
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hip Seng Yim | + 3780 word(s) | 3780 | 2021-08-04 04:46:08 | | | |
| 2 | Vivi Li | Meta information modification | 3780 | 2021-09-15 06:10:50 | | |
Video Upload Options
The risk of macular degeneration can be reduced through the consumption of antioxidant-rich foods, supplements, and nutraceutical formulas. This study focuses on the antioxidants, vitamins, and minerals that have been reported for reducing the risk of macular degeneration and other eye-related diseases. Antioxidants including anthocyanins, carotenoids, flavonoids, and vitamins have been shown to reduce the risk of eye-related diseases. Anthocyanins extracted from berries are powerful antioxidants. Cyanidin, delphinidin, malvidin, pelargonidin, peonidin, and petunidin are anthocyanin aglycones detected in berries, currants, and other colored fruits and vegetables. β-Carotene, as well as xanthophyll lutein and zeaxanthin, have been reported to reduce the risk of macular degeneration. Flavonoids from plants help in the prevention of eye-related diseases through anti-inflammatory mechanisms. A combination of these antioxidants, vitamins, and minerals possess a synergistic effect on the prevention or risk reduction of macular degeneration. Formulas have been developed as dietary supplements to cater to the high demand from consumers and patients with eye problems.
1. Introduction
2. Antioxidant Nutrients
2.1. Anthocyanins
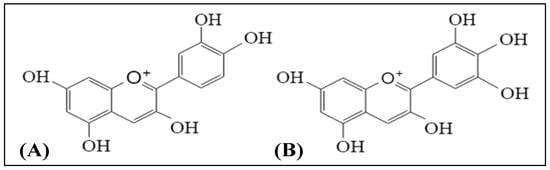
| Compounds | Study Design | Doses | Outcomes | Ref. |
|---|---|---|---|---|
| Anthocyanins | ||||
| Cyanidin 3-glucoside, cyanidin 3-rutinoside, delphinidin 3-glucoside, and delphinidin 3-rutinoside | In vitro bioassays: Rod outer segment and opsin membranes of frog | 10–50 µM | Positive outcomes: Cyanidin 3-glucoside and cyanidin 3-rutinoside stimulated regeneration of rhodopsin | [14] |
| Cyanidin 3-glucoside and delphinidin 3-glucoside | Cell culture: ARPE-19 cells (human retinal pigment epithelial cell line) | 5 μM | Positive outcome: Anthocyanins pre-treatment attenuated apoptosis of ARPE-19 cells induced by UVB irradiation. | [16] |
| Bilberry anthocyanin extract | In vivo study: Retinal degeneration model in pigmented rabbits (seven days) | 250 and 500 mg/kg/day | Positive outcomes: Attenuated changes caused by light to Bax, Bcl-2, and caspase-3. Increased the levels of superoxide dismutase, glutathione peroxidase, catalase, and total antioxidant capacity. Decreased malondialdehyde level in the retinal cells. Inhibited light-induced elevation in the levels of pro-inflammatory cytokines and angiogenic parameters (IL-1β and VEGF). |
[15] |
| Anthocyanin supplement | Randomized, parallel study. Postmenopausal, one woman (eight months) | 60 mg/day | Negative outcome (compared to baseline): No significant increase in macular pigment optical density | [19] |
| Carotenoids | ||||
| Lutein and zeaxanthin | Cell culture: ARPE-19 cells | 5 μM | Positive outcome: Anthocyanins pre-treatment attenuated apoptosis of ARPE-19 cells induced by UVB irradiation. | [16] |
| Lutein and zeaxanthin | Prospective, randomized, double-blind, placebo-controlled human study (12 months) | 10 mg/day lutein and 2 mg/day zeaxanthin | Positive outcomes: Significantly increased macular pigment optical density for treatment group compared to placebo. Significantly increased levels of serum lutein and zeaxanthin. Significantly improved chromatic contrast and photo stress recovery time for treatment group compared to placebo. |
[20] |
| Zeaxanthin-containing spirulina (4–5 g) | Human feeding trials (45 days) | 2.6–3.7 mg zeaxanthin | Positive outcome: Increased mean serum zeaxanthin concentration from 0.06 to 0.15 μmol/L. | [21] |
| Lutein, zeaxanthin, and meso-zeaxanthin in sunflower oil suspension | Double-blind, placebo-controlled, block-randomized human trial (12 months) | 10 mg lutein, 10 mg meso-zeaxanthin, and 2 mg zeaxanthin | Positive outcomes: Significantly improved contrast sensitivity of the visual function after 12 months supplementation compared to baseline. Treatment group had significant increase in serum concentrations of the xanthophylls in retina and macular pigment optical density compared to placebo. |
[22] |
| Lutein vs. α-tocopherol | Randomized, double-blind, placebo-controlled supplementation study (24 months) | 12 mg lutein mixtures and 100 mg α-tocopherol | Positive outcomes: Significantly increased serum concentration of lutein. Increased visual performance (visual acuity and glare sensitivity) in lutein group only. No toxic effect found—no significant changes in hematological and biochemical profiles. |
[23] |
| Oral total daily supplementation of antioxidants (mixture of β-carotene with other vitamins) | Randomized, placebo-controlled clinical trial (followed up for up to 10 years) | 15 mg β-carotene | Positive primary outcome (compared to baseline): Reduced risk of visual acuity lost. Negative secondary outcomes: No significant differences for all the secondary outcomes between the treatment group and placebo. | [24] |
| Nutrient intake (β-carotene, β-cryptoxathin, lutein, zeaxanthin, and lycopene) | Epidemiological study (Self-report data) | - | Positive outcome: Participants with the highest self-reported dietary intake of lutein and zeaxanthin were inversely associated with advancedage-related macular degeneration (AMD). | [25] |
| Total carotenoids (lutein/zeaxanthin, α-carotene, β-carotene, cryptoxanthin, and lycopene | Eye Disease Case-Control Study | - | Positive outcome: Serum carotenoid level significantly associated with the risk of AMD | [26] |
| Xanthophyll supplement | Randomized, parallel study. Postmenopausal women (8 months) | 6 mg lutein and 2 mg zeaxanthin daily) | Positive outcome: Dietary supplementation of lutein and zeaxanthin significantly increased the serum lutein and zeaxanthin levels. Negative outcome (compared to baseline): No significant increase in macular pigment optical density |
[19] |
2.2. Xanthophylls
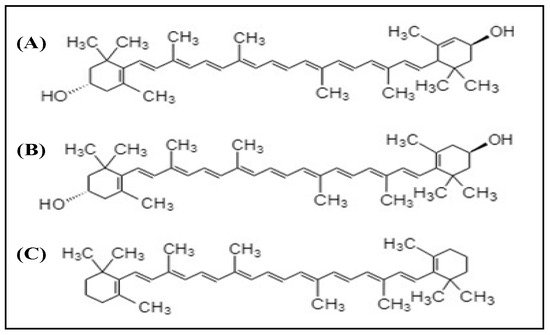
3. Vitamins
| Compounds | Study Design | Doses | Outcomes | Ref. |
|---|---|---|---|---|
| Vitamins | ||||
| Mixture of vitamin C and vitamin E with provitamin A carotenoid | Randomized, placebo-controlled clinical trials (followed-up for up to 10 years) | Vitamin C (500 mg) and vitamin E (400 IU) daily | Positive primary outcomes (compared to baseline): Increase in nuclear, cortical, or posterior subcapsular opacity grades or cataract surgery. Moderate visual acuity lost (≥15 letters). Negative secondary outcomes: No significant differences for all the secondary outcomes between the treatment group and placebo. |
[21] |
| Provitamin A β-carotene, vitamin C, and vitamin E | Age-Related Eye Disease Study | - | Positive outcomes: Increased intake of β-carotene, vitamin C, and vitamin E associated with a reduced risk of neovascular AMD. | [39] |
| Vitamin A, vitamin C, and vitamin E | Systematic review and meta-analysis | - | Positive outcomes: Dietary intake of a mixture of vitamin A, vitamin C, and vitamin E had a larger effect on the reduction of AMD risk than the individual vitamin. | [40] |
| Vitamin A, vitamin C, and vitamin E | Case-control study | - | Positive outcomes: Low dietary intake of vitamin C and vitamin E was associated with neovascular AMD. Negative outcome: Dietary vitamin A showed no association with neovascular AMD. |
[41] |
| Vitamin E | Randomized controlled trial (four years) | 500 IU daily | Negative outcomes: Failed to prevent the development and progression of AMD. | [42] |
| Vitamin E | Randomized placebo controlled 4-arm trial (follow-up of 5.6 ± 1.2 years) | 400 IU daily (DL-α-tocopherol acetate) | Negative outcome: Vitamin supplementation showed no protective effect against cataracts among the participants (elderly men). | [43] |
| Vitamin A, vitamin C, and vitamin E | Multicenter eye disease case-control study (Epidemiological study) | - | Negative outcomes: Vitamins A, C, and E consumptions were not associated with the reduced risk of AMD. | [36] |
| Vitamin A (retinol), vitamin C (ascorbic acid), and vitamin E (α-tocopherol) | POLA (Pathologies Oculaires Liées à l’Age) study | - | Negative outcomes: Plasma vitamin A and vitamin C showed no association with reduction in macular degeneration risk. Plasma vitamin E was negatively associated with early signs of AMD and late AMD. |
[37] |
| Vitamin C | Cochrane Review | - | Negative outcomes: Vitamin C supplementation did not prevent any AMD or late AMD. | [44] |
| Vitamin C and vitamin E | Eye Disease Case-Control Study | - | Negative outcome: No statistically significant overall association was found between serum vitamin status and neovascular AMD. | [45] |
| Minerals | ||||
| Zinc | Case-control study | - | Positive outcome: Low dietary intake of zinc was associated with neovascular AMD. | [41] |
| Zinc | Randomized, placebo-controlled clinical trials (followed-up for up to 10 years) | Zinc oxide (80 mg daily) | Positive outcome: Significantly reduced the risk of developing advanced AMD. | [21] |
| Zinc | Randomized double-blinded, placebo-controlled trials (2 years intervention) | Zinc sulfate (200 mg daily) | Positive outcome: Significantly reduced visual loss in treatment group compared to placebo. | [46] |
| Zinc | Randomized, prospective, placebo-controlled clinical trial (three and six months intervention) | Zinc monocysteine (25 mg daily) | Positive outcomes: Significantly improved visual acuity and contrast sensitivity. Significantly shortened macular light flash recovery time both at three months and at six months. |
[47] |
| Zinc | Randomized, double-blinded, placebo-controlled study (two years intervention) | Zinc sulfate (200 mg daily) | Positive outcome: Significantly increased serum zinc. Negative outcome: No significant improvement of eye conditions for patients with AMD. |
[48] |
| Selenium | Randomized, placebo-controlled, 4-arm trial (follow-up of 5.6 ± 1.2 years) | 200 μg daily (from l-selenomethionine) | Negative outcome: Selenium supplementation did not show significant effect in reducing risk of cataracts among the participants (elderly men). | [43] |
4. Minerals
References
- Chew, E.Y. Nutrition effects on ocular diseases in the aging eye. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF42–ORSF47.
- Schleicher, M.; Weikel, K.; Garber, C.; Taylor, A. Diminishing risk for age-related macular degeneration with nutrition: A current view. Nutrients 2013, 5, 2405–2456.
- Andreatta, W.; El-Sherbiny, S. Evidence-based nutritional advice for patients affected by age-related macular degeneration. Ophthalmologica 2014, 231, 185–190.
- Gorusupudi, A.; Nelson, K.; Bernstein, P.S. The Age-Related Eye Disease 2 Study: Micronutrients in the treatment of macular degeneration. Adv. Nutr. 2017, 8, 40–53.
- Bourne, R.R.; Jonas, J.B.; Bron, A.M.; Cicinelli, M.V.; Das, A.; Flaxman, S.R.; Friedman, D.S.; Keeffe, J.E.; Kempen, J.H.; Leasher, J.; et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: Magnitude, temporal trends and projections. Br. J. Ophthalmol. 2018, 102, 575–585.
- Hogg, R.; Chakravarthy, U. AMD and micronutrient antioxidants. Curr. Eye Res. 2004, 29, 387–401.
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779.
- Khoo, H.E.; Chew, L.Y.; Ismail, A.; Azlan, A. Anthocyanins in purple colored fruits. In Polyphenols: Chemistry, Dietary Sources and Health Benefits; Sun, J., Prasad, K.N., Ismail, A., Yang, B., You, X., Li, L., Eds.; Nova Science Publisher: New York, NY, USA, 2012; pp. 133–152. ISBN 978-1-62081-809-1.
- Sin, H.P.; Liu, D.T.; Lam, D.S. Lifestyle modification, nutritional and vitamins supplements for age-related macular degeneration. Acta Ophthalmol. 2013, 91, 6–11.
- Madhavi, D.; Bomser, J.; Smith, M.; Singletary, K. Isolation of bioactive constituents from Vaccinium myrtillus (bilberry) fruits and cell cultures. Plant Sci. 1998, 131, 95–103.
- Jang, Y.P.; Zhou, J.; Nakanishi, K.; Sparrow, J.R. Anthocyanins protect against A2E photooxidation and membrane permeabilization in retinal pigment epithelial cells. Photochem. Photobiol. 2005, 81, 529–536.
- Müller, D.; Schantz, M.; Richling, E. High performance liquid chromatography analysis of anthocyanins in bilberries (Vaccinium myrtillus L.), blueberries (Vaccinium corymbosum L.), and corresponding juices. J. Food Sci. 2012, 77, C340–C345.
- Duan, X.; Jiang, Y.; Su, X.; Zhang, Z.; Shi, J. Antioxidant properties of anthocyanins extracted from litchi (Litchi chinenesis Sonn.) fruit pericarp tissues in relation to their role in the pericarp browning. Food Chem. 2007, 101, 1365–1371.
- Matsumoto, H.; Nakamura, Y.; Tachibanaki, S.; Kawamura, S.; Hirayama, M. Stimulatory effect of cyanidin 3-glycosides on the regeneration of rhodopsin. J. Agric. Food Chem. 2003, 51, 3560–3563.
- Wang, Y.; Zhao, L.; Lu, F.; Yang, X.; Deng, Q.; Ji, B.; Huang, F. Retinoprotective effects of bilberry anthocyanins via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms in a visible light-induced retinal degeneration model in pigmented rabbits. Molecules 2015, 20, 22395–22410.
- Silván, J.M.; Reguero, M.; de Pascual-Teresa, S. A protective effect of anthocyanins and xanthophylls on UVB-induced damage in retinal pigment epithelial cells. Food Funct. 2016, 7, 1067–1076.
- Ghosh, D.; Konishi, T. Anthocyanins and anthocyanin-rich extracts: Role in diabetes and eye function. Asia Pac. J. Clin. Nutr. 2007, 16, 200–208.
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.; Graf, B.A.; O’Leary, J.M.; Milbury, P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 56, 705–712.
- Olmedilla-Alonso, B.; Estévez-Santiago, R.; Silván, J.M.; Sánchez-Prieto, M.; de Pascual-Teresa, S. Effect of long-term xanthophyll and anthocyanin supplementation on lutein and zeaxanthin serum concentrations and macular pigment optical density in postmenopausal women. Nutrients 2018, 10, 959.
- Hammond, B.R.; Fletcher, L.M.; Roos, F.; Wittwer, J.; Schalch, W. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on photostress recovery, glare disability, and chromatic contrast. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8583–8589.
- Yu, B.; Wang, J.; Suter, P.M.; Russell, R.M.; Grusak, M.A.; Wang, Y.; Yin, S.; Tang, G. Spirulina is an effective dietary source of zeaxanthin to humans. Br. J. Nutr. 2012, 108, 611–619. [Green Version]
- Nolan, J.M.; Power, R.; Stringham, J.; Dennison, J.; Stack, J.; Kelly, D.; Moran, R.; Akuffo, K.O.; Corcoran, L.; Beatty, S. Enrichment of macular pigment enhances contrast sensitivity in subjects free of retinal disease: Central Retinal Enrichment Supplementation Trials–Report 1. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3429–3439.
- Olmedilla, B.; Granado, F.; Blanco, I.; Vaquero, M. Lutein, but not alpha-tocopherol, supplementation improves visual function in patients with age-related cataracts: A 2-y doubleblind, placebo-controlled pilot study. Nutrition 2003, 19, 21–24.
- Stringham, J.M.; Hammond, B.R. Macular pigment and visual performance under glare conditions. Optom. Vis. Sci. 2008, 85, 82–88.
- Delcourt, C.; Cristol, J.-P.; Tessier, F.; Léger, C.L.; Descomps, B.; Papoz, L. Age-related macular degeneration and antioxidant status in the POLA study. Arch. Ophthalmol. 1999, 117, 1384–1390.
- Chong, E.W.; Wong, T.Y.; Kreis, A.J.; Simpson, J.A.; Guymer, R.H. Dietary antioxidants and primary prevention of age related macular degeneration: Systematic review and meta-analysis. BMJ 2007, 335, 755.
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66.
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophy. 2018, 652, 18–26.
- Ma, L.; Lin, X.M. Effects of lutein and zeaxanthin on aspects of eye health. J. Sci. Food Agric. 2010, 90, 2–12.
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201.
- Zhao, L.; Sweet, B.V. Lutein and zeaxanthin for macular degeneration. Am. J. Health Syst. Pharm. 2008, 65, 1232–1238.
- Nolan, J.M.; Stack, J.; O’ Donovan, O.; Loane, E.; Beatty, S. Risk factors for age-related maculopathy are associated with a relative lack of macular pigment. Exp. Eye Res. 2007, 84, 61–74.
- Johnson, E.J.; Maras, J.E.; Rasmussen, H.M.; Tucker, K.L. Intake of lutein and zeaxanthin differ with age, sex, and ethnicity. J. Am. Diet. Assoc. 2010, 110, 1357–1362.
- Chung, H.Y.; Rasmussen, H.M.; Johnson, E.J. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J. Nutr. 2004, 134, 1887–1893.
- Chew, E.Y.; Clemons, T.E.; SanGiovanni, J.P.; Danis, R.P.; Ferris, F.L.; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; Bressler, S.B.; et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 Report No. 3. JAMA Ophthalmol. 2014, 132, 142–149.
- Goldberg, J.; Flowerdew, G.; Smith, E.; Brody, J.A.; Tso, M.O.M. Factors associated with age-related macular degeneration: An analysis of data from the First National Health and Nutrition Examination Survey. Am. J. Epidemiol. 1988, 128, 700–710.
- De Koning-Backus, A.P.; Buitendijk, G.H.; Kiefte-de Jong, J.C.; Colijn, J.M.; Hofman, A.; Vingerling, J.R.; Haverkort, E.B.; Franco, O.H.; Klaver, C.C. Intake of vegetables, fruit, and fish is beneficial for Age-related Macular Degeneration. Am. J. Ophthalmol. 2019, 198, 70–79.
- Seddon, J.M.; Ajani, U.A.; Sperduto, R.D.; Hiller, R.; Blair, N.; Burton, T.C.; Farber, M.D.; Gragoudas, E.S.; Haller, J.; Miller, D.T.; et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA 1994, 272, 1413–1420.
- Zampatti, S.; Ricci, F.; Cusumano, A.; Marsella, L.T.; Novelli, G.; Giardina, E. Review of nutrient actions on age-related macular degeneration. Nutr. Res. 2014, 34, 95–105. [Green Version]
- SanGiovanni, J.P.; Chew, E.Y.; Clemons, T.E.; Ferris, F.L., II; Gensler, G.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; Sperduto, R.D. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study. Arch Ophthalmol. 2007, 125, 1225–1232.
- Aoki, A.; Inoue, M.; Nguyen, E.; Obata, R.; Kadonosono, K.; Shinkai, S.; Hashimoto, H.; Sasaki, S.; Yanagi, Y. Dietary n-3 fatty acid, α-tocopherol, zinc, vitamin D, vitamin C, and β-carotene are associated with age-related macular degeneration in Japan. Sci. Rep. 2016, 6, 20723.
- Taylor, H.R.; Tikellis, G.; Robman, L.D.; McCarty, C.A.; McNeil, J.J. Vitamin E supplementation and macular degeneration: Randomised controlled trial. Br. Med. J. 2002, 325, 11.
- Christen, W.G.; Glynn, R.J.; Gaziano, J.M.; Darke, A.K.; Crowley, J.J.; Goodman, P.J.; Lippman, S.M.; Lad, T.E.; Bearden, J.D.; Goodman, G.E.; et al. Age-related cataract in men in the selenium and vitamin E cancer prevention trial eye endpoints study: A randomized clinical trial. JAMA Ophthalmol. 2015, 133, 17–24.
- Evans, J.R.; Lawrenson, J.G. Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration. Cochrane Database Syst. Rev. 2017, 7, CD000253.
- Eye Disease Case-Control Study Group. Antioxidant status and neovascular age-related macular degeneration. Arch. Ophthalmol. 1993, 111, 104–109.
- Newsome, D.A.; Swartz, M.; Leone, N.C.; Elston, R.C.; Miller, E. Oral zinc in macular degeneration. Arch. Ophthalmol. 1988, 106, 192–198.
- Newsome, D.A. A randomized, prospective, placebo-controlled clinical trial of a novel zinc-monocysteine compound in age-related macular degeneration. Curr. Eye Res. 2008, 33, 591–598.
- Stur, M.; Tittl, M.; Reitner, A.; Meisinger, V. Oral zinc and the second eye in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1225–1235.
- Christen, W.G.; Ajani, U.A.; Glynn, R.J.; Manson, J.E.; Schaumberg, D.A.; Chew, E.C.; Buring, J.E.; Hennekens, C.H. Prospective cohort study of antioxidant vitamin supplement use and the risk of age-related maculopathy. Am. J. Epidemiol. 1999, 149, 476–484.
- Klein, M.L.; Francis, P.J.; Rosner, B.; Reynolds, R.; Hamon, S.C.; Schultz, D.W.; Ott, J.; Seddon, J.M. CFH and LOC387715/ARMS2 genotypes and treatment with antioxidants and zinc for age-related macular degeneration. Ophthalmology 2008, 115, 1019–1025.
- Robison, W.G.; Kuwabara, T.; Bieri, J.G. The roles of vitamin E and unsaturated fatty acids in the visual process. Retina 1982, 2, 263–281.
- Tanito, M.; Yoshida, Y.; Kaidzu, S.; Chen, Z.-H.; Cynshi, O.; Jishage, K.-I.; Niki, E.; Ohira, A. Acceleration of age-related changes in the retina in a-tocopherol transfer protein null mice fed a vitamin E–deficient diet. Investig. Ophthalmol. Vis. Sci. 2007, 48, 396–404.
- Katz, M.L.; Eldred, G.E. Failure of vitamin E to protect the retina against damage resulting from bright cyclic light exposure. Investig. Ophthalmol. Vis. Sci. 1989, 30, 29–36.
- Infante, J.P. Vitamin E and selenium participation in fatty acid desaturation. A proposal for an enzymatic function of these nutrients. Mol. Cell. Biochem. 1986, 69, 93–108.
- Handelman, G.J.; Machlin, L.J.; Fitch, K.; Weiter, J.J.; Dratz, E.A. Oral α-tocopherol supplements decrease plasma γ-tocopherol levels in humans. J. Nutr. 1985, 115, 807–813.
- Alvarez, R.; Liou, F.; Fong, S. Levels of alpha-, and gamma-tocopherol in human eyes: Evaluation of the possible role of IRBP in intraocular alpha-tocopherol transport. Am. J. Clin. Nutr. 1987, 46, 481–487.
- Belda, J.I.; Romá, J.; Vilela, C.; Puertas, F.J.; Díaz-Llopis, M.; Bosch-Morell, F.; Romero, F.J. Serum vitamin E levels negatively correlate with severity of age-related macular degeneration. Mech. Ageing Dev. 1999, 107, 159–164.
- Thurnham, D.I. Macular zeaxanthins and lutein—A review of dietary sources and bioavailability and some relationships with macular pigment optical density and age-related macular disease. Nutr. Res. Rev. 2007, 20, 163–179.
- Grahn, B.H.; Paterson, P.G.; Gottschall-Pass, K.T.; Zhang, Z. Zinc and the eye. J. Am. Coll. Nutr. 2001, 20, 106–118.
- King, J.C. Zinc: An essential but elusive nutrient. Am. J. Clin. Nutr. 2011, 94, 679S–684S.
- Ugarte, M.; Osborne, N.N. Zinc in the retina. Prog. Neurobiol. 2001, 64, 219–249.
- Age-Related Eye Disease Study Research Group (AREDS). A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS Report No. 8. Arch. Ophthalmol. 2001, 119, 1417–1436.
- Vishwanathan, R.; Chung, M.; Johnson, E.J. A systematic review on zinc for the prevention and treatment of age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3985–3998.
- Assel, M.J.; Li, F.; Wang, Y.; Allen, A.S.; Baggerly, K.A.; Vickers, A.J. Genetic polymorphisms of CFH and ARMS2 do not predict response to antioxidants and zinc in patients with age-related macular degeneration: Independent statistical evaluations of data from the Age-Related Eye Disease Study. Ophthalmol. 2018, 125, 391–397.
- Ursini, F.; Bindoli, A. The role of selenium peroxidases in the protection against oxidative damage of membranes. Chem. Phys. Lipids 1987, 44, 255–276.
- Tsang, N.C.; Penfold, P.L.; Snitch, P.J.; Billson, F. Serum levels of antioxidants and age-related macular degeneration. Doc. Ophthalmol. 1992, 81, 387–400.
- Farnsworth, C.C.; Stone, W.L.; Dratz, E.A. Effects of vitamin E and selenium deficiency on the fatty acid composition of rat retinal tissues. Biochim. Biophys. Acta 1979, 552, 281–293.





