseeded air-dried immature (I) fruits. | Not determined | IC50, ranged from 9.1 to 28.6 µg/mL (I) | A selective α-amylase inhibitory activity, Fiesta > Cayenne Golden > Acuminatum > Orange Thai. No α-glucosidase inhibitory activity. | [53] | |||
| Anti-obesity | In vitro | Antiadipogenic on murine 3T3-L1 pre-adipocytes | Not determined | Capsanthin purified from commercialised red pepper powder. | Capsanthin, 100% | IC50, 2.5 ± 0.45 µM |
The activities resulted from potent adrenoceptor-β2-agonistic which is linked to the activation of hormone sensitive lipase. | [54] |
| Lipolytic in differentiated 3T3-L1 adipocytes | ED50, 0.872 ± 0.06 µM |
|||||||
| In vivo | Inhibition of weight gain in high-fat-diet-induced obese female C57BL/6C mice | Not determined | Capsanthin purified from commercialised red pepper powder. | Capsanthin, 100% | 1, 5, and 10 µmol | A dose-dependent enhancement of locomotive activity associated with excessive production of ATP with progressive weight loss. | [54] | |
| Skin photoprotective | In vitro | Anti UVB-induced cytotoxicity on normal human dermal fibroblasts | Not determined | Purified capsanthin, capsorubin and lutein from commercialised paprika oleoresin. | Capsanthin, 100% Capsorubin, 100% Lutein, 100% |
1 µM | Protective effects by producing significant decrease in the formation of UVB-induced DNA strand break and counteracting caspase-3 cleavage. | [55] |
| Clinical | Anti-UV-induced skin damage in a double-blind placebo-controlled study (Japanese male and female, aged 30 to 50 years with skin phototype II). | Not determined | A commercial paprika-xanthophyll preparation (PapriX- oil). | 333 mg of PapriX-oil gelatine capsule (l 9 mg total xanthophylls, 5 mg capsanthin, and 0.5 mg cryptoxanthin) |
One capsule orally with a meal every evening for 5 weeks. | Suppression of UV-induced erythema and pigmentation by the xanthophylls’ strong singlet oxygen quenching activity that counteracted UV-induced photooxidative stress and acute inflammation response. | [56] | |
| Anti-Inflammatory | In vitro | Anti-inflammatory in obesity-induced inflammation in 3T3-L1 adipocytes co-cultured with RAW264.7 macrophages. | Not determined | Purified paprika pigments from commercialised paprika carotenoids. | Capsanthin, 44.3% Capsorubin, 12.8% Capsanthin analog |
15, 30, 60 µg/mL | Attenuation of inflammation in the 3T3-L1 adipocytes by dose dependant suppression of adipocytokine mRNA gene expression for IL-6, TNF-α, MCP-1 and resistin, and significant (p < 0.05) deceased versus control in nitric oxide release. | [57] |
| In vivo | Anti-inflammatory in carrageenan-induced mice paw oedema. | C. annuum var. guajillo (Guajilo 15660) | Petroleum ether fraction of acetone extract from dried fruits. | β-carotene, 10.01% β-cryptoxanthin, 11.96% Violaxanthin, 49.06% |
5, 20 and 80 mg/kg | Significant (p < 0.05) reduction of oedema at 5 hr time point, comparable to indomethacine (7 mg/kg) | [3] | |
| Anti-inflammatory in adjuvant-induced mice paw oedema. | C. annuum (Ukrainian cayenne bitter pepper) |
Petroleum ether fraction of acetone extract from air-dried fruits. | Carotenoid extract containing 69.3% yellow and 30.7% red fractions rich in capsanthin, lutein and β-carotene. | Topical application of ointment containing 2 mg extract/g daily for 20 days. | Reduction of serum cholinesterase activity by 1.3 times and double decrease in the serum seromucoid concentration that indicated good inhibitory activity. | [58] | ||
| Anti-Hyperlipidemic | In vivo | Inhibition of CETP activity and anti-atherosclerotic in cholesterol-fed male New Zealand white rabbits. | Not determined | Mixture of red pepper powder, Purina lab Chow (Purina Chemical, Korea) and 1% cholesterol. | Red pepper powder, 1% | 100 g supplement/day for 12 weeks | Significant (p < 0.05) lowered in total cholesterol, triglyceride, LDL-C, VLDL-C, and VLDL-TG levels, atherogenic index and CETP activity whereas higher HDL-C level than in the control group during the experimental period. | [59] |
| Promotion of plasma LDL-C levels and hepatic gene expression in young male Wistar rats. | Not determined | Purified capsanthin from non-acylated capsanthin powder. | Capsanthin, 100% | 0·25 and 0·49 mmol/kg diet ad libitum for 2 weeks | A dose-dependent increment of HDL-C associated with up-regulation of mRNA for apoA5 and LCAT. | [60] | ||
| Hepatoprotective | In vivo | Liver protectant against paracetamol induced hepatotoxicity in male albino Wistar rats. | C. annuum | Ethanol extract of dried red fruit powder. | Lycopene, 4.69 ± 0.01 mg/g tissue |
250 and 500 mg/kg body weight | Improvement of liver functions as indicated by significant decreased in liver weight and lipid levels while increased in glycogen and glycoprotein in liver tissue as well as decreased in serum liver markers (AST, ALT ALP) and bilirubin, (p < 0.001) versus untreated rats. | [61] |
| Chemopreventive | In vitro | Protective activity against H2O2-induced inhibition of GJIC in WB-F344 rat liver epithelial cells. | C. annuum | Diethyl ether fraction of the red fruits (RPE), capsanthin (CST) and β-carotene (BCT). | RPE CST, 100% BCT, 100% |
RPE, 10 and 25 µg/mL CST, 0.5 and 1.0 µM BCT, 0.1 and 0.5 µM |
RPE, CST and BCT prevented GJIC inhibition by blocking the generation and actions of ROS and enhancing Cx43 mRNA expression, protein levels and the activity of ERK1/2, p38 and JNK MAP kinases. | [62] |
| MDR-efflux protein inhibitory in human MDR-1 gene-transfected L1210 mouse lymphoma cells and human breast cancer cells MDA-MB-231 (HTB-26). | C. annuum | Purified capsanthin and capsorubin from red paprika. | Capsanthin, 100% Capsorubin, 100% |
4 µg/mL | Capsanthin and capsorubin enhanced rhodamine 123 accumulation 30-fold relative to nontreated lymphoma cells that suggested their MDR reversal effect on the cells. | [63] | ||
| Apoptosis induction in human breast cancer cells MDA- MB-231 (HTB-26). | C. annuum | Purified capsanthin from red paprika. | Capsanthin, 100% | 2 µg/mL | Capsanthin induced apoptosis in both tumour cells comparable to that of the positive control, M627 (50 µg/mL). | [63] |
3.1. Antidiabetic Potential
3.2. Antiadipogenic and Anti-Obesity
3.3. Skin Photoprotective
3.4. Macula Pigments
3.5. Antinociceptive/Analgesic and Anti-Inflammatory
3.6. Antihyperlipidemic and Cardioprotective
3.7. Hepatoprotective
3.8. Chemopreventive
3.9. Provitamin A Activity
References
- Pugliese, A.; Loizzo, M.R.; Tundis, R.; O’Callaghan, Y.; Galvin, K.; Menichini, F.; O’Brien, N. The effect of domestic processing on the content and bioaccessibility of carotenoids from chili peppers (Capsicum species). Food Chem. 2013, 141, 2606–2613.
- Ludy, M.J.; Moore, G.E.; Mattes, R.D. The Effects of capsaicin and capsiate on energy balance: Critical review and meta-analyses of studies in humans. Chem. Sens. 2012, 37, 103–121.
- Hernández-Ortega, M.; Ortiz-Moreno, A.; Hernández-Navarro, M.D.; Chamorro-Cevallos, G.; Dorantes-Alvarez, L.; Necoechea-Mondragón, H. Antioxidant, antinociceptive, and anti-Inflammatory effects of carotenoids extracted from dried pepper (Capsicum annuum L.). J. Biomed. Biotechnol. 2012, 2012, 524019.
- Pugliese, A.; O’Callaghan, Y.; Tundis, R.; Galvin, K.; Menichini, F.; O’Brien, N.; Loizzo, M.R. In vitro investigation of the bioaccessibility of carotenoids from raw, frozen and boiled red chili peppers (Capsicum annuum). Eur. J. Nutr. 2014, 53, 501–510.
- Eroglu, A.; Harrison, E.H. Carotenoid metabolism in mammals, including man: Formation, occurrence, and function of apocarotenoids. J. Lipid Res. 2013, 54, 1719–1730.
- Westphal, A.; Jena, V.B. Carotenoids: Properties, distribution, bioavailability, metabolism and health effects. Ernaehrungs Umschau Int. 2015, 11, 196–207.
- Bhandari, S.R.; Jung, B.-D.; Baek, H.-Y.; Lee, Y.-S. Ripening-dependent changes in phytonutrients and antioxidant activity of red pepper (Capsicum annuum L.) fruits cultivated under open-field conditions. Hortic. Sci. 2013, 48, 1275–1282.
- Zimmer, A.R.; Leonardi, B.; Miron, D.; Schapoval, E.; Oliveira, J.R.D.; Gosmann, G. Antioxidant and anti-inflammatory properties of Capsicum baccatum: From traditional use to scientific approach. J. Ethnopharmacol. 2012, 139, 228–233.
- Arimboor, R.; Natarajan, R.B.; Menon, K.R.; Chandrasekhar, L.P.; Vidya Moorkoth, V. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: Analysis and stability—A review. J. Food Sci. Technol. 2015, 52, 1258–1271.
- Chengaiah, B.; Rao, K.M.; Kumar, K.M.; Alagusundaram, M.; Chetty, C.M. Medicinal importance of natural dyes-a review. Int. J. PharmTech. Res. 2010, 2, 144–154.
- Nadeem, M.; Muhammad Anjum, F.; Rafiq Khan, M.; Saeed, M.; Riaz, A. Antioxidant potential of bell pepper (Capsicum annuum L.)—A review. Pak. J. Food Sci. 2011, 21, 45–51.
- Chen, Y.-H.; Zou, X.H.; Zheng, T.-Z.; Zhou, Q.; Qiu, H.; Chen, Y.-L.; He, M.; Du, J.; Lei, H.K.; Zhou, P. High spicy food intake and risk of cancer: A meta-analysis of case–control studies. Chin. Med. J. 2017, 130, 2241–2250.
- The Biochemistry of Peppers. Available online: www.chemistryviews.org/details/ezine/6108461/The_Biochemistry_of_Peppers.html (accessed on 26 November 2018).
- Deli, J.; Molnár, P.; Matus, Z.; Tóth, G. Carotenoid composition in the fruits of red paprika (Capsicum annuum var. lycopersiciforme rubrum) during ripening; biosynthesis of carotenoids in red paprika. J. Agric. Food Chem. 2001, 49, 1517–1523.
- Kiokias, S.; Proestos, C.; Varzakas, T. A review of structure, biosynthesis, absorption of carotenoids-analysis and properties of their common natural extracts. Curr. Res. Nutr. Food Sci. 2016, 4, 25–37.
- Egea, I.; Barsan, C.; Bian, W.; Purgatto, E.; Latché, A.; Chervin, C.; Bouzayen, M.; Pech, J.-C. Chromoplast differentiation: Current status and perspectives. Plant Cell Physiol. 2010, 51, 1601–1611.
- Gómez-García, M.R.; Ochoa-Alejo, N. Biochemistry and molecular biology of carotenoid biosynthesis in chili peppers (Capsicum spp.). Int. J. Mol. Sci. 2013, 14, 19025–19053.
- Ha, S.H.; Kim, J.B.; Park, J.S.; Lee, S.W.; Cho, K.J. A comparison of the carotenoid accumulation in Capsicum varieties that show different ripening colours: Deletion of the capsanthin-capsorubin synthase gene is not a prerequisite for the formation of a yellow pepper. J. Exp. Bot. 2007, 58, 3135–3144.
- Rodriguez-Burruezo, A.; Gonzalez-Mas Mdel, C.; Nuez, F. Carotenoid composition and vitamin A value in aji (Capsicum baccatum L.) and rocoto (C. pubescens R. and P.), 2 pepper species from the Andean region. J. Food Sci. 2010, 75, S446–S453.
- Cervantes-Paz, B.; Yahia, E.M.; Ornelas-Paz, J.J.; Victoria-Campos, C.I.; Ibarra-Junquera, V.; Pérez-Martínez, J.D.; Escalante-Minakata, P. Antioxidant activity and content of chlorophylls and carotenoids in raw and heat-processed Jalapeño peppers at intermediate stages of ripening. Food Chem. 2014, 146, 188–196.
- Kim, J.-S.; An, C.G.; Park, J.-S.; Lim, Y.P.; Kim, S. Carotenoid profiling from 27 types of paprika (Capsicum annuum L.) with different colors, shapes, and cultivation methods. Food Chem. 2016, 201, 64–71.
- Wall, M.M.; Waddell, C.A.; Bosland, P.W. Variation in β-carotene and total carotenoid content in fruits of Capsicum. Hortic. Sci. 2001, 36, 746–749.
- Umigai, N.; Murakami, K.; Shimizu, R.; Takeda, R.; Azuma, T. Safety evaluation and plasma carotenoid accumulation in healthy adult subjects after 12 weeks of paprika oleoresin supplementation. J. Oleo Sci. 2018, 67, 225–234.
- Schweiggert, U.; Kammerer, D.R.; Carle, R.; Schieber, A. Characterization of carotenoids and carotenoid esters in red pepper pods (Capsicum annuum L.) by high-performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2617–2628.
- Nishino, A.; Ichihara, T.; Takaha, T.; Kuriki, T.; Nihei, H.; Kawamoto, K.; Yasui, H.; Maoka, T. Accumulation of paprika carotenoids in human plasma and erythrocytes. J. Oleo Sci. 2015, 64, 1135–1142.
- Giuffrida, D.; Dugo, P.; Torre, G.; Bignardi, C.; Cavazza, A.; Corradini, C.; Dugo, G. Characterization of 12 Capsicum varieties by evaluation of their carotenoid profile and pungency determination. Food Chem. 2013, 140, 794–802.
- Rodriguez-Uribe, L.; Guzman, I.; Rajapakse, W.; Richins, R.D.; O’Connell, M.A. Carotenoid accumulation in orange-pigmented Capsicum annuum fruit, regulated at multiple levels. J. Exp. Bot. 2012, 63, 517–526.
- Muhammad Shah, S.N.; Tian, S.-L.; Gong, Z.-H.; Mohamed Hamid, A. Studies on metabolism of capsanthin and its regulation under different conditions in pepper fruits (Capsicum spp.). Ann. Res. Rev. Biol. 2014, 4, 1106–1120.
- Pugliese, A.; O’Callaghan, Y.; Tundis, R.; Galvin, K.; Menichini, F.; O’Brien, N.; Loizzo, M.R. In vitro assessment of the bioaccessibility of carotenoids from sun-dried chilli peppers. Plant Foods Hum. Nutr. 2014, 69, 8–17.
- Topuz, A.; Dincer, C.; Özdemir, K.S.; Feng, H.; Kushad, M. Influence of different drying methods on carotenoids and capsaicinoids of paprika (Cv., Jalapeno). Food Chem. 2011, 129, 860–865.
- Guzman, I.; Hamby, S.; Romero, J.; Bosland, P.W.; O’Connell, M.A. Variability of carotenoid biosynthesis in orange colored Capsicum spp. Plant Sci. 2010, 179, 49–59.
- Acunha, T.D.S.; Crizel, R.L.; Tavares, I.B.; Barbieri, R.L.; de Pereira, C.M.P.; Cesar Valmor Rombaldi, C.V.; Chaves, F.C. Bioactive compound variability in a Brazilian Capsicum pepper collection. Crop Sci. 2017, 57, 1–13.
- Carvalho, A.V.; de Andrade Mattietto, R.; de Oliveira Rios, A.; de Almeida Maciel, R.; Moresco, S.K.; de Souza Oliveira, T.C. Bioactive compounds and antioxidant activity of pepper (Capsicum sp.) genotypes. J. Food Sci. Technol. 2015, 52, 7457–7464.
- Chávez-Mendoza, C.; Sanchez, E.; Muñoz-Marquez, E.; Sida-Arreola, J.P.; Flores-Cordova, M.A. Bioactive compounds and antioxidant activity in different grafted varieties of bell pepper. Antioxidants 2015, 4, 427–446.
- Vinković, T.; Gluščić, V.; Mendaš, G.; Vinković, V.I.; Parađiković, N.; Tkalec, M.; Štolfa Camagajevac, S.I. Phytochemical composition of ground paprika from Eastern Danube region. Agriculture 2018, 24, 3–12.
- Keyhaninejad, N.; Richins, R.D.; O’Connell, M.A. Carotenoid content in field-grown versus greenhouse-grown peppers: Different responses in leaf and fruit. Hort. Sci. 2012, 47, 852–855.
- Zhang, Y.; Navarro, E.; Cánovas-Márquez, J.T.; Almagro, L.; Chen, H.; Chen, Y.Q.; Zhang, H.; Torres-Martínez, S.; Chen, W.; Garre, V. A new regulatory mechanism controlling carotenogenesis in the fungus Mucor circinelloides as a target to generate β-carotene over-producing strains by genetic engineering. Microb. Cell Fact. 2016, 15, 99.
- Perucka, I.; Materska, M. Antioxidant vitamin contents of Capsicum annuum fruit extracts as affected by processing and varietal factors. Acta Sci. Pol. Technol. Aliment 2007, 6, 67–74.
- Suzuki, K.; Mori, M.; Ishikawa, K.; Takizawa, K.; Nunomura, O. Carotenoids composition in mature Capsicum annuum. Food Sci. Technol. Res. 2007, 17, 77–80.
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M.R.; Conforti, F.; Statti, G.; de Cindio, B.; Houghton, P.J.; Menichini, F. The influence of fruit ripening on the phytochemical content and biological activity of Capsicum chinense Jacq. cv Habanero. Food Chem. 2009, 114, 553–560.
- Bunea, A.; Socaciu, C.; Pintea, A. Xanthophyll esters in fruits and vegetables. Not. Bot. Hortic. Agrobot. 2014, 42, 310–324.
- Khoo, H.; Prasad, K.N.; Kong, K.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738.
- Giuffrida, D.; Zoccali, B.; Giofrè, S.V.; Dugo, P.; Mondello, L. Apocarotenoids determination in Capsicum chinense Jacq. cv. Habanero, by supercritical fluid chromatography-triple-quadrupole/mass spectrometry. Food Chem. 2017, 231, 316–323.
- Maoka, T.; Fujiwara, Y.; Hashimoto, K.; Akimoto, N. Isolation of a series of apocarotenoids from the fruits of the red paprika Capsicum annuum L. J. Agric. Food Chem. 2001, 49, 1601–1606.
- Maoka, T.; Akimoto, T.N.; Fujiwara, Y.; Hashimoto, K. Structure of new carotenoids with the 6-oxo-κ end group from the fruits of paprika, Capsicum annuum. J. Nat. Prod. 2004, 67, 115–117.
- Lv, J.; Qi, L.; Yu, C.; Yang, L.; Guo, Y.; Chen, Y.; Bian, Z.; Sun, D.; Du, J.; Ge, P.; et al. Consumption of spicy foods and total and cause specific mortality: Population based cohort study. BMJ 2015, 351, 3942.
- Chopan, M.; Littenberg, B. The association of hot red chili pepper consumption and mortality: A large population-based cohort study. PLoS ONE 2017.
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488.
- Kim, S.; Ha, T.Y.; Hwang, I.K. Analysis, bioavailability, and potential healthy effects of capsanthin, natural red pigment from Capsicum spp. Food Rev. Int. 2009, 25, 198–213.
- Perera, C.O.; Yen, G.M. Functional properties of carotenoids in human health. Int. J. Food Prop. 2007, 10, 201–230.
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Bonesi, M.; Conforti, F.; Statti, G.; De Luca, D.; de Cindio, B.; Menichini, F. Comparative study on the chemical composition, antioxidant properties and hypoglycaemic activities of two Capsicum annuum L. cultivars (acuminatum small and cerasiferum). Plant Foods Hum. Nutr. 2011, 66, 261–269.
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Bonesi, M.; Conforti, F.; De Luca, D.; Menichini, F. Air-dried Capsicum annuum var. acuminatum medium and big: Determination of bioactive constituents, antioxidant activity and carbohydrate-hydrolyzing enzymes inhibition. Food Res. Int. 2012, 45, 170–176.
- Tundis, R.; Menichini, F.; Bonesi, M.; Conforti, F.; Statti, G.; Menichini, F.; Loizzo, M.R. Antioxidant and hypoglycaemic activities and their relationship to phytochemicals in Capsicum annuum cultivars during fruit development. LWT Food Sci. Technol. 2013, 53, 370–377.
- Jo, S.J.; Kim, J.W.; Choi, H.O.; Kim, J.H.; Kim, H.J.; Woo, S.H.; Han, B.H. Capsanthin inhibits both adipogenesis in 3T3-L1 preadipocytes and weight gain in high-fat diet-induced obese mice. Biomol. Ther. 2017, 25, 329–336.
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. Carotenoids exclusively synthesized in red pepper (capsanthin and capsorubin) protect human dermal fibroblasts against UVB induced DNA damage. Photochem. Photobiol. Sci. 2016, 15, 1204–1211.
- Nishino, A.; Sugimoto, K.; Sambe, H.; Ichihara, T.; Takaha, T.; Kuriki, T. Effects of dietary paprika xanthophylls on ultraviolet light-induced skin damage: A double-blind placebo-controlled study. J. Oleo Sci. 2018, 67, 863–869.
- Maeda, H.; Saito, S.; Nakamura, N.; Maoka, T. Paprika pigments attenuate obesity-induced inflammation in 3T3-L1 adipocytes. ISRN Inflamm. 2013.
- Boiko, Y.A.; Kravchenko, I.A.; Shandra, A.A.; Boiko, I.A. Extraction, identification and anti-inflammatory activity of carotenoids out of Capsicum Anuum L. J. Herbmed. Pharmacol. 2017, 6, 10–15.
- Kwon, M.-J.; Song, Y.-S.; Choi, M.-S.; Song, Y.-K. Red pepper attenuates cholesteryl ester transfer protein activity and atherosclerosis in cholesterol-fed rabbits. Clin. Chim. Acta 2003, 332, 37–44.
- Aizawa, K.; Inakuma, T. Dietary capsanthin, the main carotenoid in paprika (Capsicum annuum), alters plasma high-density lipoprotein-cholesterol levels and hepatic gene expression in rats. Br. J. Nutr. 2009, 102, 1760–1766.
- Priya, A.S.; Anitha, A. In vivo hepatoprotective effect of ethanolic extract of Capsicum annuum L. red variety against paracetamol induced hepatotoxicity in male wistar albino rats. Eur. J. Biomed. Pharm. Sci. 2017, 4, 326–332.
- Kim, J.-S.; Lee, W.-M.; Rhee, H.C.; Kim, S. Red paprika (Capsicum annuum L.) and its main carotenoids, capsanthin and β-carotene, prevent hydrogen peroxide-induced inhibition of gap-junction intercellular communication. Chem. Biol. Interact. 2016, 254, 146–155.
- Molnár, J.; Gyémánt, N.; Mucsi, I.; Molnár, A.; Szabó, M.; Körtvélyesi, T.; Varga, A.; Molnár, P.; Tóth, G. Modulation of multidrug resistance and apoptosis of cancer cells by selected carotenoids. In Vivo 2004, 18, 237–244.
- Kou, L.; Du, M.; Zhang, C.; Dai, Z.; Li, X.; Zhang, B. The hypoglycemic, hypolipidemic and anti-diabetic nephritic activities of zeaxanthin in diet-streptozotocin-induced diabetic Sprague Dawley rats. Appl. Biochem. Biotechnol. 2017, 182, 944–955.
- Burrows, T.L.; Warren, J.M.; Colyvas, K.; Garg, M.L.; Collins, C.E. Validation of overweight children’s fruit and vegetable intake using plasma carotenoids. Obesity 2009, 17, 162–168.
- Canas, J.A.; Damaso, L.; Altomare, A.; Killen, K.; Hossain, J.; Balagopal, P. Insulin resistance and adiposity in relation to serum b-Carotene levels. J. Paediatr. 2012, 161, 58–64.
- Fernández-García, E. Skin protection against UV light by dietary antioxidants. Food Funct. 2014, 5, 1994–2003.
- Stahl, W.; Sies, H. β-Carotene and other carotenoids in protection from sunlight. Am. J. Clin. Nutr. 2012, 96, 1179S–1184S.
- Palombo, P.; Fabrizi, G.; Ruocco, V.; Ruocco, E.; Fluhr, J.; Roberts, R.; Morganti, P. Beneficial long-term effects of combined oral/topical antioxidant treatment with the carotenoids lutein and zeaxanthin on human skin: A double-blind, placebo-controlled study. Skin Pharmacol. Physiol. 2007, 20, 199–210.
- Ma, L.; Yan, S.F.; Huang, Y.M.; Lu, X.R.; Qian, F.; Pang, H.L.; Xu, X.R.; Zou, Z.Y.; Dong, P.C.; Xiao, X.; et al. Effect of lutein and zeaxanthin on macular pigment and visual function in patients with early age-related macular degeneration. Ophthalmology 2012, 119, 2290–2297.
- Mozaffarieh, M.; Sacu, S.; Wedrich, A. The role of the carotenoids, lutein and zeaxanthin, in protecting against age-related macular degeneration: A review based on controversial evidence. Nutr. J. 2003, 2, 20.
- Connolly, E.E.; Beatty, S.; Loughman, J.; Howard, A.N.; Louw, M.S.; Nolan, J.M. Supplementation with all three macular carotenoids: Response, stability, and safety. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9207–9217.
- Wu, J.; Cho, E.; Willett, W.C.; Sastry, S.M.; Schaumberg, D.A. Intakes of lutein, zeaxanthin, and other carotenoids and age-related macular degeneration during 2 decades of prospective follow-up. JAMA Ophthalmol. 2015, 133, 1415–1424.
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014.
- Abdel-Aal, E.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients 2013, 5, 1169–1185.
- Voutilainen, S.; Nurmi, T.; Mursu, J.; Rissanen, T.H. Carotenoids and cardiovascular health. Am. J. Clin. Nutr. 2006, 83, 1265–1271.
- Ciccone, M.M.; Cortese, F.; Gesualdo, M.; Carbonara, S.; Zito, A.; Ricci, G.; De Pascalis, F.; Scicchitano, P.; Riccioni, G. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediat. Inflamm. 2013.
- Di Pietro, N.; Di Tomo, P.; Pandolfi, A. Carotenoids in cardiovascular disease prevention. JSM Atheroscler. 2016, 1, 1002.
- Karathanasis, S.K.; Freeman, L.A.; Gordon, S.M.; Remaley, A.T. The changing face of HDL and the best way to measure it. Clin. Chem. 2017, 63, 196–210.
- Murillo, A.G.; DiMarco, D.M.; Luz Fernandez, M. The potential of non-provitamin A carotenoids for the prevention and treatment of non-alcoholic fatty liver disease. Biology 2016, 5, 42.
- Ni, Y.; Zhuge, F.; Nagashimada, M.; Ota, T. Novel action of carotenoids on non-alcoholic fatty liver disease: Macrophage polarization and liver homeostasis. Nutrients 2016, 8, 391.
- Cao, Y.; Wang, C.; Liu, J.; Liu, Z.; Ling, W.; Chen, Y. Greater serum carotenoid levels associated with lower prevalence of nonalcoholic fatty liver disease in Chinese adults. Sci. Rep. 2015, 5, 12951.
- Yilmaz, B.; Sahin, K.; Bilen, H.; Bahcecioglu, I.H.; Bilir, B.; Ashraf, S.; Halazun, K.J.; Kucuk, O. Carotenoids and non-alcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 2015, 4, 161–171.
- González-Ponce, H.A.; Rincón-Sánchez, A.R.; Jaramillo-Juárez, F.; Moshage, H. Natural dietary pigments: Potential mediators against hepatic damage induced by over-the-counter non-steroidal anti-inflammatory and analgesic drugs. Nutrients 2018, 10, 117.
- Bhattacharya, S. Anticarcinogenic property of medicinal plants: Involvement of antioxidant role. In Medicinal Plants as Antioxidant Agents: Understanding Their Mechanism of Action and Therapeutic Efficacy; Capasso, A., Ed.; Research Signpost: Kerala, India, 2012; pp. 83–96. ISBN 978-81-308-0509-2.
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242.
- Nishino, H.; Murakoshi, M.; Ii, T.; Takemura, M.; Kuchide, M.; Kanazawa, M.; Mou, X.Y.; Wada, S.; Masuda, M.; Ohsaka, Y.; et al. Carotenoids in cancer chemoprevention. Cancer Metastasis Rev. 2002, 21, 257–264.
- De Flora, S.; Ferguson, L.R. Overview of mechanisms of cancer chemopreventive agents. Mutat. Res. 2005, 591, 8–15.
- Maoka, T.; Mochida, K.; Kozuka, M.; Ito, Y.; Fujiwara, Y.; Hashimoto, K.; Enjo, F.; Ogata, M.; Nobukuni, Y.; Tokuda, H.; et al. Cancer chemopreventive activity of carotenoids in the fruits of red paprika Capsicum annuum L. Cancer Lett. 2001, 172, 103–109.
- Huang, X.; Gao, Y.; Zhi, X.; Ta, N.; Jiang, H.; Zheng, J. Association between vitamin A, retinol and carotenoid intake and pancreatic cancer risk: Evidence from epidemiologic studies. Sci. Rep. 2016.
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.; Biesalski, H.K. β-Carotene as an important vitamin A source for humans. Nutrition 2010, 140, 2268S–2285S.
- Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005. WHO Global Database on Vitamin A Deficiency. 2009. Available online: www.who.int/vmnis/database/vitamina/x/en/ (accessed on 26 November 2018).
- Weber, D.; Grune, T. The contribution of beta-carotene to vitamin A supply of humans. Mol. Nutr. Food Res. 2012, 56, 251–258.
- Tomlekova, N.B.; White, P.J.; Thompson, J.A.; Penchev, E.A.; Nielen, S. Mutation increasing β-carotene concentrations does not adversely affect concentrations of essential mineral elements in pepper fruit. PLoS ONE 2017, 12, e0172180.
- Burri, B.J. Beta-cryptoxanthin as a source of vitamin A. J. Sci. Food Agric. 2015, 95, 1786–1794.
- Marin, A.; Ferreres, F.; Tomas-Barberaan, F.A.; Gil, M.I. Characterization and quantitation of antioxidant constituents of sweet pepper (Capsicum annuum L.). J. Agric. Food Chem. 2004, 52, 3861–3869.
- Blumhoff, R. Vitamin A and carotenoid toxicity. Food Nutr. Bull. 2001, 22, 320–334.
- Vitamin, A.U.S. Department of Health and Human Services. Available online: https://ods.od.nih.gov/factsheets/VitaminA-Consumer/ (accessed on 9 September 2018).