
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antimo Moretti | + 1302 word(s) | 1302 | 2020-07-24 05:34:23 | | | |
| 2 | Nicole Yin | -1 word(s) | 1301 | 2020-07-28 04:27:19 | | |
Video Upload Options
Choline is an essential micronutrient with a pivotal role in several metabolic pathways contributing to liver, neurological, and hematological homeostasis. Although choline is commonly administered to improve physical performance, its effects on muscle are still unclear. Our scoping review elucidates and summarizes the
crucial role of choline in modulating muscle fat metabolism, muscle proteins homeostasis, and the
modulation of inflammation and autophagy.
1. Definition
Choline is a water-soluble quaternary amine of the vitamin B group considered as an essential nutrient by the Food and Nutrition Board of the Institute of Medicine[1].
2. Introduction
Choline endogenous synthesis from amino acid methionine is insufficient to support human choline requirements, so it is essential to maintain an appropriate dietary intake of choline, consuming fish, eggs, and meats[2]. Adequate choline daily intakes ranged from 330 to 468 mg for men, 269 to 444 mg for women, and 356 mg/day as mean estimate intake in pregnant women[3]. This micronutrient plays an important role in phospholipid synthesis and triglycerides metabolism contributing to structure and function of cell membranes, including skeletal muscle cells[4]. Low concentration of choline is associated with several changes in myoblasts up to muscle wasting as demonstrated by increased serum creatine kinase (CK)[5]. Moreover, choline seems to exert an ancillary role in inflammatory muscle diseases with anti-fibrotic effects[6]. From a functional point of view, choline is involved in muscle contraction being a precursor of the main neurotransmitter of α-motor neurons, acetylcholine (ACh)[7]. These effects might have clinical implications as suggested by the observation of poor physical performance in healthy people with low serum choline[8][9][10].
Despite available knowledge about effects of choline on both muscle histomorphology and function, pathways involved in these processes are still poorly investigated.
3. Choline: An Essential Nutrient for Skeletal Muscle
3.1. Choline and Muscle Fat Metabolism
Adequate amount of choline improves mitochondrial energy metabolism and lipid metabolism by decreasing FA synthesis. Choline deficiency impairs the incorporation of FAs in PC, increasing their availability for DAG and TAG synthesis, and consequently favoring accumulation of TAG in muscle cells[11]. Choline influences fat metabolism also modulating expression of genes involved in FA genesis (ACC and FASN) as well as those involved in fat intracellular transportation (LPL and CD36)[12]. Moreover, high levels of choline reduce biosynthesis of long-chain FAs in muscle lowering intramuscular fat content. This effect could be explained also by an insulin-mediated gene modulation. In fact, choline supplementation improves insulin signaling that upregulates AMPK, downregulates mTORC1, a modulating factor of proteostasis in skeletal muscle[13], and decreasing lipogenesis[14].
Choline also downregulates Diacylglycerol O-acyltransferase (Dgat1/2), reducing FA esterification and TAG formation. On the other side, an experimental study reported that additional choline supplementation did not result in significant difference in “carcass fat levels” when compared to control[15]. Accumulation of lipid intermediates in skeletal muscle (intermuscular and/or intramuscular adipose tissue, IMAT) leads to cellular dysfunction and death. An excess of lipids in diet could impair choline metabolism. This effect reduces the consumption of lipids for intracellular synthesis of PC, increasing DAG and FA accumulation, causing adipocyte hyperplasia and hypertrophy, chronic inflammation, lipotoxicity, and insulin resistance, key mechanisms involved in sarcopenic obesity[16]. However, it should be underlined that polyunsaturated FAs, such as PAM, reduce the expression of plasma membrane choline transporter CTL1/SLC44A1 inducing cell membrane fragility because of poor choline availability for PC synthesis. Moreover, choline deficiency shifts CDP-choline pathway toward TAG formation and lipid droplets accumulation resulting in lipotoxicity. Conversely, monounsaturated FAs, such as OLA, seem to exert a protective activity on muscle by increasing mitochondrial FA oxidation and stimulating PC synthesis. In a clinical scenario, Gao et al. demonstrated that choline supplementation improved body composition by lowering body fat and increasing lean mass, suggesting a pivotal role of this micronutrient in promoting FA β-oxidation and translocation into the mitochondria[17].
3.2. Choline and Muscle Proteins
Choline is an essential nutrient for protein metabolism. As methyl-group donor, this micronutrient influences protein homeostasis, increasing synthesis and reducing breakdown. Robinson et al. reported a reduced whole-body protein synthesis in piglets fed with methyl deficient diet, due to 50% reduction of protein synthesis in skeletal muscle[18]. In the early stages, impaired protein synthesis did not lead to a poor body growth, probably because of a simultaneous slow protein catabolism. However, a chronic choline diet restriction undoubtedly determines a low muscle protein content, resulting in impaired muscle growth and function. Vice versa, choline-rich diet increases serum IGF2 and decreases IGFBP-2 in skeletal muscle fibers. IGF2 enhances proliferation and growth of muscle tissue promoting amino acid and glucose uptake[19][20]. It has been also hypothesized that methyl-donors nutrients, including choline, increases the expression of follistatin (FST), a member of the TGF-β family that causes hypertrophy and hyperplasia of muscle cells through binding ACTIIB receptor, and thus inhibiting myostatin[21][22](Figure 1).
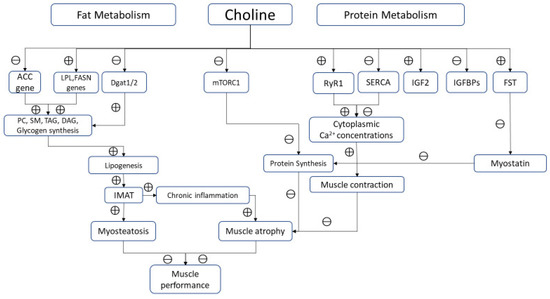
Figure 1. Biological pathways modulated by choline in skeletal muscle. Note: ⊕ and ⊖ indicate positive and negative modulation, respectively.
From a functional point of view, potential actions of choline in modulating key mechanisms of muscle contraction beyond its role as precursor of Ach should be considered. Ions replacement of K+ with choline+ results in a potent inhibition of SERCA in sarcoplasmic/endoplasmic reticulum of skeletal muscle as demonstrated in animal models[23]. This effect is reached through a dual mechanism: choline+ inhibits both the cytosolic Ca2+ uptake into SR and, at the same time, SERCA ATPase activity, probably uncoupling Ca2+ transport from ATP hydrolysis.
Choline regulates intracellular calcium and therefore muscle contraction also by modulating the binding of calmodulin and RYR1[24]. This mechanism could increase the cytoplasmic calcium concentrations into myofibrillar spaces improving its bioavailability for muscle contraction. However, this seems not true in muscle affected by pathological conditions. Alves et al. reported that choline supplementation (5 g/kg choline) paradoxically increased SERCA activity reducing calcium cytosolic content in mice models of Duchenne Muscular Dystrophy (mdx)[6].
3.3. Choline and Inflammation
Choline influences inflammation through different mechanisms. In animal exposed to endotoxin choline improves activation of vagal anti-inflammatory system[25][26][27]. In the same animal model, choline counteracts the endotoxin-induced tissue damage reducing serum levels of urea, uric acid, lactate dehydrogenase (LDH), creatine kinase (CK), and creatine kinase myocardial isoform (CK-MB)[19], probably through improved tissue perfusion as well as enhanced cholinergic neurotransmission.
3.4. Choline, Apoptosis, and Autophagy
Choline modulates cell apoptosis and autophagy; thus, contributing to maintain intercellular homeostasis. Da Costa et al.[28] reported that choline deficiency was associated with significant DNA damage resulting in lymphocyte apoptosis. Authors found an increase of activated caspase-3 in lymphocytes in patients fed with choline deficient diet. This could be the main mechanism of apoptosis, probably occurring earlier than the reduced cytoplasmatic availability of choline for PC synthesis. Lower membrane PC concentration seems to contribute to plasma membrane fragility of myocytes[5].
Choline is an important regulator of autophagy. Taylor et al. reported that choline supplementation restores insulin receptor substrate 1 (IRS1) levels, a key factor in the IGF1-Akt-mTOR pathway with significant anabolic effects on skeletal muscle[14]. Choline prevents phosphorylation of mTORC1, defined as “the main gateway to autophagy”[29]. Autophagy is a critical survival mechanism of cells and its alteration conduce to skeletal muscle damage in different disorders. For example, Pompe disease, an inherited deficiency of the lysosomal enzyme acid α-glucosidase, is characterized by significant accumulation of autophagosomes containing LC3 (microtubule-associated protein 1 light chain 3), a pivotal factor for autophagy in type I muscle fibers. Two forms of LC3 have been described: a cytoplasmic protein (LC3-I), and a specifically associated autophagosomes form (LC3-II). Higher levels of LC3-II provoke a dramatic and disruptive autophagic effect on skeletal muscle[30]. Choline reduces lipidation of LC3, lowering LC3-II/LC3-I ratio and its accumulation in autophagy-lysosomal system[31]. This ratio is also influenced by FAs. PAM increases intracellular content of PC precursor providing more lipids for LC3 lipidation and autophagosome formation stimulating autophagy.
References
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press (US): Washington, DC, USA, 1998; pp. 390–442.
- Nan, A. Miscellaneous Drugs, Materials, Medical Devices and Techniques. In Side Effects of Drugs Annual, 1st ed.; Sidhartha, D.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 38, pp. 603–619.
- EFSA. NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific opinion on Dietary Reference Value for choline. EFSA J. 2016, 14, 1–70.
- Cindy X. Cai; Stella Carlos; Pejman Solaimani; Bansari J. Trivedi; Chuong Tran; Shobha Castelino-Prabhu; Nutritional and Dietary Interventions for Nonalcoholic Fatty Liver Disease. Dietary Interventions in Liver Disease 2019, 29, 357-372, 10.1016/b978-0-12-814466-4.00029-x.
- Kerry-Ann Da Costa; Mihaela Badea; Leslie M Fischer; Steven H. Zeisel; Elevated serum creatine phosphokinase in choline-deficient humans: mechanistic studies in C2C12 mouse myoblasts. The American Journal of Clinical Nutrition 2004, 80, 163-170, 10.1093/ajcn/80.1.163.
- Francesca M. Alves; Marissa Caldow; Jennifer Trieu; Tim Naim; Magdalene Montgomery; Matthew J. Watt; Gordon S. Lynch; Rene Koopman; Choline administration attenuates aspects of the dystrophic pathology in mdx mice. Clinical Nutrition Experimental 2019, 24, 83-91, 10.1016/j.yclnex.2018.12.005.
- Di Zhao; Michael Frohman; Jan Krzysztof Blusztajn; Generation of choline for acetylcholine synthesis by phospholipase D isoforms. BMC Neuroscience 2001, 2, 16-16, 10.1186/1471-2202-2-16.
- Penry, J.T.; Manore, M.M. Choline: An important micronutrient for maximal endurance-exercise performance? Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 191–203.
- Hongu, N.; Sachan, D.S. Carnitine and choline supplementation with exercise alter carnitine profiles, biochemical markers of fat metabolism and serum leptin concentration in healthy women. J. Nutr. 2003, 133, 84–89.
- Jäger, R.; Purpura, M.; Kingsley, M. Phospholipids and sports performance. J. Int. Soc. Sports Nutr. 2007, 4, 5.
- Vera Michel; Ratnesh Kumar Singh; Marica Bakovic; The impact of choline availability on muscle lipid metabolism. Food & Function 2011, 2, 53-62, 10.1039/c0fo00069h.
- Huawei Li; Hongrong Wang; Lihuai Yu; Mengzhi Wang; Shimin Liu; Lisha Sun; Qing Chen; Effects of supplementation of rumen-protected choline on growth performance, meat quality and gene expression inlongissimus dorsimuscle of lambs. Archives of Animal Nutrition 2015, 69, 340-350, 10.1080/1745039x.2015.1073001.
- Giselle A. Joseph; Sharon X. Wang; Cody E. Jacobs; Weihua Zhou; Garrett C. Kimble; Herman W. Tse; John K. Eash; Tea Shavlakadze; David J. Glass; Partial Inhibition of mTORC1 in Aged Rats Counteracts the Decline in Muscle Mass and Reverses Molecular Signaling Associated with Sarcopenia. Molecular and Cellular Biology 2019, 39, e00141-19, 10.1128/mcb.00141-19.
- Giovanni Iolascon; Antimo Moretti; The relationship between serum IGF-1, handgrip strength, physical performance and falls in elderly men and women. Journal of Laboratory and Precision Medicine 2018, 3, 103-103, 10.21037/jlpm.2018.12.05.
- J. Kenney; K. Carlberg; The Effect of Choline and Myo-Inositol on Liver and Carcass Fat Levels in Aerobically Trained Rats. International Journal of Sports Medicine 1995, 16, 114-116, 10.1055/s-2007-972975.
- Alexander Kalinkovich; G. Livshits; Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Research Reviews 2017, 35, 200-221, 10.1016/j.arr.2016.09.008.
- Xiang Gao; Yongbo Wang; Edward Randell; Pardis Pedram; Yanqing Yi; Wayne Gulliver; Guang Sun; Higher Dietary Choline and Betaine Intakes Are Associated with Better Body Composition in the Adult Population of Newfoundland, Canada. PLoS ONE 2016, 11, e0155403, 10.1371/journal.pone.0155403.
- Jason L Robinson; Scott V. Harding; Janet A. Brunton; Robert F. P. Bertolo; Dietary Methyl Donors Contribute to Whole-Body Protein Turnover and Protein Synthesis in Skeletal Muscle and the Jejunum in Neonatal Piglets. The Journal of Nutrition 2016, 146, 2007-2012, 10.3945/jn.115.226035.
- Michael Oster; W. Nuchchanart; Nares Trakooljul; Eduard Muráni; A. Zeyner; E. Wirthgen; A Hoeflich; S. Ponsuksili; Klaus Wimmers; Methylating micronutrient supplementation during pregnancy influences foetal hepatic gene expression and IGF signalling and increases foetal weight. European Journal of Nutrition 2015, 55, 1717-1727, 10.1007/s00394-015-0990-2.
- D. L. Devol; P. Rotwein; J. L. Sadow; J. Novakofski; P. J. Bechtel; Activation of insulin-like growth factor gene expression during work-induced skeletal muscle growth. American Journal of Physiology-Endocrinology and Metabolism 1990, 259, E89-E95, 10.1152/ajpendo.1990.259.1.e89.
- Hamid Arazi; Lida Salek; Elham Nikfal; Mani Izadi; James J. Tufano; Bradley T. Elliott; Matt Brughelli; Comparable endocrine and neuromuscular adaptations to variable vs. constant gravity-dependent resistance training among young women. Journal of Translational Medicine 2020, 18, 1-12, 10.1186/s12967-020-02411-y.
- Helge Amthor; Gina Nicholas; Iain McKinnell; C.Fred Kemp; Mridula Sharma; Ravi Kambadur; Ketan Patel; Follistatin complexes Myostatin and antagonises Myostatin-mediated inhibition of myogenesis. Developmental Biology 2004, 270, 19-30, 10.1016/s0012-1606(04)00118-6.
- Sanja Beca; Roozbeh Aschar-Sobbi; Dragana Ponjevic; Robert J. Winkfein; Margaret E. Kargacin; Gary J. Kargacin; Effects of monovalent cations on Ca2+ uptake by skeletal and cardiac muscle sarcoplasmic reticulum. Archives of Biochemistry and Biophysics 2009, 490, 110-117, 10.1016/j.abb.2009.08.014.
- Erika Kovacs; Le Xu; Daniel A. Pasek; Károly Liliom; Gerhard Meissner; Regulation of ryanodine receptors by sphingosylphosphorylcholine: Involvement of both calmodulin-dependent and -independent mechanisms. Biochemical and Biophysical Research Communications 2010, 401, 281-286, 10.1016/j.bbrc.2010.09.050.
- Yesim Ozarda Ilcol; Zeki Yilmaz; Ismail H Ulus; ENDOTOXIN ALTERS SERUM-FREE CHOLINE AND PHOSPHOLIPID-BOUND CHOLINE CONCENTRATIONS, AND CHOLINE ADMINISTRATION ATTENUATES ENDOTOXIN-INDUCED ORGAN INJURY IN DOGS. Shock 2005, 24, 288-293, 10.1097/01.shk.0000174018.02688.4b.
- Yesim Ozarda Ilcol; M. Sibel Gurun; Yavuz Taga; Ismail H. Ulus; Choline increases serum insulin in rat when injected intraperitoneally and augments basal and stimulated aceylcholine release from the rat minced pancreas in vitro.. JBIC Journal of Biological Inorganic Chemistry 2003, 270, 991-999, 10.1046/j.1432-1033.2003.03472.x.
- James E.G Downing; Jaleel A. Miyan; Neural immunoregulation: emerging roles for nerves in immune homeostasis and disease. Immunology Today 2000, 21, 281-289, 10.1016/s0167-5699(00)01635-2.
- Kerry-Ann Da Costa; Mihai D Niculescu; Corneliu N Craciunescu; Leslie M Fischer; Steven H. Zeisel; Choline deficiency increases lymphocyte apoptosis and DNA damage in humans2,3. The American Journal of Clinical Nutrition 2006, 84, 88-94, 10.1093/ajcn/84.1.88.
- Yoana Rabanal-Ruiz; Elsje G. Otten; mTORC1 as the main gateway to autophagy. Essays in Biochemistry 2017, 61, 565-584, 10.1042/ebc20170027.
- L. Shea; N Raben; Autophagy in skeletal muscle: implications for Pompe disease.. Int. Journal of Clinical Pharmacology and Therapeutics 2009, 47, S42-S47, 10.5414/cpp47042.
- Pengzhou Hang; Jing Zhao; Zhenli Su; Hanqi Sun; Tingting Chen; Lihui Zhao; Zhimin Du; Choline Inhibits Ischemia-Reperfusion-Induced Cardiomyocyte Autophagy in Rat Myocardium by Activating Akt/mTOR Signaling. Cellular Physiology and Biochemistry 2018, 45, 2136-2144, 10.1159/000488049.




