
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cristina Rodríguez Varela | + 2179 word(s) | 2179 | 2021-09-12 04:12:47 | | | |
| 2 | Conner Chen | Meta information modification | 2179 | 2021-09-16 03:54:09 | | |
Video Upload Options
Acquiring oocyte competence requires optimal mitochondrial function and adequate ATP levels. In this context, CoQ10 supplementation may improve human oocyte quality and subsequent reproductive performance given its role in ATP synthesis and mitochondrial protection from ROS oxidative damage. In infertility treatments, CoQ10 therapy can be orally supplied to promote a more favorable environment for oocyte development in vivo or by its addition to culture media in an attempt to improve its quality in vitro.
1. Introduction
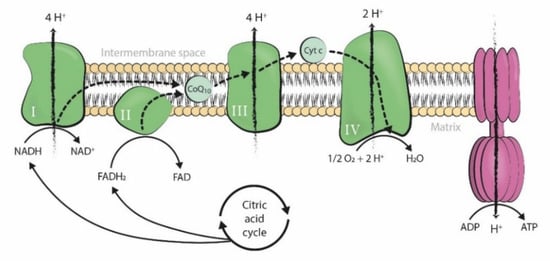
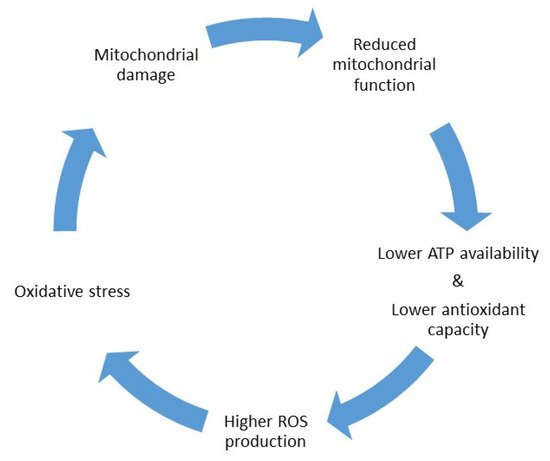
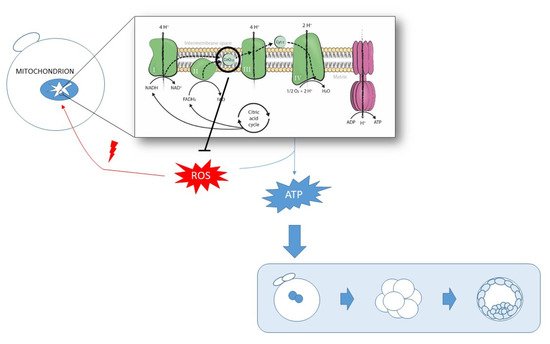
2. CoQ10 Supplementation in IVF Treatments
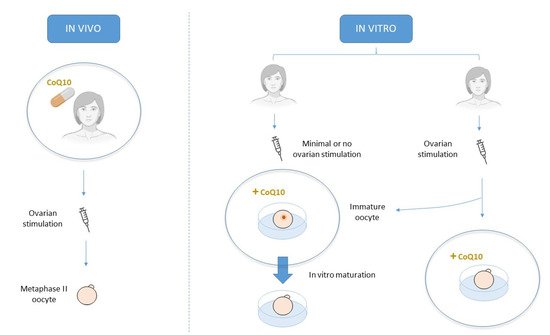
3. CoQ10 supplementation in human clinical trials
CoQ10 has been shown as a safe and well tolerated antioxidant treatment in humans [1]. Some adverse effects, such as nausea, diarrhea and abdominal pain, have been described after CoQ10 intake in the treatment of other diseases [25][26]. However, they are mild and occasionally-occurring side effects [27].
It is also a versatile therapy because it can be administered following a wide variety of protocols and at different ART treatment time points. Oral CoQ10 may benefit women with poor ovarian reserve, poor response to ovarian stimulation, advanced age or PCOS. What they all have in common are fewer and, usually less competent, mature oocytes [28][29]. However, promising results have been found mostly in follicular terms [30][31][32], and an enhancement at the oocyte level has been achieved only in a population of young poor responders [33]. This finding suggests that the lower age-related CoQ10 levels might be too low to be rescued after this antioxidant treatment. These patients may need higher doses or a different administration protocol, which have not yet been defined.
Regarding the beneficial effects of CoQ10 supplementation at the follicular level, higher levels of this molecule may create a more favorable environment for developing competent follicles. It has been proven that oxidative stress leads to higher apoptotic processes in granulosa cells [34]. CoQ10, by means of counteracting oxidative stress, can reduce this programmed granulosa cell death and, thus, reduce follicular atresia. This is evidenced by the higher antral follicle counts and larger number of mature follicles recorded in some reviewed studies [30][31]. However, this improvement did not suffice to significantly enhance oocyte quality, which has been directly evaluated in only a few studies [35][36][30], but is indirectly evidenced by similar pregnancy outcomes in others [36][30][33]. It is important to bear in mind that, although CoQ10 can have an impact at the follicular level, the ultimate objective of every ART treatment is to achieve successful pregnancy, which means that clear upgrades in pregnancy rates are needed to introduce this treatment into our routine clinical practice. A recent systematic review and meta-analysis of five randomized controlled trials (RCTs) concluded that CoQ10 oral supplementation increased clinical pregnancy rates (CPR) compared to a placebo or no treatment [28.8% vs. 14.1%; odds ratio (OR) 2.44, 95% confidence interval (CI) 1.30–4.59, p = 0.006] [37]. However, these results lose relevance given the high heterogeneity in the analyzed RCTs.
Another approach is to supplement CoQ10 directly in vitro during IVF treatment. High levels of this antioxidant come into close contact with the oocyte, although its apparent positive action at the follicular level is absent. In this context, CoQ10 supplementation does not offer any advantage over the standard culture of fertilized oocytes from women of advanced age [38], which seems logical if we consider that these oocytes had already undergone two consecutive meiotic divisions with age-related damaged cell machinery. For this reason, CoQ10 supplementation during the IVM of immature aged oocytes, which are arrested in the prophase of the first meiosis, seems more plausible. Indeed promising results have been shown in this line [39], which suggest that CoQ10 might help these aged oocytes to properly resume meiosis, as evidenced by lower aneuploidy rates. CoQ10 might achieve this by improving the mitochondrial function [40], as evidenced by the increased mitochondrial mass in treated oocytes [41] and, thus, provides the energy they lacked due to the aging process, which is essential for acquiring final maturation. In any case, the improvement was not fully achieved as more age-related factors contribute to this poor oocyte quality [42] and CoQ10 treatment itself may not be enough to overcome them. In contrast, CoQ10 addition during IVM of oocytes from young women did not show any advantage [39], which suggests that these oocytes already had the sufficient energy needed to resume meiosis, and higher CoQ10 levels did not lead to any advantage. Thus other strategies to improve maturation rates in such patients should be investigated.
Nevertheless, MitoQ supplementation during IVM culture showed significant improved oocyte quality regardless of patients’ age [43]. We hypothesize that the advantageous location of this targeted molecule and its ability to concentrate at higher rates in mitochondria may favor its mechanism of action and, thus, exert significant changes on young oocytes. MitoQ, or any other mitochondria-targeted antioxidant, supplementation deserves further research in human clinical trials.
In any case, the majority of the studies herein discussed focused on clinical outcomes, and did not evaluate the effects of CoQ10 on the oxidative stress status or at the mitochondrial level in oocytes. Ma et al. in 2018 and Al-Zubaidi et al. in 2021 were the only ones to analyze such parameters, and proved higher mitochondrial mass and mitochondrial membrane potential, respectively, after CoQ10/MitoQ addition to IVM medium [39][41][43]. However, they did not evaluate oxidative stress markers or any other indicator of oocyte energy status as many animal studies have previously done [15][18][19][23].
Therefore, further research is needed in this field, and should focus mainly on the molecular level to understand the exact mechanism by which CoQ10 enhances mitochondrial function. By solving this research question, we would be able to establish the best protocol, dose, molecular form and approach for its administration. Presently, our recommendation is to continue investigating this antioxidant in the reproductive field, but mostly as oral treatment or during IVM. Its addition to fertilized oocytes during standard culture seems worthless as its main role in improving oocyte competence should be performed prior to completing the second meiosis, and probably even earlier. In addition, more attention should be paid to mitochondria-targeted antioxidants, which have been poorly studied in human clinical trials and seem more efficient than the isolated CoQ10 form.
4. Conclusions
CoQ10 constitutes a safe well tolerated therapy capable of improving oocyte quality through oxidative stress counteraction and mitochondrial function enhancement. In humans, oral CoQ10 supplementation seems to exert positive effects, especially at the follicular level, by creating a more favorable environment for competent follicle development. However, these benefits are not necessarily translated to substantial oocyte improvements and subsequent gestational results. Indeed, no improvement has been reported regarding finally pregnancy outcome using this therapy. CoQ10 addition to culture media appears effective if performed in immature stages. In this scenario, mitochondria-targeted molecules may confer a certain advantage and offer a better prognosis.
Hence, the available data reviewed in this work do not clearly prove the advantage of CoQ10 supplementation in improving human oocyte quality. It seems promising, thus it deserves further research, specially using these modified CoQ10 forms, as well as molecular studies evaluating the impact of this therapy on oxidative stress status and mitochondrial function in human gametes.
References
- Isabella Peixoto De Barcelos; Richard H. Haas; CoQ10 and Aging. Biology 2019, 8, 28, 10.3390/biology8020028.
- Cristina Rodríguez-Varela; Elena Labarta; Clinical Application of Antioxidants to Improve Human Oocyte Mitochondrial Function: A Review. Antioxidants 2020, 9, 1197, 10.3390/antiox9121197.
- UyenPhuong C. Tran; Catherine F. Clarke; Endogenous synthesis of coenzyme Q in eukaryotes. Mitochondrion 2007, 7, S62-S71, 10.1016/j.mito.2007.03.007.
- Leo Marcoff; Paul D. Thompson; The Role of Coenzyme Q10 in Statin-Associated Myopathy: A Systematic Review. Journal of the American College of Cardiology 2007, 49, 2231-2237, 10.1016/j.jacc.2007.02.049.
- Catarina M. Quinzii; Michio Hirano; Coenzyme Q and mitochondrial disease. Developmental Disabilities Research Reviews 2010, 16, 183-188, 10.1002/ddrr.108.
- Serena Bianchi; Guido Macchiarelli; Giulietta Micara; Cesare Aragona; Marta Maione; Stefania Annarita Nottola; Ultrastructural and morphometric evaluation of aged cumulus-oocyte-complexes. IJAE 2014, 118, 28, 10.13128/ijae-13929.
- Hiroyuki Sasaki; Toshio Hamatani; Shintaro Kamijo; Maki Iwai; Masato Kobanawa; Seiji Ogawa; Kenji Miyado; Mamoru Tanaka; Impact of Oxidative Stress on Age-Associated Decline in Oocyte Developmental Competence. Frontiers in Endocrinology 2019, 10, 811, 10.3389/fendo.2019.00811.
- Li-Ya Wang; Da-Hui Wang; Xiang-Yang Zou; Chen-Ming Xu; Mitochondrial functions on oocytes and preimplantation embryos. Journal of Zhejiang University SCIENCE B 2009, 10, 483-492, 10.1631/jzus.b0820379.
- Serena Bianchi; Stefania Annarita Nottola; Diana Torge; Maria Grazia Palmerini; Stefano Necozione; Guido Macchiarelli; Association between Female Reproductive Health and Mancozeb: Systematic Review of Experimental Models. International Journal of Environmental Research and Public Health 2020, 17, 2580, 10.3390/ijerph17072580.
- Cai-Xia Yang; Zhi-Qiang Song; Su-Rui Pei; Xiao-Xia Yu; Jia-Kun Miao; Hao Liang; Yi-Liang Miao; Zhi-Qiang Du; Single cell RNA-seq reveals molecular pathways altered by 7, 12-dimethylbenz[a]anthracene treatment on pig oocytes. Theriogenology 2020, 157, 449-457, 10.1016/j.theriogenology.2020.08.020.
- Alberto Revelli; Luisa Delle Piane; Simona Casano; Emanuela Molinari; Marco Massobrio; Paolo Rinaudo; Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reproductive Biology and Endocrinology 2009, 7, 40-40, 10.1186/1477-7827-7-40.
- Angelo Turi; Stefano Raffaele Giannubilo; Francesca Brugè; Federica Principi; Silvia Battistoni; Fabrizia Santoni; Andrea Luigi Tranquilli; Gianpaolo Littarru; Luca Tiano; Coenzyme Q10 content in follicular fluid and its relationship with oocyte fertilization and embryo grading. Archives of Gynecology and Obstetrics 2011, 285, 1173-1176, 10.1007/s00404-011-2169-2.
- Süleyman Akarsu; Funda Gode; Ahmet Zeki Isik; Zeliha Günnur Dikmen; Mustafa Agah Tekindal; The association between coenzyme Q10 concentrations in follicular fluid with embryo morphokinetics and pregnancy rate in assisted reproductive techniques. Journal of In Vitro Fertilization and Embryo Transfer 2017, 34, 599-605, 10.1007/s10815-017-0882-x.
- Juan D. Hernández-Camacho; Michel Bernier; Guillermo López-Lluch; Plácido Navas; Coenzyme Q10 Supplementation in Aging and Disease. Frontiers in Physiology 2018, 9, 44, 10.3389/fphys.2018.00044.
- Assaf Ben‐Meir; Elizabeth Burstein; Aluet Borrego‐Alvarez; Jasmine Chong; Ellen Wong; Tetyana Yavorska; Taline Naranian; Maggie Chi; Ying Wang; Yaakov Bentov; et al.Jennifer AlexisJames MerianoHoon-Ki SungDavid L. GasserKelle H. MoleySiegfried HekimiRobert F. CasperAndrea Jurisicova Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 2015, 14, 887-895, 10.1111/acel.12368.
- Assaf Ben-Meir; Kyunga Kim; Rosanne McQuaid; Navid Esfandiari; Yaakov Bentov; Robert F. Casper; Andrea Jurisicova; Co-Enzyme Q10 Supplementation Rescues Cumulus Cells Dysfunction in a Maternal Aging Model. Antioxidants 2019, 8, 58, 10.3390/antiox8030058.
- Mianqun Zhang; Xiayan ShiYang; Yuwei Zhang; Yilong Miao; Ying Chen; Zhaokang Cui; Bo Xiong; Coenzyme Q10 ameliorates the quality of postovulatory aged oocytes by suppressing DNA damage and apoptosis. Free Radical Biology and Medicine 2019, 143, 84-94, 10.1016/j.freeradbiomed.2019.08.002.
- Ying-Jie Niu; Wenjun Zhou; Zheng-Wen Nie; Dongjie Zhou; Yong-Nan Xu; Sun A. Ock; Chang-Guo Yan; Xiang-Shun Cui; Ubiquinol-10 delays postovulatory oocyte aging by improving mitochondrial renewal in pigs. Aging 2020, 12, 1256-1271, 10.18632/aging.102681.
- C.E. Boots; A. Boudoures; W. Zhang; A. Drury; K.H. Moley; Obesity-induced oocyte mitochondrial defects are partially prevented and rescued by supplementation with co-enzyme Q10 in a mouse model. Human Reproduction 2016, 31, 2090-2097, 10.1093/humrep/dew181.
- Pınar Özcan; Cem Fıçıcıoğlu; Ozge Kizilkale; Mert Yesiladali; Olgu Enis Tok; Ferda Ozkan; Mukaddes Esrefoglu; Can Coenzyme Q10 supplementation protect the ovarian reserve against oxidative damage?. Journal of Assisted Reproduction and Genetics 2016, 33, 1223-1230, 10.1007/s10815-016-0751-z.
- Maria Fernanda Hornos Carneiro; Nara Shin; Rajendiran Karthikraj; Fernando Barbosa; Kurunthachalam Kannan; Monica P. Colaiácovo; Antioxidant CoQ10 Restores Fertility by Rescuing Bisphenol A-Induced Oxidative DNA Damage in the Caenorhabditis elegans Germline. Genetics 2020, 214, 381-395, 10.1534/genetics.119.302939.
- Carolina Maside; Cristina A Martinez; Josep M. Cambra; Xiomara Lucas; Emilio A. Martinez; María Antonia Gil; Heriberto Rodriguez-Martinez; Inmaculada Parrilla; Cristina Cuello; Supplementation with exogenous coenzyme Q10 to media for in vitro maturation and embryo culture fails to promote the developmental competence of porcine embryos. Reproduction in Domestic Animals 2019, 54, 72-77, 10.1111/rda.13486.
- M. K. Abdulhasan; Q. Li; J. Dai; H. M. Abu-Soud; E. E. Puscheck; D. A. Rappolee; CoQ10 increases mitochondrial mass and polarization, ATP and Oct4 potency levels, and bovine oocyte MII during IVM while decreasing AMPK activity and oocyte death. Journal of In Vitro Fertilization and Embryo Transfer 2017, 34, 1595-1607, 10.1007/s10815-017-1027-y.
- Sergio Romero; Ricardo Pella; Ingrid Zorrilla; Paola Berrío; Francisco Escudero; Ygor Pérez; Mario García; Carla Gonzalez; Patricia Orihuela; Coenzyme Q10 improves the in vitro maturation of oocytes exposed to the intrafollicular environment of patients on fertility treatment. JBRA Assisted Reproduction 2020, 24, 283-288, 10.5935/1518-0557.20200003.
- Catherine K. Yeung; Frederic T. Billings IV; Adam J. Claessens; Baback Roshanravan; Lori Linke; Mary B. Sundell; Suhail Ahmad; Baohai Shao; Danny D. Shen; T. Alp Ikizler; et al.Jonathan Himmelfarb Coenzyme Q10 dose-escalation study in hemodialysis patients: safety, tolerability, and effect on oxidative stress. BMC Nephrology 2015, 16, 1-8, 10.1186/s12882-015-0178-2.
- Serena L Orr; Sunita Venkateswaran; Nutraceuticals in the prophylaxis of pediatric migraine: Evidence-based review and recommendations. Cephalalgia 2014, 34, 568-583, 10.1177/0333102413519512.
- Gaku Yamanaka; Kanako Kanou; Tomoko Takamatsu; Mika Takeshita; Shinichiro Morichi; Shinji Suzuki; Yu Ishida; Yusuke Watanabe; Soken Go; Shingo Oana; et al.Hisashi Kawashima Complementary and Integrative Medicines as Prophylactic Agents for Pediatric Migraine: A Narrative Literature Review. Journal of Clinical Medicine 2021, 10, 138, 10.3390/jcm10010138.
- Daniel A. Dumesic; Joanne S. Richards; Ontogeny of the ovary in polycystic ovary syndrome. Fertility and Sterility 2013, 100, 23-38, 10.1016/j.fertnstert.2013.02.011.
- Sandro C. Esteves; Matheus Roque; Giuliano Bedoschi; Alessandro Conforti; Peter Humaidan; Carlo Alviggi; Defining Low Prognosis Patients Undergoing Assisted Reproductive Technology: POSEIDON Criteria—The Why. Frontiers in Endocrinology 2018, 9, 461, 10.3389/fendo.2018.00461.
- Itai Gat; Sonia Blanco Mejia; Hanna Balakier; Clifford Librach; Anne Claessens; Edward A.J. Ryan; The use of coenzyme Q10 and DHEA during IUI and IVF cycles in patients with decreased ovarian reserve. Gynecological Endocrinology 2016, 32, 534-537, 10.3109/09513590.2015.1137095.
- Abdelaziz El Refaeey; Amal Selem; Ahmed Badawy; Combined coenzyme Q10 and clomiphene citrate for ovulation induction in clomiphene-citrate-resistant polycystic ovary syndrome. Reproductive BioMedicine Online 2014, 29, 119-124, 10.1016/j.rbmo.2014.03.011.
- Sen Sharma, D; Category C: Oral Presentations: Fertility and Reproductive Medicine. BJOG: An International Journal of Obstetrics & Gynaecology 2017, 124, 9-11, 10.1111/1471-0528.2_14571.
- Yangying Xu; Victoria Nisenblat; Cuiling Lu; Rong Li; Jie Qiao; Xiumei Zhen; Shuyu Wang; Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: a randomized controlled trial. Reproductive Biology and Endocrinology 2018, 16, 1-11, 10.1186/s12958-018-0343-0.
- Hongyan Yang; Yan Xie; Dongyu Yang; Decheng Ren; Oxidative stress-induced apoptosis in granulosa cells involves JNK, p53 and Puma. Oncotarget 2017, 8, 25310-25322, 10.18632/oncotarget.15813.
- Yaakov Bentov; Thomas Hannam; Andrea Jurisicova; Navid Esfandiari; Robert F. Casper; Coenzyme Q10 Supplementation and Oocyte Aneuploidy in Women Undergoing IVF-ICSI Treatment. Clinical Medicine Insights: Reproductive Health 2014, 8, 31-6, 10.4137/cmrh.s14681.
- T. Caballero; F. Fiameni; A. Valcarcel; J. Buzzi; Dietary supplementation with coenzyme Q10 in poor responder patients undergoing IVF-ICSI Treatment. Fertility and Sterility 2016, 106, e58, 10.1016/j.fertnstert.2016.07.177.
- Panagiota Florou; Panagiotis Anagnostis; Patroklos Theocharis; Michail Chourdakis; Dimitrios G. Goulis; Does coenzyme Q10 supplementation improve fertility outcomes in women undergoing assisted reproductive technology procedures? A systematic review and meta-analysis of randomized-controlled trials. Journal of Assisted Reproduction and Genetics 2020, 37, 2377-2387, 10.1007/s10815-020-01906-3.
- Rebecca Kile; Deirdre M. Logsdon; Catherine Nathanson; Sue McCormick; William B. Schoolcraft; Rebecca L. Krisher; MITOCHONDRIAL SUPPORT OF EMBRYOS FROM WOMEN OF ADVANCED MATERNAL AGE DURING ART. Fertility and Sterility 2020, 114, e122, 10.1016/j.fertnstert.2020.08.363.
- Long Ma; Lingbo Cai; Mengting Hu; Jing Wang; Jiazi Xie; Yan Xing; Jiandong Shen; Yugui Cui; X. Johné Liu; Jiayin Liu; et al. Coenzyme Q10 supplementation of human oocyte in vitro maturation reduces postmeiotic aneuploidies. Fertility and Sterility 2020, 114, 331-337, 10.1016/j.fertnstert.2020.04.002.
- Xupeng Xing; Jinjing Zhang; Jingcheng Zhang; Yongsheng Wang; Jingyi Wang; Jian Kang; Fusheng Quan; Jianmin Su; Yong Zhang; Coenzyme Q10 supplement rescues postovulatory oocyte aging by regulating SIRT4 expression. Current Molecular Pharmacology 2021, 14, 1-1, 10.2174/1874467214666210420112819.
- L. Ma; M. Hu; X. Ma; Y. Cui; J. Liu; CoQ10 decreases aneuploidy rate and increases mitochondrial mass during in vitro maturation of human immature oocytes. Fertility and Sterility 2018, 110, e312, 10.1016/j.fertnstert.2018.07.878.
- Jie Qiao; Zhen-Bo Wang; Huai-Liang Feng; Yi-Liang Miao; Qiang Wang; Yang Yu; Yan-Chang Wei; Jie Yan; Wei-Hua Wang; Wei Shen; et al.Shao-Chen SunHeide SchattenQing-Yuan Sun The root of reduced fertility in aged women and possible therapentic options: Current status and future perspects. Molecular Aspects of Medicine 2013, 38, 54-85, 10.1016/j.mam.2013.06.001.
- Usama Al-Zubaidi; Deepak Adhikari; Ozgur Cinar; Qing-Hua Zhang; Wai Shan Yuen; Michael P Murphy; Luk Rombauts; Rebecca L Robker; John Carroll; Mitochondria-targeted therapeutics, MitoQ and BGP-15, reverse aging-associated meiotic spindle defects in mouse and human oocytes. Human Reproduction 2020, 36, 771-784, 10.1093/humrep/deaa300.




