
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mariacristina Filice | + 4040 word(s) | 4040 | 2021-09-08 04:26:38 |
Video Upload Options
Teleost fish are often regarded with interest for the remarkable ability of several species to tolerate even dramatic stresses, either internal or external, as in the case of fluctuations in O2 availability and temperature regimes. These events are naturally experienced by many fish species under different time scales, but they are now exacerbated by growing environmental changes. This further challenges the intrinsic ability of animals to cope with stress. The heart is crucial for the stress response, since a proper modulation of the cardiac function allows blood perfusion to the whole organism, particularly to respiratory organs and the brain. In cardiac cells, key signalling pathways are activated for maintaining molecular equilibrium, thus improving stress tolerance. In fish, the nitric oxide synthase (NOS)/nitric oxide (NO) system is fundamental for modulating the basal cardiac performance and is involved in the control of many adaptive responses to stress, including those related to variations in O2 and thermal regimes.
1. Introduction
2. The NOS/NO System and the Fish Heart
2.1. Hypoxia
Water hypoxia (O2 levels: ±2.8mg L-1; [32] is a major limiting environmental factor. It is routinely experienced by aquatic animals, either chronically or on a diel or seasonal basis, and results from complex processes including mixing, air–water exchange, and fluctuations in the pattern of O2 production and consumption [33] [34]. In recent years, aquatic hypoxia is increasing due to anthropogenic influences and climate changes [35][36], with severe impact on individual organisms, communities, and ecosystems.
In the heart, a general response to hypoxia is bradycardia, together with the depression of myocardial contractility, and the O2 consumption rate. These effects are particularly evident in hypoxia-intolerant species [37][38][39]. In contrast, hypoxia-tolerant species such as the crucian carp and the goldfish retain a normal cardiac function and even potentiate it by activating a complex molecular machinery that is only partially known [8][40][41].
2.2. The NOS/NO System in the Fish Heart under Hypoxia
A general effect of the activation of the NOS/NO system in the heart under hypoxia is the limitation of mitochondrial O2 consumption for the NO competitive binding with O2 to CytC oxidase [42]. When O2 availability is reduced, this contributes to preserving myocardial efficiency by enhancing the force generated per O2 consumed [43][44]. A comparison between the cardiac response to low O2 in hypoxia-intolerant vs. hypoxia-tolerant fish has been carried out by using ventricular strips from trout and goldfish [38]. In both species, NO generated by NOS activation inhibits respiration rates and contributes to improving myocardial efficiency. However, when NO is generated from nitrite conversion, different behaviors are observed in the two species. In fact, in trout but not in goldfish, myocardial O2 consumption is reduced without changes in force development. This is attributed to differences in oxygen affinity and then in the nitrite reductase capacity of myocardial Mb. With less O2 available, trout Mb may readily de-oxygenate, thus generating NO from nitrite, while the goldfish Mb, by remaining saturated with O2, is prevented by reducing nitrite [38]. In the steelhead trout, the role of the nitrergic system in the mechanical response of the heart to low O2 has been further investigated [22]. By exposing spongy ventricular strips from animals acclimated to low oxygen (PO2 = 8 kPa) to the NO donor (SNP), they observed that hypoxic acclimation scarcely influences the frequency-related NO-dependent effect on twitch duration and muscle contractility. The above studies suggest that, in trout, hypoxia exposure does not significantly influence the cardiac isometric contractility in response to NO. However, the authors do not exclude the possibility that the use of muscle strips may fail to reveal additional effects of NO that may be preserved in the whole heart preparation, closer to the in vivo situation [22][38]. In line with this, evidence obtained in goldfish, by using ex vivo isolated and perfused working heart preparations, a technique that prevents the constraints imposed by the use of limited parts of the organ, shows that the potentiated basal performance, typical of acute O2 limitation, is accompanied by an increased myocardial NOS expression [8]. The possibility that a more expressed enzyme generates a higher amount of NO and this in turn affects the myocardial performance is confirmed by the evidence that NO scavenging with PTIO, as well as NOS inhibition by L-NMMA, reduces the hypoxia-dependent increase of contractility. Moreover, under hypoxia, NOS inhibition by L-NMMA unchanges the Frank–Starling response of the goldfish heart. In contrast, a significant reduction of the myocardial sensitivity to stretch is observed if NO is removed from the tissue by PTIO and if sGC is inhibited by ODQ. This is of relevance, since it indicates that the effects elicited by NO involve the cGMP cascade but are NOS-independent, thus requiring other routes for NO generation [45].
In the hypoxic goldfish heart, the molecular events of downstream NO generation exclude the involvement of the cGMP-dependent signalling [45]. Non cGMP-dependent pathways represent an important route for NO to control its molecular targets. These pathways are mainly represented by protein S-nitrosylation, the covalent attachment of NO to the thiol group of cysteine (Cys) residues [46]. A significant reduction in the degree of S-nitrosylated proteins has been reported in the hypoxic goldfish heart with respect to the normoxic counterpart. In mammals, the significance of a dysregulated protein S-nitrosylation is correlated with both cardiac disorders [47] and with protective mechanisms against the development of myocardial dysfunction under stress [48]. Proteins encountering denitrosylation in the hypoxic goldfish heart and the related functional significance have not yet been identified. However, it is reasonable to hypothesize that this event, by activating still undefined protective programs, contributes to preserving myocardial function when challenged by hypoxia [45].
Under hypoxia, NO may determine protein nitration. Data obtained in the hypoxic goldfish heart suggest the presence of hypoxia-induced nitration, since an increased expression of Nox2, the catalytic subunit of NADPH oxidase [45], and 3-nitrotyrosine [49] has been reported. If further data confirm the occurrence of nitration, putative targets must be identified. Based on the available information, some proteins can be hypothesized. One is the SERCA2a pump, the integral membrane protein controlling cardiac Ca2+ homeostasis by actively transporting the ion into the sarcoplasmic reticulum. It is susceptible to nitrosative and oxidative modifications for the presence of several cysteine and tyrosine residues [50][51]. The structural proximity to mitochondria exposes SERCA2a pumps to reactive O2/nitrogen species generated as by-products of the oxidative phosphorylation [52]. The nitrotyrosine modification of SERCA2a has been observed in several pathophysiological conditions, and nitrated SERCA2a is utilized as a cardiac marker of nitrative stress [50]. Although direct evidence on SERCA2a pumps nitration in fish is not available, the significant reduction of the hypoxia-induced time-course increase of the goldfish heart performance observed under conditions of SERCA2a inhibition [45] points to SERCA2a as a putative target of nitration in the hypoxic heart.
In general, it appears that, in the goldfish heart, NO activates a protective program that sustains the performance under hypoxic challenge. Consistent with this, it was found that NO positively modulates cardiac sarcolemmal KATP channels, a response that, like the KATP-dependent protection observed in the ischemic mammalian myocardium [53], may contribute to the cardiac hypoxia tolerance of this species [5]. In addition, the hypoxic goldfish heart also shows an enhanced expression of the hypoxia inducible factor (HIF-1α) [8]. In mammals, HIF1α/NO interaction is involved in hypoxia-elicited cardio-protective responses. Under hypoxia, HIF-1α activates genes critical for cell survival, including NOS [54]; at the same time, high NO concentrations (>1 µM) stabilize HIF-1α, leading to an increase in the dimeric form of protein which, by binding HREs sites, and enhances NOS gene expression and thus NO generation [55].
2.3. Temperature
Aquatic ectotherms depend on the thermal milieu to regulate their metabolic rate. Apart from species living in extremely stable environments, many fish routinely face temperature fluctuations associated not only with ontogenetic and/or seasonal changes, but also with diurnal changes especially in shallow water bodies. Nevertheless, their phenotypic plasticity (developmental or reversible acclimation) allows compensation by altering tolerance limits for optimizing the performance under changed temperature regimes [56]. While eurythermal fish, naturally subjected to large temperature changes, develop acclimation strategies for preserving their fitness, stenothermal species show specific evolutionary adaptations at the expense of reduced plasticity.
In many eurytherm fish, temperature changes importantly influence the cardiac function that requires to be modulated to ensure an adequate CO. In addition, the upper thermal tolerance is partly determined by the capacity of the heart to ensure an adequate systemic O2 delivery [57]. This occurs by changing the heart rate (HR) more than the stroke volume [58][59]. When temperature acutely rises, the HR increases before declining at temperatures preceding the critical thermal maximum [58][59] and this compromises the cardiac function [57]. On the other hand, when the temperature drops, bradycardia occurs [60], and this is associated with an increased diastolic duration to maintain CO by increasing filling time and with little modifications of the systolic duration [61].
Different from eurythermal species, stenotherm fish scarcely tolerate thermal challenges. This is the case of Antarctic teleost Channichthyidae that live in the extremely stable, frigid, and highly oxygenated Antarctic waters [62][63]. Some of them are unique among adult vertebrates, since they lack hemoglobin (Hb; [64]) and, in some species, also Mb [65]. This is compensated by extensive cardiocirculatory remodelling such as hypervolemia, low blood viscosity, large capillaries, cardiomegaly, and high blood flow with low systemic pressure and systemic resistance ([62] and references therein).
2.4. The NOS/NO System in the Fish Heart under Temperature Challenges
In fish, the NOS/NO system plays a role in the regulation of the cardiac function in species adapted to both temperate and extreme thermal regimes, as well as in animals differently tolerant to thermal stress.In the eurythermal eel A. anguilla, the NOS/NO-dependent modulation of the Frank–Starling response [23][25] is impaired by temperature changes [66]. The positive modulation elicited by the intracardiac NO release on the Frank–Starling response disappears when animals are acutely exposed at temperatures lower orhigher than the acclimation one, both in the case of spring- and winter-like (acclimationtemperature: 20 °C and 10 °C, respectively) conditions. These effects are paralleled by reduced expression levels of NOS and pAkt, suggesting that the NO production via the Akt/NOS axis is temperature-dependent [66].
Another example is provided by salmonids. In the eurythermal Atlantic salmon, longterm exposure to temperature enhancement is accompanied by an increased expression of iNOS in both compact and spongy ventricular myocardium, indicative of an enhanced NO production. At the same time, also VEGF expression increases [67]. This is interesting, since the two effects, if considered together, may call for a potentiated blood supply to the myocardium obtained by increasing vascularization (via VEGF) and/or by dilating the vessels (via NO). In fact, in salmonids, NO is known to induce vasodilation and reduce coronary resistance [13], thus contributing to compensating for the increased O2 demand under elevated temperature. The relationship between temperature variations and the cardiac nitrergic control may be of great importance in fish living under extreme temperatures. Unfortunately, this aspect remains unexplored, although the information so far available indicates that the NOS/NO system plays a role in the modulation of the basal cardiac performance of these animals. In Antarctic teleosts, functional NOSs are present in the heart of the hemoglobinless C. aceratus and C. hamatus and the red blooded T. bernacchii. An eNOS-like enzyme is mainly present in the lacunae of the spongy ventricle, while iNOS is basally expressed in the cytoplasm of myocardiocytes [19][20]. Despite the similar distribution, physio-pharmacological studies show that NO differently affects the contractility of the three species [20]. In fact, endogenous NO (L-arginine administration) reduces contractility in T. bernacchii, contrary to the stimulatory effect observed in the two icefish species. In addition, while in C. hamatus the NO-induced effects are cGMP-dependent, in T. bernacchii and C. aceratus these effects are cGMP-independent. The authors suggest that, in the absence of respiratory pigments, the loss of NO-oxygenase activities associates with Hb/Mb and the consequent increased NO levels may account for the observed differences [20].
Tropical lungfish represent a peculiar model organism, since during warm seasons they undergo aestivation, a metabolic adaptation associated with functional modifications in tissues and organs including heart, kidney, gills, lung, and skeletal muscle [7][21][68][69][70]. A very interesting aspect of the lungfish is the ability of the myocardium to ensure contractility during warm aestivation, maintaining an appropriate blood perfusion to the whole organism. The lungfish heart, as observed in Protopterus dolloi [21] and Protopterus annectens [7], expresses NOS enzymes, and this expression increases under aestivation [21]. It has been proposed that the consequent enhanced NO release preserves the heart by sustaining cardiac bioenergetics in the presence of metabolic depression and reduced myocardial O2 consumption [7][21][69].
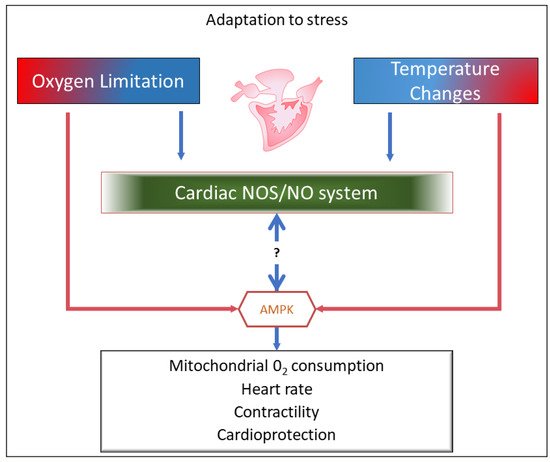
3. Upstream and Downstream the NOS/NO System: AMP-Activated Protein Kinase (AMPK) as a Candidate in Fish
References
- Selye, H. The Stress of Life; McGraw-Hill: Oxford, UK, 1978.
- Selye, H. Stress without Distress. In Psychopathology of Human Adaptation; Serban, G., Ed.; Springer: Boston, MA, USA, 1976; pp. 137–146.
- Balasch, J.C.; Tort, L. Netting the Stress Responses in Fish. Front. Endocrinol. 2019, 10, 62.
- Allmon, E.; Serafin, J.; Chen, S.; Rodgers, M.L.; Griffitt, R.; Bosker, T.; de Guise, S.; Sepulveda, M.S. Effects of polycyclic aromatic hydrocarbons and abiotic stressors on Fundulus grandis cardiac transcriptomics. Sci. Total. Environ. 2021, 752, 142156.
- Cameron, J.S.; Hoffmann, K.E.; Zia, C.; Hemmett, H.M.; Kronsteiner, A.; Lee, C.M. A role for nitric oxide in hypoxia-induced activation of cardiac KATP channels in goldfish (Carassius auratus). J. Exp. Biol. 2003, 206, 4057–4065.
- Cameron, J.S.; DeWitt, J.P.; Ngo, T.T.; Yajnik, T.; Chan, S.; Chung, E.; Kang, E. Cardiac K(ATP) channel alterations associated with acclimation to hypoxia in goldfish (Carassius auratus L.). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 164, 554–564.
- Amelio, D.; Garofalo, F.; Wong, W.P.; Chew, S.F.; Ip, Y.K.; Cerra, M.C.; Tota, B. Nitric oxide synthase-dependent “on/off” switch and apoptosis in freshwater and aestivating lungfish, Protopterus annectens: Skeletal muscle versus cardiac muscle. Nitric Oxide 2013, 32, 1–12.
- Imbrogno, S.; Capria, C.; Tota, B.; Jensen, F.B. Nitric oxide improves the hemodynamic performance of the hypoxic goldfish (Carassius auratus) heart. Nitric Oxide 2014, 42, 24–31.
- Gattuso, A.; Garofalo, F.; Cerra, M.C.; Imbrogno, S. Hypoxia Tolerance in Teleosts: Implications of Cardiac Nitrosative Signals. Front. Physiol. 2018, 9, 366.
- Eddy, F.B. Role of nitric oxide in larval and juvenile fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 142, 221–230.
- Hill, B.G.; Dranka, B.P.; Bailey, S.M.; Lancaster, J.R., Jr.; Darley-Usmar, V.M. What part of NO don’t you understand? Some answers to the cardinal questions in nitric oxide biology. J. Biol. Chem. 2010, 285, 19699–19704.
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167.
- Agnisola, C. Role of nitric oxide in the control of coronary resistance in teleosts. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 142, 178–187.
- Olson, K.R.; Donald, J.A. Nervous control of circulation-the role of gasotransmitters, NO, CO, and H2S. Acta Histochem. 2009, 111, 244–256.
- Imbrogno, S.; Tota, B.; Gattuso, A. The evolutionary functions of cardiac NOS/NO in vertebrates tracked by fish and amphibian paradigms. Nitric Oxide 2011, 25, 1–10.
- Imbrogno, S.; Filice, M.; Cerra, M.C.; Gattuso, A. NO, CO and H2S: What about gasotransmitters in fish and amphibian heart? Acta Physiol. 2018, 223, e13035.
- Tota, B.; Amelio, D.; Pellegrino, D.; Ip, Y.K.; Cerra, M.C. NO modulation of myocardial performance in fish hearts. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 142, 164–177.
- Filice, M.; Amelio, D.; Garofalo, F.; David, S.; Fucarino, A.; Jensen, F.B.; Imbrogno, S.; Cerra, M.C. Angiotensin II dependent cardiac remodeling in the eel Anguilla anguilla involves the NOS/NO system. Nitric Oxide 2017, 65, 50–59.
- Amelio, D.; Garofalo, F.; Pellegrino, D.; Giordano, F.; Tota, B.; Cerra, M.C. Cardiac expression and distribution of nitric oxide synthases in the ventricle of the cold-adapted Antarctic teleosts, the hemoglobinless Chionodraco hamatus and the red-blooded Trematomus bernacchii. Nitric Oxide 2006, 15, 190–198.
- Garofalo, F.; Amelio, D.; Cerra, M.C.; Tota, B.; Sidell, B.D.; Pellegrino, D. Morphological and physiological study of the cardiac NOS/NO system in the Antarctic (Hb-/Mb-) icefish Chaenocephalus aceratus and in the red-blooded Trematomus bernacchii. Nitric Oxide 2009, 20, 69–78.
- Amelio, D.; Garofalo, F.; Brunelli, E.; Loong, A.M.; Wong, W.P.; Ip, Y.K.; Tota, B.; Cerra, M.C. Differential NOS expression in freshwater and aestivating Protopterus dolloi (lungfish): Heart vs. kidney readjustments. Nitric Oxide 2008, 18, 1–10.
- Carnevale, C.; Syme, D.A.; Gamperl, A.K. Effects of hypoxic acclimation, muscle strain, and contraction frequency on nitric oxide-mediated myocardial performance in steelhead trout (Oncorhynchus mykiss). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R588–R610.
- Imbrogno, S.; De Iuri, L.; Mazza, R.; Tota, B. Nitric oxide modulates cardiac performance in the heart of Anguilla anguilla. J. Exp. Biol. 2001, 204, 1719–1727.
- Gattuso, A.; Mazza, R.; Imbrogno, S.; Sverdrup, A.; Tota, B.; Nylund, A. Cardiac performance in Salmo salar with infectious salmon anaemia (ISA): Putative role of nitric oxide. Dis. Aquat. Organ. 2002, 52, 11–20.
- Garofalo, F.; Parisella, M.L.; Amelio, D.; Tota, B.; Imbrogno, S. Phospholamban S-nitrosylation modulates Starling response in fish heart. Proc. Biol. Sci. 2009, 276, 4043–4052.
- Jensen, F.B. The role of nitrite in nitric oxide homeostasis: A comparative perspective. Biochim. Biophys. Acta 2009, 1787, 841–848.
- Hansen, M.N.; Lundberg, J.O.; Filice, M.; Fago, A.; Christensen, N.M.; Jensen, F.B. The roles of tissue nitrate reductase activity and myoglobin in securing nitric oxide availability in deeply hypoxic crucian carp. J. Exp. Biol. 2016, 219, 3875–3883.
- Sandvik, G.K.; Nilsson, G.E.; Jensen, F.B. Dramatic increase of nitrite levels in hearts of anoxia-exposed crucian carp supporting a role in cardioprotection. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R468–R477.
- Cerra, M.C.; Angelone, T.; Parisella, M.L.; Pellegrino, D.; Tota, B. Nitrite modulates contractility of teleost (Anguilla anguilla and Chionodraco hamatus, i.e., the Antarctic hemoglobinless icefish) and frog (Rana esculenta) hearts. Biochim. Biophys. Acta 2009, 1787, 849–855.
- Angelone, T.; Gattuso, A.; Imbrogno, S.; Mazza, R.; Tota, B. Nitrite is a positive modulator of the Frank-Starling response in the vertebrate heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R1271–R1281.
- Chapman, L.J.. Low-Oxygen Lifestyles; Springer International Publishing: Cham, Switzerland, 2015; pp. 9-33.
- Early life co-exposures to a real-world PAH mixture and hypoxia result in later life and next generation consequences in medaka (Oryzias latipes). . https://www.sciencedirect.com/science/article/abs/pii/S0166445X17301807?via%3Dihub. Retrieved 2021-9-10
- Oxygen declines and the shoaling of the hypoxic boundary in the California Current. . https://agupubs.onlinelibrary.wiley.com/doi/full/10.1029/2008GL034185. Retrieved 2021-9-10
- The change in oceanic O2 inventory associated with recent global warming . https://www.pnas.org/content/99/12/7848. Retrieved 2021-9-10
- Declining oxygen in the global ocean and coastal waters . https://www.science.org/lookup/doi/10.1126/science.aam7240. Retrieved 2021-9-10
- Adverse impacts of hypoxia on aquatic invertebrates: A meta-analysis. Sci. Total Environ. 2019, 652, 736–743 . https://www.sciencedirect.com/science/article/abs/pii/S0048969718341214?via%3Dihub. Retrieved 2021-9-10
- Cardiovascular responses to hypoxia in the Adriatic sturgeon (Acipenser naccarii) . https://onlinelibrary.wiley.com/doi/10.1111/j.1439-0426.1999.tb00209.x. Retrieved 2021-9-10
- Roles of nitric oxide, nitrite and myoglobin on myocardial efficiency in trout (Oncorhynchus mykiss) and goldfish (Carassius auratus): Implications for hypoxia tolerance. . https://journals.biologists.com/jeb/article/213/16/2755/9856/Roles-of-nitric-oxide-nitrite-and-myoglobin-on. Retrieved 2021-9-10
- Hypoxic acclimation negatively impacts the contractility of steelhead trout (Oncorhynchus mykiss) spongy myocardium. . https://journals.physiology.org/doi/full/10.1152/ajpregu.00107.2019. Retrieved 2021-9-10
- Maintained cardiac pumping in anoxic crucian carp . https://www.science.org/lookup/doi/10.1126/science.1100763. Retrieved 2021-9-10
- MS-Based proteomic analysis of cardiac response to hypoxia in the goldfish (Carassius auratus) . https://www.nature.com/articles/s41598-019-55497-w#citeas. Retrieved 2021-9-10
- Nitric oxide and mitochondrial signaling: From physiology to pathophysiology . https://www.ahajournals.org/doi/10.1161/ATVBAHA.107.151167. Retrieved 2021-9-12
- Endogenous nitric oxide enhances coupling between O2 consumption and ATP synthesis in guinea pig hearts . https://journals.physiology.org/doi/full/10.1152/ajpheart.2001.281.2.H838. Retrieved 2021-9-12
- Nitric oxide increases myocardial efficiency in the hypoxia-tolerant turtle Trachemys scripta . https://journals.biologists.com/jeb/article/212/7/954/19083/Nitric-oxide-increases-myocardial-efficiency-in. Retrieved 2021-9-12
- The Hypoxia Tolerance of the Goldfish (Carassius auratus) Heart: The NOS/NO System and Beyond . https://www.mdpi.com/2076-3921/9/6/555. Retrieved 2021-9-12
- Protein S-nitrosylation: Purview and parameters . https://www.nature.com/articles/nrm1569#citeas. Retrieved 2021-9-12
- Protein S-nitrosylation in health and disease: A current perspective . https://www.cell.com/trends/molecular-medicine/fulltext/S1471-4914(09)00119-1?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1471491409001191%3Fshowall%3Dtrue. Retrieved 2021-9-12
- Reduction of cardiomyocyte S-nitrosylation by S-nitrosoglutathione reductase protects against sepsis-induced myocardial depression . https://journals.physiology.org/doi/full/10.1152/ajpheart.00887.2012. Retrieved 2021-9-12
- Selenoprotein T as a new positive inotrope in the goldfish, Carassius auratus . https://journals.biologists.com/jeb/article/222/11/jeb201202/20462/Selenoprotein-T-as-a-new-positive-inotrope-in-the. Retrieved 2021-9-12
- Nitrotyrosine-modified SERCA2: A cellular sensor of reactive nitrogen species . https://link.springer.com/article/10.1007%2Fs00424-007-0429-6#citeas. Retrieved 2021-9-12
- SERCA2a tyrosine nitration coincides with impairments in maximal SERCA activity in left ventricles from tafazzin-deficient mice . https://physoc.onlinelibrary.wiley.com/doi/full/10.14814/phy2.14215. Retrieved 2021-9-12
- Mitochondrial free radical production and cell signaling . https://www.sciencedirect.com/science/article/abs/pii/S0098299704000068?via%3Dihub. Retrieved 2021-9-12
- ATP-Regulated K+ channels in cardiac muscle . https://www.nature.com/articles/305147a0#citeas. Retrieved 2021-9-12
- Hypoxia-Inducible factor as a therapeutic target for cardioprotection . https://www.sciencedirect.com/science/article/abs/pii/S0163725812001301?via%3Dihub. Retrieved 2021-9-12
- Regulation of hypoxia-inducible factor-1alpha by nitric oxide through mitochondria-dependent and -independent pathways . https://portlandpress.com/biochemj/article-abstract/376/2/537/40930/Regulation-of-hypoxia-inducible-factor-1-by-nitric?redirectedFrom=fulltext. Retrieved 2021-9-12
- Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure . https://academic.oup.com/icb/article/51/5/691/630399. Retrieved 2021-9-12
- Eliason, E.J.; Anttila, K.. Temperature and the Cardiovascular System; Gamperl, A.K., Gillis, T.E., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 235–297.
- The effects of acute changes in temperature and oxygen availability on cardiac performance in winter flounder (Pseudopleuronectes americanus) . https://www.sciencedirect.com/science/article/pii/S1095643309011702?via%3Dihub. Retrieved 2021-9-12
- Farrell, A.P.; Smith, F.. Cardiac Form, Function and Physiology; Gamperl, A.K., Gillis, T.E., Farrell, A.P., Brauner, C.J., Eds.; Academic Press : Cambridge, MA, USA, 2017; pp. 155–264.
- Temperature-Induced cardiac remodelling in fish . https://journals.biologists.com/jeb/article/220/2/147/18615/Temperature-induced-cardiac-remodelling-in-fish. Retrieved 2021-9-12
- Effects of seasonal acclimatization on temperature dependence of cardiac excitability in the roach, Rutilus rutilus . https://journals.biologists.com/jeb/article/219/10/1495/14649/Effects-of-seasonal-acclimatization-on-temperature. Retrieved 2021-9-12
- B. Tota; Maria Carmela Cerra; R. Mazza; D. Pellegrino; J. Icardo; The heart of the Antarctic icefish as paradigm of cold adaptation. Journal of Thermal Biology 1997, 22, 409-417, 10.1016/s0306-4565(97)00060-0.
- F. Garofalo; D. Pellegrino; Daniela Amelio; B. Tota; The Antarctic hemoglobinless icefish, fifty five years later: A unique cardiocirculatory interplay of disaptation and phenotypic plasticity. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 2009, 154, 10-28, 10.1016/j.cbpa.2009.04.621.
- Johan T. Ruud; Vertebrates without Erythrocytes and Blood Pigment. Nature 1954, 173, 848-850, 10.1038/173848a0.
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016, 26, 190–201.
- Daniela Amelio; Filippo Garofalo; C. Capria; B. Tota; S. Imbrogno; Effects of temperature on the nitric oxide-dependent modulation of the Frank–Starling mechanism: the fish heart as a case study. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 2013, 164, 356-362, 10.1016/j.cbpa.2012.10.037.
- Sven Martin Jørgensen; Vicente Castro; Aleksei Krasnov; Jacob Torgersen; Gerrit Timmerhaus; Ernst Morten Hevrøy; Tom Johnny Hansen; Sissel Susort; Olav Breck; Harald Takle; et al. Cardiac responses to elevated seawater temperature in Atlantic salmon. BMC Physiology 2014, 14, 2-2, 10.1186/1472-6793-14-2.
- F. Garofalo; D. Amelio; J.M. Icardo; S.F. Chew; B. Tota; M.C. Cerra; Y.K. Ip; Signal molecule changes in the gills and lungs of the African lungfish Protopterus annectens, during the maintenance and arousal phases of aestivation. Nitric Oxide 2015, 44, 71-80, 10.1016/j.niox.2014.11.017.
- Daniela Amelio; Filippo Garofalo; The NOS/NO system in an example of extreme adaptation: The African lungfish. Journal of Thermal Biology 2020, 90, 102594, 10.1016/j.jtherbio.2020.102594.
- Renato Filogonio; William Joyce; Tobias Wang; Nitrergic cardiovascular regulation in the African lungfish, Protopterus aethiopicus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 2017, 207, 52-56, 10.1016/j.cbpa.2016.12.030.
- D. Grahame Hardie; Bethany E. Schaffer; Anne Brunet; AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends in Cell Biology 2015, 26, 190-201, 10.1016/j.tcb.2015.10.013.
- Dengler, F. Activation of AMPK under Hypoxia: Many Roads Leading to Rome. Int. J. Mol. Sci. 2020, 21, 2428.
- Dan Shao; Shin-Ichi Oka; Tong Liu; Peiyong Zhai; Tetsuro Ago; Sebastiano Sciarretta; Hong Li; Junichi Sadoshima; A Redox-Dependent Mechanism for Regulation of AMPK Activation by Thioredoxin1 during Energy Starvation. Cell Metabolism 2014, 19, 232-245, 10.1016/j.cmet.2013.12.013.
- Cardaci, S.; Filomeni, G.; Ciriolo, M.R. Redox implications of AMPK-mediated signal transduction beyond energetic clues. J. Cell Sci. 2012, 125, 2115–2125.
- Kar, R.; Kellogg, D.L., 3rd; Roman, L.J. Oxidative stress induces phosphorylation of neuronal NOS in cardiomyocytes through AMP-activated protein kinase (AMPK). Biochem. Biophys. Res. Commun. 2015, 459, 393–397.
- Sartoretto, J.L.; Kalwa, H.; Pluth, M.D.; Lippard, S.J.; Michel, T. Hydrogen peroxide differentially modulates cardiac myocyte nitric oxide synthesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15792–15797.
- Zhang, Y.; Lee, T.S.; Kolb, E.M.; Sun, K.; Lu, X.; Sladek, F.M.; Kassab, G.S.; Garland, T., Jr.; Shyy, J.Y. AMP-Activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1281–1287.
- Hu, L.; Wang, J.; Zhu, H.; Wu, X.; Zhou, L.; Song, Y.; Zhu, S.; Hao, M.; Liu, C.; Fan, Y.; et al. Ischemic postconditioning protects the heart against ischemia-reperfusion injury via neuronal nitric oxide synthase in the sarcoplasmic reticulum and mitochondria. Cell Death Dis. 2016, 7, e2222.
- Salt, I.P.; Hardie, D.G. AMP-Activated Protein Kinase: An Ubiquitous Signaling Pathway With Key Roles in the Cardiovascular System. Circ. Res. 2017, 120, 1825–1841.
- Stenslokken, K.O.; Ellefsen, S.; Stecyk, J.A.; Dahl, M.B.; Nilsson, G.E.; Vaage, J. Differential regulation of AMP-activated kinase and AKT kinase in response to oxygen availability in crucian carp (Carassius carassius). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1803–R1814.
- Jibb, L.A.; Richards, J.G. AMP-Activated protein kinase activity during metabolic rate depression in the hypoxic goldfish, Carassius auratus. J. Exp. Biol. 2008, 211, 3111–3122.
- Mitochondria: a multimodal hub of hypoxia tolerance . https://cdnsciencepub.com/doi/10.1139/cjz-2013-0247. Retrieved 2021-9-12
- Anttila, K.; Casselman, M.T.; Schulte, P.M.; Farrell, A.P. Optimum temperature in juvenile salmonids: Connecting subcellular indicators to tissue function and whole-organism thermal optimum. Physiol. Biochem. Zool. 2013, 86, 245–256.
- Nie, M.; Lu, Y.; Zou, C.; Wang, L.; Zhang, P.; You, F. Insight into AMPK regulation mechanism in vivo and in vitro: Responses to low temperatures in the olive flounder Paralichthys olivaceus. J. Therm. Biol. 2020, 91, 102640.
- Nilsson, G.E.; Vaage, J.; Stensløkken, K.-O. Oxygen- and temperature-dependent expression of survival protein kinases in crucian carp (Carassius carassius) heart and brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R50–R61.





