
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ines Cordeiro Filipe | + 1812 word(s) | 1812 | 2021-08-30 08:19:08 | | | |
| 2 | Vicky Zhou | Meta information modification | 1812 | 2021-09-13 11:00:32 | | | | |
| 3 | Sirius Huang | + 3 word(s) | 1815 | 2023-05-06 08:21:52 | | |
Video Upload Options
Enteroviruses (EVs) from the D species are the causative agents of a diverse range of infectious diseases in spite of comprising only five known members. This small clade has a diverse host range and tissue tropism. It contains types infecting non-human primates and/or humans, and for the latter, they preferentially infect the eye, respiratory tract, gastrointestinal tract, and nervous system. Although several Enterovirus D members, in particular EV-D68, have been associated with neurological complications, including acute myelitis, there is currently no effective treatment or vaccine against any of them.
1. Introduction
Enteroviruses (EVs) are among the most prevalent viruses worldwide and several of them are important human pathogens. This group of viruses is characterized by a high genetic and phenotypic diversity, although the determinants behind it are still poorly understood. The Enterovirus D species includes five members with distinct symptomatology, illustrating EV diversity.
Enterovirus D Classification
Enterovirus genus includes 15 different species, each further subdivided in numerous types. Seven species, Enterovirus A to D and Rhinovirus A to C , contain human-infecting viruses [1][2][3], while the remaining eight species contain viruses with a wide host range from camelids to rodents and even marine mammals [4].
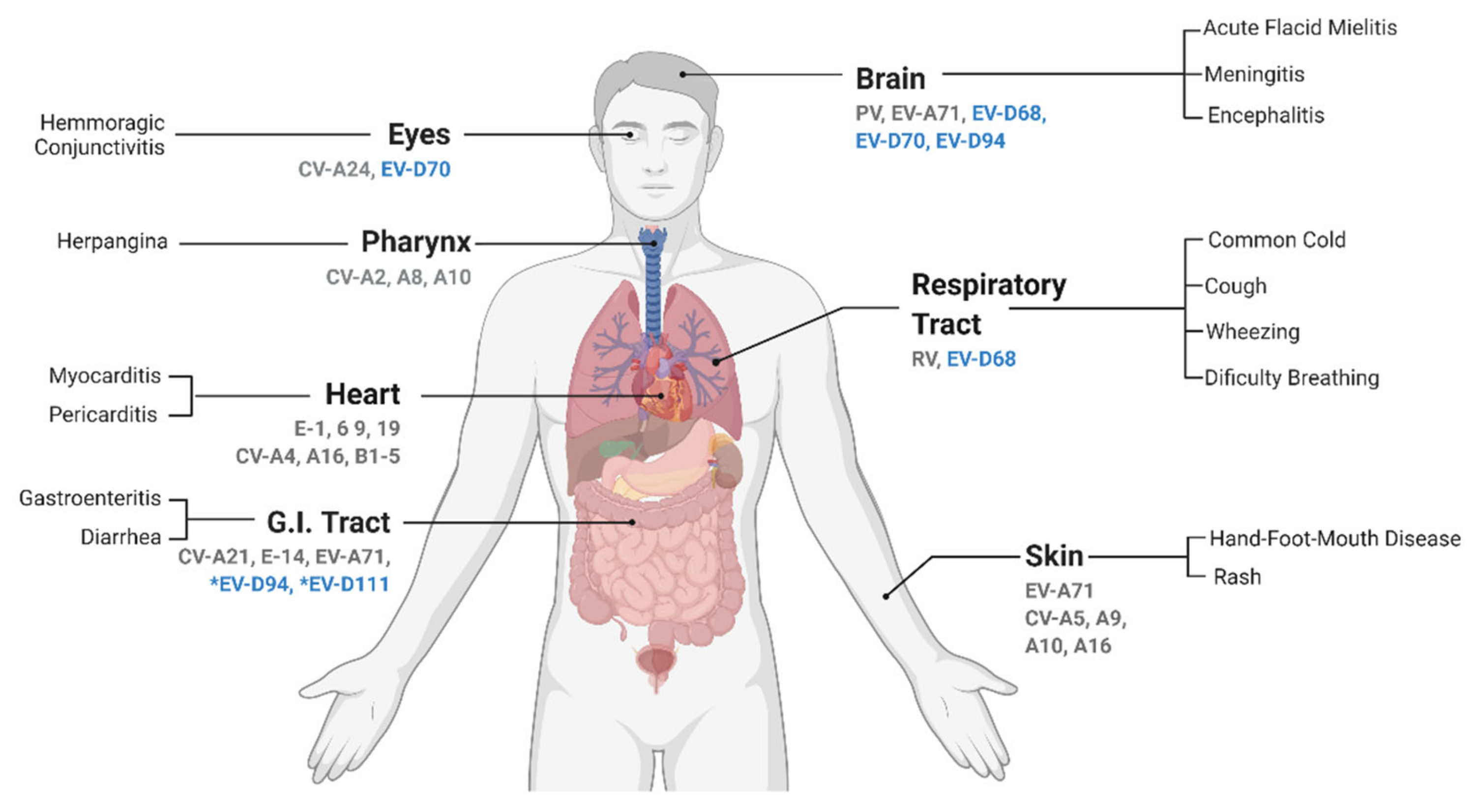
Enterovirus D is among the smallest species of the Enterovirus genus, counting only five types, but the host range, tissue tropism, and associated diseases are very diverse. EV-D68 and EV-D70, the first discovered EV-Ds, infect only humans but, unlike most EVs from the A to C species, they are not considered enterotropic viruses and are rather associated with respiratory and eye infections, respectively [5][6]. Although the EV-D68 genome has been detected in stools samples, no infectious viruses were isolated [7]. EV-D68 is also the only acid-sensitive in the Enterovirus D species [8][9]. The other three EV-D types, EV-D94, EV-D111, and EV-D120, were detected in Africa and remain poorly characterized. EV-D94 was discovered in 2007 in samples from sewage in Egypt and from acute flaccid paralysis (AFP) cases in the Democratic Republic of the Congo [10]. EV-D111 was identified both in human and primates stool samples, while EV-D120 was only detected in stool samples of wild non-human primates [11][12]. Important to note, the circulation of these viruses in humans may be underestimated as exemplified by seroprevalence studies highlighting the high prevalence of anti-EV-D94 antibodies in the Finnish population [10].
Based on the full genome sequence, EV-D94 is more closely related to EV-D111 and EV-D70 than to EV-D68 ( Figure 1 A). In fact, it has been proposed that EV-D94 and EV-D111 evolved by intertypic recombination, whereas no evidence has been found to support such recombination between these two viruses and EV-D68 [11]. In contrast, based on VP1 sequences, EV-D94 is closely related to EV-D68 and EV-D70 to EV-D120, while EV-D111 appears to be the most distinct member of the species ( Figure 1 B). This variation in VP1 may reflect different selective pressures. Important to note, the VP1 sequence divergence does not account for the observed intraspecies phenotypic diversity and is not specific to Enterovirus D . Indeed, other EV species infecting humans also contain viruses capable of causing a wide range of diseases (reviewed in Reference [3]) with the exception of the three rhinovirus (RV) species that contain only viruses causing respiratory infections. Even though recombination has been determined as a crucial mechanism for EV evolution, it seems to be a rare event among human-infecting EV-Ds probably due to distinct in vivo tropism [11][13].
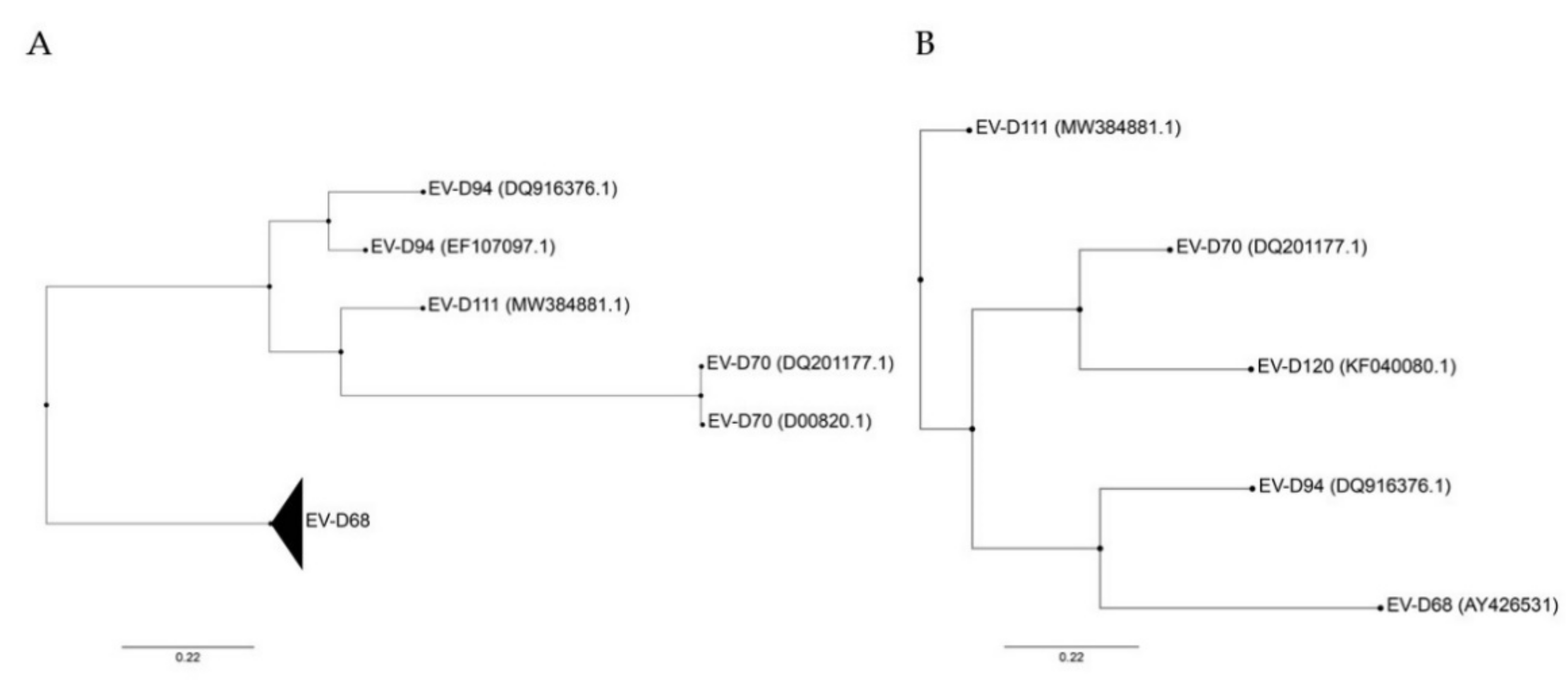
Figure 1. Phylogenetic trees constructed from complete genomes (A) and VP1 (B) of members of the Enterovirus D species. Both trees were rooted on porcine enterovirus 8 (AF406813), not shown. Only VP1 sequence was available for EV-D120, excluding this genotype from (A). Phylogenetic trees were constructed with PhyML using the Smart Model Selection, based on ungapped multiple sequence alignments produced with Muscle [14][15][16][17]. GenBank accession number are detailed in parentheses for each virus except for EV-D68 that includes several strains (AY426531, AB601882.2, AB601883.2, JX070222.1, JX10184.1, KF726085, KM851231.1, KM892500.1, KP240936, KP745755.1, KP745766.1, KP745767.1, KT285319.1, MK105982.1, MN240505, MN245981).
2. Enterovirus D Pathogenesis and Associated Symptoms
3. Conclusions
References
- Simmonds, P.; Gorbalenya, A.E.; Harvala, H.; Hovi, T.; Knowles, N.J.; Lindberg, A.M.; Oberste, M.S.; Palmenberg, A.C.; Reuter, G.; Skern, T.; et al. Recommendations for the nomenclature of enteroviruses and rhinoviruses. Arch. Virol. 2020, 165, 793–797.
- ICTV. Picornaviridae, Genus Enterovirus. Available online: https://talk.ictvonline.org/ictv-reports/ictv_online_report/positive-sense-rna-viruses/picornavirales/w/picornaviridae/681/genus-enterovirus (accessed on 15 July 2021).
- Tapparel, C.; Siegrist, F.; Petty, T.J.; Kaiser, L. Picornavirus and Enterovirus Diversity with Associated Human Diseases. Infect. Genet. Evol. 2013, 14, 282–293.
- Pirbright Institute. Picornaviridae.com. Available online: https://www.picornaviridae.com/hosts.htm (accessed on 15 July 2021).
- Khan, F. Enterovirus D68: Acute Respiratory Illness and the 2014 Outbreak. Emerg. Med. Clin. 2015, 33, e19–e32.
- Mirkovic, R.R.; Kono, R.; Yin-Murphy, M.; Sohier, R.; Schmidt, N.J.; Melnick, J.L. Enterovirus type 70: The etiologic agent of pandemic acute haemorrhagic conjunctivitis. Bull. World Health Organ. 1973, 49, 341–346.
- Pham, N.T.K.; Thongprachum, A.; Baba, T.; Okitsu, S.; Trinh, Q.D.; Komine-Aizawa, S.; Shimizu, H.; Hayakawa, S.; Ushijima, H. A 3-Month-Old Child with Acute Gastroenteritis with Enterovirus D68 Detected from Stool Specimen. Natl. Lab. Med. 2017, 63, 1269–1272.
- Royston, L.; Essaidi-Laziosi, M.; Pérez-Rodríguez, F.J.; Piuz, I.; Geiser, J.; Krause, K.-H.; Huang, S.; Constant, S.; Kaiser, L.; Garcin, D.; et al. Viral chimeras decrypt the role of enterovirus capsid proteins in viral tropism, acid sensitivity and optimal growth temperature. PLoS Pathog. 2018, 14, e1006962.
- Blomqvist, S.; Savolainen, C.; Råman, L.; Roivainen, M.; Hovi, T. Human Rhinovirus 87 and Enterovirus 68 Represent a Unique Serotype with Rhinovirus and Enterovirus Features. J. Clin. Microbiol. 2002, 40, 4218–4223.
- Smura, T.; Junttila, N.; Blomqvist, S.; Norder, H.; Kaijalainen, S.; Paananen, A.; Magnius, L.O.; Hovi, T.; Roivainen, M. Enterovirus 94, a proposed new serotype in human enterovirus species D. J. Gen. Virol. 2007, 88, 849–858.
- Sadeuh-Mba, S.A.; Joffret, M.-L.; Mazitchi, A.; Endegue-Zanga, M.-C.; Njouom, R.; Delpeyroux, F.; Gouandjika-Vasilache, I.; Bessaud, M. Genetic and phenotypic characterization of recently discovered enterovirus D type 111. PLoS Neglected Trop. Dis. 2019, 13, e0007797.
- Harvala, H.; Van Nguyen, D.; McIntyre, C.; Ahuka-Mundeke, S.; Ngole, E.M.; Delaporte, E.; Peeters, M.; Simmonds, P. Co-circulation of enteroviruses between apes and humans. J. Gen. Virol. 2014, 95, 403–407.
- Kyriakopoulou, Z.; Pliaka, V.; Amoutzias, G.; Markoulatos, P. Recombination among human non-polio enteroviruses: Implications for epidemiology and evolution. Virus Genes 2014, 50, 177–188.
- Lefort, V.; Longueville, J.-E.; Gascuel, O. SMS: Smart Model Selection in PhyML. Mol. Biol. Evol. 2017, 34, 2422–2424.
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321.
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113.
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278.
- Holm-Hansen, C.C.; Midgley, S.E.; Fischer, T.K. Global emergence of enterovirus D68: A systematic review. Lancet Infect. Dis. 2016, 16, e64–e75.
- Moss, R.B. Enterovirus 68 Infection–Association with Asthma. J. Allergy Clin. Immunol. Pr. 2016, 4, 226–228.
- Chen, B.-S.; Lee, H.-C.; Lee, K.-M.; Gong, Y.-N.; Shih, S.-R. Enterovirus and Encephalitis. Front. Microbiol. 2020, 11, 261.
- Smura, T.; Ylipaasto, P.; Klemola, P.; Kaijalainen, S.; Kyllönen, L.; Sordi, V.; Piemonti, L.; Roivainen, M. Cellular tropism of human enterovirus D species serotypes EV-94, EV-70, and EV-68 in vitro: Implications for pathogenesis. J. Med Virol. 2010, 82, 1940–1949.
- Palacios, G.; Oberste, M.S. Enteroviruses as agents of emerging infectious diseases. J. NeuroVirology 2005, 11, 424–433.
- Hixon, A.M.; Frost, J.; Rudy, M.J.; Messacar, K.; Clarke, P.; Tyler, K.L. Understanding Enterovirus D68-Induced Neurologic Disease: A Basic Science Review. Viruses 2019, 11, 821.
- Chatterjee, S.; O Quarcoopome, C.; Apenteng, A. Unusual type of epidemic conjunctivitis in Ghana. Br. J. Ophthalmol. 1970, 54, 628–630.
- Higgins, P.G. Enteroviral conjunctivitis and its neurological complications. Arch. Virol. 1982, 73, 91–101.
- Chen, D.; Texada, D.E.; Duggan, C.; Deng, Y.; Redens, T.B.; Langford, M.P. Caspase-3 and -7 mediate apoptosis of human Chang’s conjunctival cells induced by enterovirus 70. Virology 2006, 347, 307–322.
- Haddad, A.; Nokhbeh, M.R.; Alexander, D.A.; Dawe, S.J.; Grisé, C.; Gulzar, N.; Dimock, K. Binding to Decay-Accelerating Factor Is Not Required for Infection of Human Leukocyte Cell Lines by Enterovirus 70. J. Virol. 2004, 78, 2674–2681.
- Bharucha, E.; Mondkar, V.; Wadia, N.; Irani, P.; Katrak, S. Neurological complications of a new conjunctivitis. Lancet 1972, 300, 970–971.
- Junttila, N.; Leveque, N.; Kabue, J.P.; Cartet, G.; Mushiya, F.; Muyembe-Tamfum, J.-J.; Trompette, A.; Lina, B.; Magnius, L.O.; Chomel, J.-J.; et al. New enteroviruses, EV-93 and EV-94, associated with acute flaccid paralysis in the Democratic Republic of the Congo. J. Med Virol. 2007, 79, 393–400.
- Schieble, J.H.; Fox, V.L.; Lennette, E.H. A probable new human picornavirus associated with resoiratory disease. Am. J. Epidemiol. 1967, 85, 297–310.
- Royston, L.; Tapparel, C. Rhinoviruses and Respiratory Enteroviruses: Not as Simple as ABC. Viruses 2016, 8, 16.
- Kidd, S.; Lopez, A.S.; Konopka-Anstadt, J.L.; Nix, W.A.; Routh, J.A.; Oberste, M.S. Enterovirus D68-associated acute flaccid myelitis, United States, 2020. Emerg. Infect. Dis. 2020, 26, e201630.
- Baggen, J.; Thibaut, H.J.; Staring, J.; Jae, L.; Liu, Y.; Guo, H.; Slager, J.J.; de Bruin, J.W.; van Vliet, A.L.W.; Blomen, V.A.; et al. Enterovirus D68 receptor requirements unveiled by haploid genetics. Proc. Natl. Acad. Sci. USA 2016, 113, 1399–1404.
- Smith, B.D.; Pekosz, A. Contemporary enterovirus D68 strains show enhanced replication and translation at 37 °C. bioRxiv 2020.
- Jiang, Y.; Liu, S.; Shen, S.; Guo, H.; Huang, H.; Wei, W. Methyl-β-cyclodextrin inhibits EV-D68 virus entry by perturbing the accumulation of virus particles and ICAM-5 in lipid rafts. Antivir. Res. 2020, 176, 104752.
- Brown, B.A.; Nix, W.A.; Sheth, M.; Frace, M.; Oberste, M.S. Seven Strains of Enterovirus D68 Detected in the United States during the 2014 Severe Respiratory Disease Outbreak. Genome Announc. 2014, 2, e01201-14.
- Hixon, A.M.; Yu, G.; Leser, J.S.; Yagi, S.; Clarke, P.; Chiu, C.Y.; Tyler, K.L. A mouse model of paralytic myelitis caused by enterovirus D68. PLoS Pathog. 2017, 13, e1006199.
- Hixon, A.M.; Clarke, P.; Tyler, K.L. Contemporary Circulating Enterovirus D68 Strains Infect and Undergo Retrograde Axonal Transport in Spinal Motor Neurons Independent of Sialic Acid. J. Virol. 2019, 93.
- Hu, Y.; Musharrafieh, R.; Zheng, M.; Wang, J. Enterovirus D68 Antivirals: Past, Present, and Future. ACS Infect. Dis. 2020, 6, 1572–1586.
- Elrick, M.J.; Pekosz, A.; Duggal, P. Enterovirus D68 molecular and cellular biology and pathogenesis. J. Biol. Chem. 2021, 296, 100317.
- Liu, Y.; Sheng, J.; Fokine, A.; Meng, G.; Shin, W.-H.; Long, F.; Kuhn, R.J.; Kihara, D.; Rossmann, M.G. Structure and inhibition of EV-D68, a virus that causes respiratory illness in children. Science 2015, 347, 71–74.
- Sun, L.; Meijer, A.; Froeyen, M.; Zhang, L.; Thibaut, H.J.; Baggen, J.; George, S.; Vernachio, J.; Van Kuppeveld, F.J.M.; Leyssen, P.; et al. Antiviral Activity of Broad-Spectrum and Enterovirus-Specific Inhibitors against Clinical Isolates of Enterovirus D68. Antimicrob. Agents Chemother. 2015, 59, 7782–7785.
- Rhoden, E.; Zhang, M.; Nix, W.A.; Oberste, M.S. InVitroEfficacy of Antiviral Compounds against Enterovirus D68. Antimicrob. Agents Chemother. 2015, 59, 7779–7781.
- Smee, D.F.; Evans, W.J.; Nicolaou, K.; Tarbet, E.B.; Day, C.W. Susceptibilities of enterovirus D68, enterovirus 71, and rhinovirus 87 strains to various antiviral compounds. Antivir. Res. 2016, 131, 61–65.
- Ma, C.; Hu, Y.; Zhang, J.; Musharrafieh, R.; Wang, J. A Novel Capsid Binding Inhibitor Displays Potent Antiviral Activity against Enterovirus D68. ACS Infect. Dis. 2019, 5, 1952–1962.
- Arita, M.; Fuchino, H.; Kawakami, H.; Ezaki, M.; Kawahara, N. Characterization of a New Antienterovirus D68 Compound Purified from Avocado. ACS Infect. Dis. 2020, 6, 2291–2300.
- Musharrafieh, R.; Zhang, J.; Tuohy, P.; Kitamura, N.; Bellampalli, S.S.; Hu, Y.; Khanna, R.; Wang, J. Discovery of Quinoline Analogues as Potent Antivirals against Enterovirus D68 (EV-D68). J. Med. Chem. 2018, 62, 4074–4090.
- Kim, Y.; Kankanamalage, A.C.G.; Damalanka, V.C.; Weerawarna, P.; Groutas, W.C.; Chang, K.-O. Potent inhibition of enterovirus D68 and human rhinoviruses by dipeptidyl aldehydes and α-ketoamides. Antivir. Res. 2015, 125, 84–91.
- Musharrafieh, R.; Ma, C.; Zhang, J.; Hu, Y.; Diesing, J.M.; Marty, M.T.; Wang, J. Validating enterovirus D68-2Apro as an antiviral drug target and the discovery of telaprevir as a potent D68-2Apro inhibitor. J. Virol. 2019, 93, e02221-18.
- Hurst, B.L.; Evans, W.J.; Smee, D.F.; Van Wettere, A.J.; Tarbet, E.B. Evaluation of antiviral therapies in respiratory and neurological disease models of Enterovirus D68 infection in mice. Virology 2018, 526, 146–154.
- Ulferts, R.; Van Der Linden, L.; Thibaut, H.J.; Lanke, K.H.W.; Leyssen, P.; Coutard, B.; De Palma, A.M.; Canard, B.; Neyts, J.; Van Kuppeveld, F.J.M. Selective Serotonin Reuptake Inhibitor Fluoxetine Inhibits Replication of Human Enteroviruses B and D by Targeting Viral Protein 2C. Antimicrob. Agents Chemother. 2013, 57, 1952–1956.
- Gao, Q.; Yuan, S.; Zhang, C.; Wang, Y.; Wang, Y.; He, G.; Zhang, S.; Altmeyer, R.; Zou, G. Discovery of Itraconazole with Broad-SpectrumIn VitroAntienterovirus Activity That Targets Nonstructural Protein 3A. Antimicrob. Agents Chemother. 2015, 59, 2654–2665.
- Xu, N.; Yang, J.; Zheng, B.; Zhang, Y.; Cao, Y.; Huan, C.; Wang, S.; Chang, J.; Zhang, W. The Pyrimidine Analog FNC Potently Inhibits the Replication of Multiple Enteroviruses. J. Virol. 2020, 94.
- Van Der Linden, L.; Adrián, L.V.; Selisko, B.; Ferrer-Orta, C.; Liu, X.; Lanke, K.; Ulferts, R.; De Palma, A.M.; Tanchis, F.; Goris, N.; et al. The RNA Template Channel of the RNA-Dependent RNA Polymerase as a Target for Development of Antiviral Therapy of Multiple Genera within a Virus Family. PLoS Pathog. 2015, 11, e1004733.
- Tang, Q.; Li, S.; Du, L.; Chen, S.; Gao, J.; Cai, Y.; Xu, Z.; Zhao, Z.; Lan, K.; Wu, S. Emetine protects mice from enterovirus infection by inhibiting viral translation. Antivir. Res. 2019, 173, 104650.
- Hixon, A.M.; Clarke, P.; Tyler, K.L. Evaluating Treatment Efficacy in a Mouse Model of Enterovirus D68–Associated Paralytic Myelitis. J. Infect. Dis. 2017, 216, 1245–1253.
- Takeda, N.; Tanimura, M.; Miyamura, K. Molecular evolution of the major capsid protein VP1 of enterovirus 70. J. Virol. 1994, 68, 854–862.
- Smura, T.; Savolainen-Kopra, C.; Roivainen, M. Evolution of newly described enteroviruses. Future Virol. 2011, 6, 109–131.
- Sadeuh-Mba, S.A.; Bessaud, M.; Massenet, D.; Joffret, M.-L.; Endegue, M.-C.; Njouom, R.; Reynes, J.-M.; Rousset, D.; Delpeyroux, F. High Frequency and Diversity of Species C Enteroviruses in Cameroon and Neighboring Countries. J. Clin. Microbiol. 2012, 51, 759–770.
- Mombo, I.M.; Lukashev, A.N.; Bleicker, T.; Brünink, S.; Berthet, N.; Maganga, G.D.; Durand, P.; Arnathau, C.; Boundenga, L.; Ngoubangoye, B.; et al. African Non-Human Primates Host Diverse Enteroviruses. PLoS ONE 2017, 12, e0169067.




