
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Martin Desimone | + 4692 word(s) | 4692 | 2020-07-07 08:39:24 | | | |
| 2 | Rita Xu | -2086 word(s) | 2606 | 2020-07-24 07:42:25 | | |
Video Upload Options
Smart or stimuli-responsive materials are an emerging class of materials used for tissue engineering and drug delivery. A variety of stimuli (including temperature, pH, redox-state, light, and magnet fields) are being investigated for their potential to change a material’s properties, interactions, structure, and/or dimensions. The specificity of stimuli response, and ability to respond to endogenous cues inherently present in living systems provide possibilities to develop novel tissue engineering and drug delivery strategies (for example materials composed of stimuli responsive polymers that self-assemble or undergo phase transitions or morphology transformations). Herein, smart materials as controlled drug release vehicles for tissue engineering are described, highlighting their potential for the delivery of precise quantities of drugs at specific locations and times promoting the controlled repair or remodeling of tissues.
1. Definition
Smart or stimuli-responsive materials are an emerging class of materials used for tissue engineering and drug delivery. A variety of stimuli (including temperature, pH, redox-state, light, and magnet fields) are being investigated for their potential to change a material’s properties, interactions, structure, and/or dimensions. The specificity of stimuli response, and ability to respond to endogenous cues inherently present in living systems provide possibilities to develop novel tissue engineering and drug delivery strategies (for example materials composed of stimuli responsive polymers that self-assemble or undergo phase transitions or morphology transformations).
2. Introduction
The United States Food and Drug Administration (FDA) defined regenerative medicine as the capacity to: facilitate regeneration of parts of the human body, where cells and tissues can be engineered to grow healthy, functional organs to replace diseased ones; new genes can be introduced into the body to combat disease; and adult stem cells can generate replacements for cells that are lost due to injury or disease; tissue engineering and regenerative medicine aim to replace/regenerate tissues from cells and biomaterials [1]. In the case of biomaterials, they can be processed into nanocarriers [2], hydrogels [3][4] and films [5] for drug delivery, wound healing [5][6], tissue engineering and cell therapy, leading to many emerging and promising regenerative approaches for the treatment of diseases or injuries.
The number of publications in materials science has increased dramatically in recent years, in line with the introduction of new biomaterials and devices to diagnose and treat diseases, aiming to improve our quality of life and contribute to the steady increase in life expectancy (Figure 1).
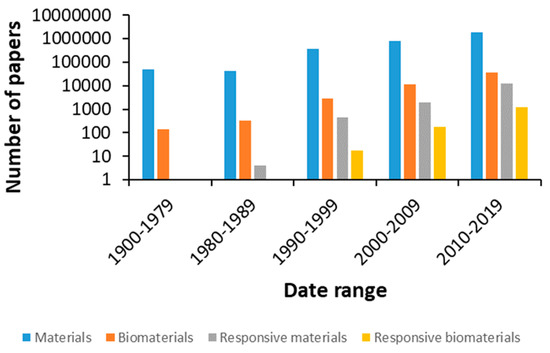
Figure 1. Number of publications on related topics in the Web of Science database with respect to time.
Different biomaterials possess different physical and chemical properties and can be processed into a variety of shapes (e.g. films, foams, gels and particles) [7][8]. They can be used on their own or as part of composites/hybrids in order to impart other functionality and tune their bulk properties, and their surface properties can be tailored through a variety of surface modification techniques [9][10][11]. The ability of a material to respond to different stimuli is related to their physico-chemical characteristics [12][13][14][15][16]. Taking advantage of such features with the recent developments in technology, we expect to be able to control the interaction between the biomaterial and its contents (e.g. cells, drugs) and surrounding environment in response to various stimuli (including but not limited to: pH, temperature, redox potential, magnetic fields and light) [12][13][17][18].
Stimuli-responsive materials have numerous applications in the biomedical field, from drug delivery systems to diagnostics and treatment. The delivery of drugs and genes requires the pharmaceutical compound or gene to reach the site of action at the right time and at an appropriate concentration, traversing obstacles like biological barriers, enzymatic or hydrolytic degradation and solubility. More often than not, secondary effects arise from non-specific interactions with cells and tissues, so that vehicles that react to specific stimuli would be promising carriers for the targeted delivery of drugs and genes [19][20]. Tissue engineering also faces numerous challenges such as a paucity of renewable sources of functional cells that are immunologically compatible; a lack of suitable materials with the desired chemical composition, mechanical properties and biological function; and an inability to generate large, vascularized tissues that can easily integrate into the circulatory system of the host with the inherently complexity of native tissues architecture, some of which can be addressed through the utilization of smart responsive biomaterials [21]. In this context, stimuli-responsive nanomaterials have received great attention. Significant progress has been made to tailor nanoparticles with stimuli-responsive properties, which have potential for future therapies for human or veterinary applications. Size, shape and surface functionalization, as well as modifications, are necessary for active targeting or stimulus-responsive drug release [22].
The stimuli can be internal or external, meaning that they can build up at the site of action or that they could be applied externally to achieve the desired effect. For example, redox conditions and pH vary in the different tissues and between intracellular and extracellular compartments. The properties of redox polymers (ionic, electrical, optical, mechanical or chemical) change depending on their oxidation state, offering potential for inclusion in actuators, biosensors and drug delivery systems [23] The review of Guo and coworkers summarized the state-of-the-art of knowledge on reduction/oxidation responsive polymeric drug carriers (specifically focusing on functional groups employed for this end goal) [24]. Drug delivery and tissue engineering strategies based on electroactive materials represents an innovative field research [25]. The effects of electrical stimulation on cell growth and differentiation and tissue growth has led to interest in using piezoelectric scaffolds for tissue repair [26], influenced by the inherent piezoelectric properties of bone [27], and studies showing enhanced bone regeneration in response to the use of piezoelectric biomaterials [28]. Consequently, piezoelectric materials have begun to find a variety of biomedical applications, including drug delivery and tissue engineering applications [29][30][31]. Of particular interest is the ability of smart polymers to differentiate between the redox potential in tumors and normal tissues (with the former exhibiting 4-fold higher glutathione concentrations), or respond to the presence of reactive oxygen species (ROS), believed to play a role in diseases like cancer, heart injury and arteriosclerosis [24]. Similarly, pH responsive polymers bearing ionizable acidic/basic residues can be employed in drug/gene delivery, sensors and membranes [32]. They are of interest as it has been shown that the pH is altered in pathological conditions such as cancer, inflammation and infection and their ability to respond to changes in the pH by undergoing changes in surface activity, chain conformation, solubility and configuration has led to the development of several drug delivery systems and wound dressings [33][34][35]. For instance, the variability of pH values between 5.6-7.0 in tumor masses has inspired the development of new pH-responsive materials [36]. The pH spectrum observed in different sites within the body in physiological conditions also provides attractive targets for use in biomedicine [37], wherein pH-responsive carriers may be able to target a specific area in the body and release their bioactives with a high therapeutic impact and minimum side-effects [38].
To generate magnetically responsive materials, magnetic nanoparticles can be incorporated into scaffolds for drug delivery, tissue regeneration and artificial muscles. Through the application of a magnetic field, the nanoparticles are able to transmit a force to the surrounding material and/or cells triggering a response. Mechanical stimulation resulting from the deformation of the magnetic scaffold can lead, for example, to an increase in GAG expression and stimulate differentiation of stem cells to chondrocytes for cartilage repair, promote axonal extension and cell migration to guide neuronal regeneration or induce localized hyperthermia in cancer therapy [39]. In addition, light is an attractive source to trigger a response, as its intensity and wavelength can be controlled to impact specific areas of tissue. Light responsive moieties can then be incorporated into the structure of polymeric materials or molecules bearing light responsive groups can be introduced into a non-light responsive material to induce the release of pharmaceutical compounds or cause shape changes upon exposure to light [40]. Furthermore, thermoresponsive polymers that react to temperature enable the use of polymer networks for the release of drugs at specific temperatures [41]. These materials have been extensively reviewed [42][43], particularly thermoresponsive polymers with interesting lower critical solution temperature (LCST) and upper critical solution temperature (UCST) behaviors in water, the chemical and physicochemical characteristics and their uses and applications as drug delivery systems, hydrogels and surfaces for cell growth, among other biomedical applications [42]. Alternatively to the design, mechanism, and behavior of thermoresponsive materials and its combination with other features, namely pH-thermoresponsive or photothermoresponsive materials among others, and their applications in different fields of biomedicine [43], and to complement the existing literature, in this review, we will focus on natural and synthetic thermoresponsive polymers and the results of their application in vitro and in vivo as biomedical materials.
Interestingly, polymers can be further engineered to tune the response to various stimuli. There are different strategies of molecular design for the incorporation of appropriate responsive building blocks [44]. Moreover, the integration of polymers with different functional groups has allowed the development of multi stimuli-responsive materials [12]. These properties would be further employed to trigger the release of therapeutic molecules in biological environments with different characteristics. Thus, the precise and controlled drug delivery of multi stimuli-responsive carriers may provide new treatment options [45].
3. Stimuli to control the response of materials for tissue regeneration and drug delivery
This work highlights some interesting examples from the literature to offer an overview of the most common stimuli-responsive materials relevant to tissue engineering and drug delivery.
3.1. pH responsive
In diseased, inflamed and infected tissues, the pH may be decreased due to dysregulated metabolism or irregular angiogenesis, which cause the rapid shortage of oxygen and nutrients, that results in a shift toward glycolytic metabolism [46]; consequently, variations of the pH in different organs, tissues and intracellular compartments can be considered during the design of stimuli-responsive nanomaterials with the ability to release a therapeutic agent at a target site in response to pH [47].
Differences in the pH can lead to the modification of crosslinking processes (important for injectable hydrogels and self-healing materials); the protonation or deprotonation of acidic/basic groups can generate distinct interactions between a therapeutic agent and a material causing a defined release profile, potentially in a particular tissue/cell. The delivery of therapeutic molecules in pH-responsive nanocarriers to target cells and tissues is important in tissue regeneration. Scaffolds which can expand according to the pH may modify oxygen and nutrient transport and cell density, enhancing cell deposition and survival, which is also crucial for the management of bone infection and to support tissue regeneration.
Sometimes, single responsiveness is not able to achieve the desired goals in a physiological or pathological microenvironment. To optimize, multi-stage pH responsiveness materials are emerging. These materials are engineered with different components, which have different sensitivity to pH changes, [45] for example, electrospun core–sheath fibers with controlled multi-pH responses within the physiological pH range [48]. This and related research aims to produce new multi-stimuli-responsive materials with active components for drugs or even sensors for targeted disease providing a real-time sensing of various threats. Possibly in the future, these materials will be included in the field of tissue engineering due to their high clinical potential. These materials which are intended to ‘‘sense’’ the surrounding physiological environments and enable on-demand release of encapsulated therapeutic cargos into highly specific targets may optimize the actual therapies in a clinically relevant way.
3.2. Thermo-responsive
Thermoresponsive materials hange their physical properties or present conformational changes in response to temperature variations. These materials are used for biomedical applications including drug delivery and tissue engineering among many others [49][50][51][52][53]. However, the transition-state temperature depends on the solvent interaction with the polymer and the hydrophilic/hydrophobic balance. Polymer thermoresponsive properties can be changed by adding reactants to the polymer/solvent system. Some additives are co-polymers, surfactants, co-solvents, plasticizers and salts. Therefore, additives can alter the solvent quality and therefore can alter the polymer–solvent interactions [54][55][56][57].
Thermoresponsive materials can be classified by their origin (natural or synthetic) and also according to their response to temperature changes: polymers that become insoluble above a critical temperature called lower critical solution temperature (LCST) and polymers that become insoluble below a critical temperature called upper critical solution temperature (UCST) [58]. Natural thermo-responsive polymers are, for example, gelatin, agarose and pectin, whereas synthetic polymers include poly(N-alkyl substituted acrylamides), poly(N-vinyl-alkyl-amides), poly(ethylene glycol)-poly(propylene glycol)-poly(ethylene glycol) copolymer (PEG–PPG–PEG), poly(ethylene glycol)-poly(d,l-lactic acid)-poly(ethylene glycol) copolymer (PEG–PLLA/PDLA–PEG), among many others.
3.3. Light-responsive materials
The strategy of constructing light-responsive smart biomaterials is very attractive to fabricate complex scaffolds for controlling cellular behavior for functional tissue regeneration and stimulating the release of encapsulated compounds [40], [59][60][61]. The light stimulus offers some advantages over other stimuli, because it can be imposed instantly and delivered in exact amounts with high precision, providing spatial and temporal control with less invasive techniques [62][63].
Light is an excellent trigger as its intensity and wavelength can be remotely and accurately controlled, quickly switched and easily focused into specific areas with a resolution of 1 μm. In photo-controlled technologies, a variety of light-induced reactions, such as photopolymerization, photoisomerization, and photodegradation, are employed in different kind of architectures for both engineer smart therapeutic delivery and create dynamic cell culture platforms that better mimic living tissues [60]. Many promising advances in the development of light-responsive biomaterials have been made. The use of externally manipulated light offers excellent control for drug delivery and engineering 3D microenvironments for tissue regeneration. These technologies are very attractive to manipulate biomaterials features in real time providing further control over cell functions, tissue restoration and delivery of therapeutics.
3.4. Redox responsive materials and electroactive polymers
Redox-responsive polymeric materials can respond to biological stimuli generated by the presence of oxidants or reductants in the media and changes in the redox conditions or by the application of an external voltage. The chemical groups which are involved in their redox responsive ability include disulfide bonds, organometallic compounds, viologens or tetrathiafulvalene.
The smart polymers which in response to an appropriate chemical or electrochemical stimulus can show different answers like swelling/contraction, bending, change of state, or conductivity are excellent candidates in engineering nanomedicines and for the development of biomaterials intended to repair or substitute a damage tissue or organ.
3.5. Magnetic responsive nanomaterials
Magnetic responsive nanomaterials and particularly magnetic nanoparticles (MNPs) have interesting features for applications in various fields as a result of many properties, such as high specific surface area, chemical stability, low intraparticle diffusion rate, high loading capacity and superparamagnetism [64][65]. In the biomedical field, and specifically in tissue regeneration, this kind of nanoparticles are useful due to their biocompatibility and long-term stability [66]. Furthermore, they have many advantages in terms of penetration and invasiveness since many materials, especially biological tissues, have a lower absorption capacity for magnetic fields than for other types of stimuli, like electric fields, making it possible to remotely activate an event at a relevant distance from the magnet [67]. Although magnetic nanoparticles have been widely used in drug delivery and hyperthermia treatments, recent applications of magnetic nanoparticles have demonstrated their promise towards decreasing implant infection and increasing tissue growth.
4. Conclusions
This emerging field of material science has generated considerable and increasing interest during the past decades. In the particular field of medicinal sciences stimuli-responsive materials offer new opportunities in the treatment of various conditions. The driving force of these and future developments is the huge versality of the field in terms of the materials employed and the stimulus applied (i.e.: redox, pH, magnetic, temperature, light). We believe that future research will capitalize on opportunities to address the challenges of toxicity posed by some of the components used for stimuli-responsive materials, the development of materials with biomimetic architectural/topological and mechanical properties, and exploration of new stimuli to deliver drugs and instruct cell behavior, perhaps also employing multi-stimuli-responsive materials.
References
- N. Lumelsky, M. O’Hayre, P. Chander, L. Shum, M.J. Somerman, Autotherapies: Enhancing Endogenous Healing and Regeneration, Trends Mol. Med. 24 (2018) 919–930. https://doi.org/10.1016/j.molmed.2018.08.004.
- P. Lavrador, V.M. Gaspar, J.F. Mano, Stimuli-responsive nanocarriers for delivery of bone therapeutics – Barriers and progresses, J. Control. Release. 273 (2018) 51–67.
- A. Rogina, A. Ressler, I. Matić, G. Gallego Ferrer, I. Marijanović, M. Ivanković, H. Ivanković, Cellular hydrogels based on pH-responsive chitosan-hydroxyapatite system, Carbohydr. Polym. 166 (2017) 173–182.
- M.I.A. Echazú, C.E. Olivetti, I. Peralta, M.R. Alonso, C. Anesini, C.J. Perez, G.S. Alvarez, M.F. Desimone, Development of pH-responsive biopolymer-silica composites loaded with Larrea divaricata Cav. extract with antioxidant activity, Colloids Surfaces B Biointerfaces. 169 (2018) 82–91.
- M. Parani, G. Lokhande, A. Singh, A.K. Gaharwar, Engineered Nanomaterials for Infection Control and Healing Acute and Chronic Wounds, ACS Appl. Mater. Interfaces. 8 (2016) 10049–10069.
- S. Hamdan, I. Pastar, S. Drakulich, E. Dikici, M. Tomic-Canic, S. Deo, S. Daunert, Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications, ACS Cent. Sci. 3 (2017) 163–175.
- S. Bose, S.F. Robertson, A. Bandyopadhyay, Surface modification of biomaterials and biomedical devices using additive manufacturing, Acta Biomater. 66 (2018) 6–22. https://doi.org/10.1016/J.ACTBIO.2017.11.003.
- G. Wu, P. Li, H. Feng, X. Zhang, P.K. Chu, Engineering and functionalization of biomaterials via surface modification, J. Mater. Chem. B. 3 (2015) 2024–2042. https://doi.org/10.1039/C4TB01934B.
- M. Pezzoni, P.N. Catalano, R.A. Pizarro, M.F. Desimone, G.J.A.A. Soler-Illia, M.G. Bellino, C.S. Costa, Antibiofilm effect of supramolecularly templated mesoporous silica coatings, Mater. Sci. Eng. C. 77 (2017). https://doi.org/10.1016/j.msec.2017.04.022.
- P.N. Catalano, M. Pezzoni, C. Costa, G.J.D.A.A. Soler-Illia, M.G. Bellino, M.F. Desimone, Optically transparent silver-loaded mesoporous thin film coating with long-lasting antibacterial activity, Microporous Mesoporous Mater. 236 (2016). https://doi.org/10.1016/j.micromeso.2016.08.034.
- M.G. Bellino, S. Golbert, M.C. De Marzi, G.J.A.A. Soler-Illia, M.F. Desimone, Controlled adhesion and proliferation of a human osteoblastic cell line by tuning the nanoporosity of titania and silica coatings, Biomater. Sci. 1 (2013). https://doi.org/10.1039/c2bm00136e.
- B.A. Badeau, C.A. DeForest, Programming Stimuli-Responsive Behavior into Biomaterials, Annu. Rev. Biomed. Eng. 21 (2019) 241–265. https://doi.org/10.1146/annurev-bioeng-060418-052324.
- H.W. Ooi, S. Hafeez, C.A. van Blitterswijk, L. Moroni, M.B. Baker, Hydrogels that listen to cells: a review of cell-responsive strategies in biomaterial design for tissue regeneration, Mater. Horizons. 4 (2017) 1020–1040. https://doi.org/10.1039/C7MH00373K.
- P. Kondiah, Y. Choonara, P. Kondiah, T. Marimuthu, P. Kumar, L. du Toit, V. Pillay, A Review of Injectable Polymeric Hydrogel Systems for Application in Bone Tissue Engineering, Molecules. 21 (2016) 1580. https://doi.org/10.3390/molecules21111580.
- K. Albert, H.-Y. Hsu, Carbon-Based Materials for Photo-Triggered Theranostic Applications, Molecules. 21 (2016) 1585. https://doi.org/10.3390/molecules21111585.
- L. Shen, Biocompatible Polymer/Quantum Dots Hybrid Materials: Current Status and Future Developments, J. Funct. Biomater. 2 (2011) 355–372. https://doi.org/10.3390/jfb2040355.
- J.M. Galdopórpora, M.F. Morcillo, A. Ibar, C.J. Perez, M. V Tuttolomondo, M.F. Desimone, Development of Silver Nanoparticles/Gelatin Thermoresponsive Nanocomposites: Characterization and Antimicrobial Activity, Curr. Pharm. Des. 25 (n.d.) 4121–4129.
- S.T. Mousavi, G.R. Harper, S. Municoy, M.D. Ashton, D. Townsend, G.H.K. Alsharif, V.K. Oikonomou, M. Firlak, S. Au-Yong, B.E. Murdock, G.R. Akien, N.R. Halcovitch, S.J. Baldock, M. Fazilati, O. V Kolosov, B.J. Robinson, M.F. Desimone, J.G. Hardy, Electroactive Silk Fibroin Films for Electrochemically Enhanced Delivery of Drugs, Macromol. Mater. Eng. n/a (2020) 2000130. https://doi.org/10.1002/mame.202000130.
- G.A.R. Gonçalves, R. de M.A. Paiva, Gene therapy: advances, challenges and perspectives, Einstein (São Paulo). 15 (2017) 369–375. https://doi.org/10.1590/s1679-45082017rb4024.
- B.S. Pattni, V.P. Torchilin, Targeted Drug Delivery Systems: Strategies and Challenges, in: P. V. Devarajan, S. Jain (Eds.), Target. Drug Deliv. Concepts Des., Springer International Publishing, Cham, 2015: pp. 3–38.
- A. Khademhosseini, R. Langer, A decade of progress in tissue engineering, Nat. Protoc. 11 (2016) 1775–1781. https://doi.org/10.1038/nprot.2016.123.
- S. Mura, J. Nicolas, P. Couvreur, Stimuli-responsive nanocarriers for drug delivery, Nat. Mater. 12 (2013) 991–1003. https://doi.org/10.1038/nmat3776.
- R. Gracia, D. Mecerreyes, Polymers with redox properties: Materials for batteries, biosensors and more, Polym. Chem. 4 (2013) 2206–2214. https://doi.org/10.1039/c3py21118e.
- M. Huo, J. Yuan, L. Tao, Y. Wei, Redox-responsive polymers for drug delivery: from molecular design to applications, Polym. Chem. 5 (2014) 1519–1528. https://doi.org/10.1039/C3PY01192E.
- J.G. Hardy, J.Y. Lee, C.E. Schmidt, Biomimetic conducting polymer-based tissue scaffolds, Curr. Opin. Biotechnol. 24 (2013) 847–854. https://doi.org/10.1016/J.COPBIO.2013.03.011.
- A.H. Rajabi, M. Jaffe, T.L. Arinzeh, Piezoelectric materials for tissue regeneration: A review, Acta Biomater. 24 (2015) 12–23. https://doi.org/https://doi.org/10.1016/j.actbio.2015.07.010.
- F.R. Baxter, C.R. Bowen, I.G. Turner, A.C.E. Dent, Electrically active bioceramics: A review of interfacial responses, Ann. Biomed. Eng. 38 (2010) 2079–2092. https://doi.org/10.1007/s10439-010-9977-6.
- C. Ribeiro, V. Sencadas, D.M. Correia, S. Lanceros-Méndez, Piezoelectric polymers as biomaterials for tissue engineering applications, Colloids Surfaces B Biointerfaces. 136 (2015) 46–55. https://doi.org/https://doi.org/10.1016/j.colsurfb.2015.08.043.
- M.T. Chorsi, E.J. Curry, H.T. Chorsi, R. Das, J. Baroody, P.K. Purohit, H. Ilies, T.D. Nguyen, Piezoelectric Biomaterials for Sensors and Actuators, Adv. Mater. 31 (2018) 1–15. https://doi.org/10.1002/adma.201802084.
- H. Yuan, T. Lei, Y. Qin, J.H. He, R. Yang, Design and application of piezoelectric biomaterials, J. Phys. D. Appl. Phys. 52 (2019) 194002–194012. https://doi.org/10.1088/1361-6463/ab0532.
- K. Kapat, Q.T.H. Shubhra, M. Zhou, S. Leeuwenburgh, Piezoelectric Nano-Biomaterials for Biomedicine and Tissue Regeneration, Adv. Funct. Mater. (2020) 1–22. https://doi.org/10.1002/adfm.201909045.
- G. Kocak, C. Tuncer, V. Bütün, pH-Responsive polymers, Polym. Chem. 8 (2017) 144–176. https://doi.org/10.1039/C6PY01872F.
- M. Omidi, A. Yadegari, L. Tayebi, Wound dressing application of pH-sensitive carbon dots/chitosan hydrogel, RSC Adv. 7 (2017) 10638–10649. https://doi.org/10.1039/C6RA25340G.
- I. Banerjee, D. Mishra, T. Das, T.K. Maiti, Wound pH-Responsive Sustained Release of Therapeutics from a Poly(NIPAAm-co-AAc) Hydrogel, J. Biomater. Sci. Polym. Ed. 23 (2012) 111–132. https://doi.org/10.1163/092050610X545049.
- N. Ninan, A. Forget, V.P. Shastri, N.H. Voelcker, A. Blencowe, Antibacterial and Anti-Inflammatory pH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing, ACS Appl. Mater. Interfaces. 8 (2016) 28511–28521. https://doi.org/10.1021/acsami.6b10491.
- Y. Qiao, J. Wan, L. Zhou, W. Ma, Y. Yang, W. Luo, Z. Yu, H. Wang, Stimuli-responsive nanotherapeutics for precision drug delivery and cancer therapy, WIREs Nanomedicine and Nanobiotechnology. 11 (2019) e1527. https://doi.org/10.1002/wnan.1527.
- N.N. Ferreira, L.M.B. Ferreira, V.M.O. Cardoso, F.I. Boni, A.L.R. Souza, M.P.D. Gremião, Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches, Eur. Polym. J. 99 (2018) 117–133. https://doi.org/https://doi.org/10.1016/j.eurpolymj.2017.12.004.
- M. Karimi, M. Eslami, P. Sahandi-Zangabad, F. Mirab, N. Farajisafiloo, Z. Shafaei, D. Ghosh, M. Bozorgomid, F. Dashkhaneh, M.R. Hamblin, pH-Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents, WIREs Nanomedicine and Nanobiotechnology. 8 (2016) 696–716. https://doi.org/10.1002/wnan.1389.
- A.A. Adedoyin, A.K. Ekenseair, Biomedical applications of magneto-responsive scaffolds, Nano Res. 11 (2018) 5049–5064. https://doi.org/10.1007/s12274-018-2198-2.
- J.S. Katz, J.A. Burdick, Light-responsive biomaterials: Development and applications, Macromol. Biosci. 10 (2010) 339–348. https://doi.org/10.1002/mabi.200900297.
- M.A. Ward, T.K. Georgiou, Thermoresponsive Polymers for Biomedical Applications, Polymers (Basel). 3 (2011) 1215–1242. https://doi.org/10.3390/polym3031215.
- M. Sponchioni, U. Capasso Palmiero, D. Moscatelli, Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering, Mater. Sci. Eng. C. 102 (2019) 589–605. https://doi.org/https://doi.org/10.1016/j.msec.2019.04.069.
- P. Zarrintaj, M. Jouyandeh, M.R. Ganjali, B.S. Hadavand, M. Mozafari, S.S. Sheiko, M. Vatankhah-Varnoosfaderani, T.J. Gutiérrez, M.R. Saeb, Thermo-sensitive polymers in medicine: A review, Eur. Polym. J. 117 (2019) 402–423. https://doi.org/https://doi.org/10.1016/j.eurpolymj.2019.05.024.
- J. Zhang, X. Jiang, X. Wen, Q. Xu, H. Zeng, Y. Zhao, M. Liu, Z. Wang, X. Hu, Y. Wang, Bio-responsive smart polymers and biomedical applications, J. Phys. Mater. 2 (2019) 32004. https://doi.org/10.1088/2515-7639/ab1af5.
- X. Fu, L. Hosta-Rigau, R. Chandrawati, J. Cui, Multi-Stimuli-Responsive Polymer Particles, Films, and Hydrogels for Drug Delivery, Chem. 4 (2018) 2084–2107.
- B.A. Webb, M. Chimenti, M.P. Jacobson, D.L. Barber, Dysregulated pH: A perfect storm for cancer progression, Nat. Rev. Cancer. 11 (2011) 671–677. https://doi.org/10.1038/nrc3110.
- F.F. Sahle, M. Gulfam, T.L. Lowe, Design strategies for physical-stimuli-responsive programmable nanotherapeutics, Drug Discov. Today. 23 (2018) 992–1006. https://doi.org/10.1016/j.drudis.2018.04.003.
- D. Han, A.J. Steckl, Selective pH-Responsive Core–Sheath Nanofiber Membranes for Chem/Bio/Med Applications: Targeted Delivery of Functional Molecules, ACS Appl. Mater. Interfaces. 9 (2017) 42653–42660. https://doi.org/10.1021/acsami.7b16080.
- T. Tagami, W.D. Foltz, M.J. Ernsting, C.M. Lee, I.F. Tannock, J.P. May, S.-D. Li, MRI monitoring of intratumoral drug delivery and prediction of the therapeutic effect with a multifunctional thermosensitive liposome, Biomaterials. 32 (2011) 6570–6578. https://doi.org/10.1016/J.BIOMATERIALS.2011.05.029.
- H.Y. Yang, Y. Li, D.S. Lee, Multifunctional and Stimuli-Responsive Magnetic Nanoparticle-Based Delivery Systems for Biomedical Applications, Adv. Ther. 1800011 (2018) 1–17. https://doi.org/10.1002/adtp.201800011.
- S.P. Miguel, M.P. Ribeiro, H. Brancal, P. Coutinho, I.J. Correia, Thermoresponsive chitosan–agarose hydrogel for skin regeneration, Carbohydr. Polym. 111 (2014) 366–373. https://doi.org/10.1016/J.CARBPOL.2014.04.093.
- H. Katas, C.Y. Wen, M.I. Siddique, Z. Hussain, F.H. Mohd Fadhil, Thermoresponsive curcumin/DsiRNA nanoparticle gels for the treatment of diabetic wounds: Synthesis and drug release, Ther. Deliv. 8 (2017) 137–150. https://doi.org/10.4155/tde-2016-0075.
- A. Mellati, C.-M. Fan, A. Tamayol, N. Annabi, S. Dai, J. Bi, B. Jin, C. Xian, A. Khademhosseini, H. Zhang, Microengineered 3D cell-laden thermoresponsive hydrogels for mimicking cell morphology and orientation in cartilage tissue engineering, Biotechnol. Bioeng. 114 (2016) 217–231. https://doi.org/10.1002/bit.26061.
- C. Zhao, Z. Zeng, N.T. Qazvini, X. Yu, R. Zhang, S. Yan, Y. Shu, Y. Zhu, C. Duan, E. Bishop, J. Lei, W. Zhang, C. Yang, K. Wu, Y. Wu, L. An, S. Huang, X. Ji, C. Gong, C. Yuan, L. Zhang, W. Liu, B. Huang, Y. Feng, B. Zhang, Z. Dai, Y. Shen, X. Wang, W. Luo, L. Oliveira, A. Athiviraham, M.J. Lee, J.M. Wolf, G.A. Ameer, R.R. Reid, T.-C. He, W. Huang, Thermoresponsive Citrate-Based Graphene Oxide Scaffold Enhances Bone Regeneration from BMP9-Stimulated Adipose-Derived Mesenchymal Stem Cells, ACS Biomater. Sci. Eng. 4 (2018) 2943–2955. https://doi.org/10.1021/acsbiomaterials.8b00179.
- M. Meewes, J. Ricka, M. De Silva, R. Nyffenegger, T. Binkert, Coil-globule transition of poly(N-isopropylacrylamide): a study of surfactant effects by light scattering, Macromolecules. 24 (1991) 5811–5816. https://doi.org/10.1021/ma00021a014.
- Y. Zhu, R. Batchelor, A.B. Lowe, P.J. Roth, Design of Thermoresponsive Polymers with Aqueous LCST, UCST, or Both: Modification of a Reactive Poly(2-vinyl-4,4-dimethylazlactone) Scaffold, Macromolecules. 49 (2016) 672–680. https://doi.org/10.1021/acs.macromol.5b02056.
- E. Mah, R. Ghosh, E. Mah, R. Ghosh, Thermo-Responsive Hydrogels for Stimuli-Responsive Membranes, Processes. 1 (2013) 238–262. https://doi.org/10.3390/pr1030238.
- N. Vanparijs, L. Nuhn, B.G. De Geest, Transiently thermoresponsive polymers and their applications in biomedicine, Chem. Soc. Rev. 46 (2017) 1193–1239. https://doi.org/10.1039/C6CS00748A.
- S.K. Bhullar, N.L. Lala, S. Ramkrishna, Smart Biomaterials- A review, Rev. Adv. Mater. Sci. 40 (2015) 303–314. https://doi.org/10.21315/tlsr2018.29.2.9.
- E.R. Ruskowitz, C.A. Deforest, Photoresponsive biomaterials for targeted drug delivery and 4D cell culture, Nat. Rev. Mater. 3 (2018) 17087–17104. https://doi.org/10.1038/natrevmats.2017.87.
- S.J. Leung, M. Romanowski, Light-activated content release from liposomes, Theranostics. 2 (2012) 1020–1036. https://doi.org/10.7150/thno.4847.
- F.A. Jerca, V. Jerca, I.-C. Stancu, Development and Characterization of Photoresponsive Polymers, in: Polym. Photonic Mater. Towar. Biomed. Break., 2018: pp. 3–47. https://doi.org/10.1007/978-3-319-75801-5_1.
- F. Ercole, T.P. Davis, R.A. Evans, Photo-responsive systems and biomaterials: Photochromic polymers, light-triggered self-assembly, surface modification, fluorescence modulation and beyond, Polym. Chem. 1 (2010) 37–54. https://doi.org/10.1039/b9py00300b.
- Z. Chen, C. Wu, Z. Zhang, W. Wu, X. Wang, Z. Yu, Synthesis, functionalization, and nanomedical applications of functional magnetic nanoparticles, Chinese Chem. Lett. 29 (2018) 1601–1608. https://doi.org/10.1016/j.cclet.2018.08.007.
- M.V. Tuttolomondo, M.E. Villanueva, G.S. Alvarez, M.F. Desimone, L.E. Díaz, Preparation of submicrometer monodispersed magnetic silica particles using a novel water in oil microemulsion: properties and application for enzyme immobilization, Biotechnol. Lett. 35 (2013) 1571–1577. https://doi.org/10.1007/s10529-013-1259-6.
- Y. Zhao, T. Fan, J. Chen, J. Su, X. Zhi, P. Pan, L. Zou, Q. Zhang, Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration, Colloids Surfaces B Biointerfaces. 174 (2019) 70–79. https://doi.org/10.1016/j.colsurfb.2018.11.003.
- F. Ridi, M. Bonini, P. Baglioni, Magneto-responsive nanocomposites: Preparation and integration of magnetic nanoparticles into films, capsules, and gels, Adv. Colloid Interface Sci. 207 (2014) 3–13. https://doi.org/10.1016/j.cis.2013.09.006.




