
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Evangelia Tsiani | + 5699 word(s) | 5699 | 2021-09-02 08:34:30 |
Video Upload Options
Different diseases and disorders that affect the kidneys include, but are not limited to, glomerulonephritis, diabetic nephropathy, polycystic kidney disease, kidney stones, renal fibrosis, sepsis, and renal cell carcinoma. Kidney disease tends to develop over many years, making it difficult to identify until much later when kidney function is severely impaired and undergoing kidney failure. Epidemiological studies have suggested that a diet rich in fruits and vegetables is associated with health benefits including protection against kidney disease and renal cancer. Resveratrol, a polyphenol found in grapes and berries, has been reported to have antioxidant, anti-inflammatory, antidiabetic, hepatoprotective, neuroprotective, and anti-cancer properties.
1. Introduction
1.1. Kidney Function in Health and Disease
The kidneys are a pair of organs located below and posterior to the liver in the peritoneal cavity whose main function is blood filtration and salt and water homeostasis[1]. The kidney is divided into three regions: the outer cortex, medulla, and inner hilum. The renal cortex contains the functional unit of the kidney known as the nephron, with approximately one million nephrons located within each kidney (Figure 1)[2]. Each nephron is responsible for filtration as blood enters the kidney, which migrates through the length of the nephron where specialized regions reabsorb water and small molecules before it is secreted as urine. The nephron can be further divided into the renal corpuscle (Bowman’s capsule) and renal tubule[2]. Located within the Bowman’s capsule is the glomerulus, a filtering unit of blood vessels which is responsible for the majority of filtration within the kidney. Throughout all these structures, the kidney is connected to a highly vascularized network of arteries, veins, and nerves, entering and exiting at the renal hilum[2]. In addition to filtration and reabsorption, the kidneys also produce hormones such as renin, erythropoietin, and calcitriol/vitamin D3, that regulate blood pressure, help control red blood cell production, and maintain bone metabolism and health[3][4].
Chronic kidney disease (CKD) is defined as kidney damage, or decreased kidney function present for longer than three months. In addition, CKD requires an estimated GFR of less than 34.68 mL/min/m2 and abnormalities in biopsy/renal imaging results[5]. Kidney disease tends to develop over many years, making it difficult to identify until much later when kidney function is severely impaired. Physiologically, CKD arises due to many pathological injuries that destroys some of the nephrons, resulting in the nephrons overcompensating by hyperfiltration. Over time, glomerular hypertension, albuminuria, and loss of renal function develop[6]. The increase in glomerular capillary pressure leads to glomerular capillary wall destruction, dysfunction of podocytes that cover the capillaries, and increased macromolecule permeability[6][7]. In conjunction, increased pro-inflammatory mediators are released that stimulate the proliferation of fibrotic cells. In addition, accumulation of ECM molecules results in scar formation and renal failure[6][7][8]. Currently, treatment strategies exist for CKD, with all options aimed at relieving or preventing the condition from worsening, including conservative care, medication, dialysis, or transplantation[9][10].
CKD is not the only form of kidney disorder that can severely affect an individual, with many other disorders severely afflicting the kidney and renal system, such as polycystic kidney disease (PKD), a genetic disorder, either autosomal dominant or recessive, characterized by cyst formation in the kidneys[11]. Glomerulonephritis is term used to describe a range of immune-mediated disorders resulting in inflammation of the glomerulus and other regions of the kidney [12]. The inflammation within the kidney disrupts blood filtration, leading to decreased urination, high blood pressure, hematuria, and albuminuria[12]. Diabetic nephropathy characterized by glomeruli damage and impaired blood filtration develops in more than 50% of people with type 2 diabetes mellitus (T2DM)[13]. In addition, renal cell carcinoma (RCC), also known as cancer of the kidney, is the sixth and tenth most common cancer in men and women, respectively, accounting for more than 140,000 deaths yearly and ranking as the 13th most common cause of cancer death worldwide[14][15]. RCC originates in the lining of the proximal convoluted tubule and encompasses approximately ninety percent of all kidney cancer cases in adults[16]. RCC is characterized by decreased kidney filtration, anemia, and increased blood pressure, resulting in complete kidney failure[17]. Current treatment strategies of RCC include surgery (partial or radical nephrectomy), chemotherapy, immunotherapy, and radiation therapy[18].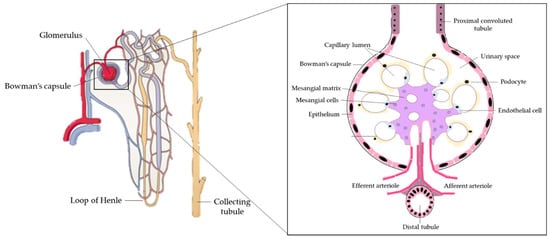
1.2. Resveratrol
2. Resveratrol’s Effects on Kidney Disease
2.1. In Vitro Studies: Effects of Resveratrol on Mesangial Cells
Glomerular mesangial cells occupy a central position in the renal glomerulus forming the central tuft-like structure of the glomerular microvasculature, involved in the generation of inflammatory mediators (such as cytokines, macromolecules and immune complexes), and are responsible for the contractile function. Mesangial cells contract or relax to modify glomerular filtration locally in response to vasoconstrictive or vasorelaxant agents, respectively[34]. Mesangial matrix expansion and vaso-mediator release result in decreased glomerular surface area and hemodynamics, reducing GFR. Mesangial cell function is affected by immunologic injury and metabolic disease, resulting in impaired filtration[35].
Overall, the studies show that the treatment of mesangial cells with RSV attenuated the basal, PDGF-, high glucose- and TGF-β1-induced cell proliferation. In addition, RSV treatment reduced the high glucose- and TGF-β1-induced oxidative stress and inflammation, reduced mitochondrial superoxide and ROS production, and increased MnSOD and mitochondrial complex III activity. The production of the extracellular matrix protein, fibronectin, was significantly inhibited by RSV treatment. RSV treatment significantly reduced the high glucose-induced effects by regulating NF-κB, JNK, Akt, and p38 signaling (Table 1).|
Cell |
Resveratrol Concentration/Duration |
Effect |
Reference |
|---|---|---|---|
|
Rat primary mesangial cells and LLCPK1 cells |
50–75 µM; 24 h |
↑NF-κB activation |
[36] |
|
Rat primary mesangial cells |
10 µM; 1 h |
↓Gentamicin-induced contraction |
[37] |
|
Rat mesangial cells |
10 µM; 1 h |
↓PDGF-induced cell proliferation ↓PDGFR Y-751 phosphorylation ↓PDGFR Y-761 phosphorylation ↓PDGF-induced PI3K, Akt, ERK1/2, c-Src activity ↑PTP1B activity |
[38] |
|
Rat primary mesangial cells |
10 μM; 6 h |
↓High glucose-induced
↑ MnSOD activity ↑ Mitochondrial complex III activity ↑ ∆Ψm hyperpolarization ↑ SIRT1 activity |
[39] |
|
CRL-2573 and primary mesangial cells |
5–10 µM; 24 h |
↓ High glucose-induced
|
[40] |
|
HBYZ-1 cells |
20 μM; 72 h |
↑High glucose-induced AdipoR1 mRNA and protein ↑FOX01 activity ↓FOX01 phosphorylation |
[41] |
|
Rat mesangial cells |
25 μM; 48 h |
↓High glucose-induced
|
[42] |
|
CRL-2573 cells |
10 µM; 48 h |
↓High glucose-induced
|
[43] |
|
SV40 MES 13 cells |
10 µM; 46 h |
↓TGF-β1-induced ROS production ↑TGF-β1-induced
|
[44] |
NF-κB: nuclear factor kappa light-chain-enhancer of activated B cells; PDGF: platelet-derived growth factor; PI3K: phosphoinositide 3-kinase; Akt: protein kinase B; ERK1/2: extracellular signal-regulated kinases 1/2; c-Src: proto-oncogene tyrosine-protein kinase Src; PTP1B: tyrosine-protein phosphatase non-receptor type 1B; MnSOD: manganese superoxide dismutase; ROS: reactive oxygen species; SIRT1: sirtuin 1; JNK: c-Jun N-terminal kinase; NADPH: nicotinamide adenine dinucleotide phosphate; FOX01: forkhead box 01; PAI-1: plasminogen activator inhibitor 1; MAPK: mitogen-activated protein kinase; TGF-β1: transforming growth factor-β1; ATP: adenosine triphosphate; NDUFB8: NADH:ubiquinone oxidoreductase subunit B8; PGC-1α: peroxisome proliferator-activated receptor gamma coactivator. ↓: decrease; ↑: increase.
2.2. In Vitro Studies: Effects of Resveratrol on Renal Epithelial Cells
|
Cell |
Resveratrol Concentration/Duration |
Effect |
Reference |
|---|---|---|---|
|
Rodent glomerular epithelial cells |
30 μM and 50 μM; 72 h |
↓High glucose-induced
|
[46] |
|
Mouse proximal tubular epithelial cells |
100 μM; 30 min |
↓Cisplatin-induced
↑SIRT1 siRNA-acetylation ↑Bcl-xL, Bax, and Bak protein |
[47] |
|
Human renal epithelial cells |
0, 40 and 80 µM; 24 h |
↓Oxalate-induced
|
[48] |
|
mpkCCDC14 cells |
25–400 µM; 30 min to 24 h |
↓Sodium transport ↑GFP-AKT-PH redistribution ↑AMPKα protein |
[49] |
|
NRK-52E cells |
10 and 100 μM; 24 h |
↓TGF-β1-induced
|
[50] |
|
HK-2 cells |
5–20 µM; 4 h |
↓High glucose-induced
|
[51] |
|
HK-2 cells |
20 μM; 48 h |
↓EMT ↓β-catenin nuclear translocation ↑E-cadherin and SIRT1 mRNA and protein ↓MMP7, α-SMA, and COLIA1 mRNA and protein |
[52] |
|
HK-2 cells |
12.5 µM; 48 h |
↓Ioxitalamate-induced
↑Bcl-2 and survivin protein ↑ Caspase 3 activity |
[53] |
|
OX161 and UCL93 human renal epithelial cells; MDCK canine renal epithelial cells |
2–50 µM; 48 h |
↓Cyst number ↓MCP-1 protein and activity ↓TNF-α protein and activity ↓CFB protein and activity ↑SOD2 protein |
[54] |
|
HK-2 cells |
20 µM; 12 h |
↑Cell viability ↓Ph-NFκB protein ↓TNF-α, IL-1β, and IL-6 mRNA and protein ↓IRE1 activation |
[55] |
|
HK-2 cells |
25 µM; 72 h |
↓High glucose-induced oxidative stress ↓MDA and ROS activity ↑CAT and SIRT1 protein ↑SIRT1 activity ↓Acetyl-FOXO3a protein |
[56] |
|
TCMK-1 cells |
25 µM; 72 h |
↓Cadmium-induced apoptosis ↓mROS production ↑mSIRT3 protein and activity ↑PGC-1α and SOD2 mRNA |
[57] |
|
HK-2 cells |
5–20, 40 µM; 72 h |
5–20 µM RSV: ↓TGF-β-induced EMT ↓Cytotoxicity ↑SIRT1 and E-cadherin protein ↓α-SMA and fibronectin protein ↓Ph-Smad3 ↓SIRT1-Smad3/4 40 µM RSV: ↑Cytotoxicity ↑mtROS release ↑Bax, fibronectin, and α-SMA protein ↓Bcl-2 protein ↓ATP production ↓PGC-1α and TFAM protein |
[58] |
LKB1: liver kinase B1; eIF: eukaryotic translation initiation factor; eEF2: eukaryotic translation elongation factor 2; p70S6K: ribosomal protein S6 kinase beta-1; AMPKα: AMP-activated protein kinase alpha; PUMA: pro-apoptotic p53 upregulated modulator of apoptosis; siRNA: small interfering RNA; GFP: green fluorescent protein; EMT: epithelial-to-mesenchymal transition; MDA: malondialdehyde; TFAM: mitochondrial transcription factor A; Bcl-xL: B-cell lymphoma-extra-large; Bax: Bcl-2-associated X; Bak protein: BCL2-antagonist/killer protein; α-SMA: α-smooth muscle actin; COLIA1: collagen type I alpha 1; NOX: NADPH oxidase; MMP: matrix metalloproteinase; MCP-1: monocyte chemoattractant protein 1; CAT: catalase; CFB: complement factor B; mROS: mitochondrial ROS; IL: interleukin; TFAM: mitochondrial transcription factor A.
2.3. In Vitro Studies: Effects of Resveratrol on Cells of the Renal Corpuscle
Renal podocytes are cells that wrap around the capillaries of the glomerulus in the Bowman’s capsule. Functionally, podocytes, together with renal endothelial cells, form the filtration barrier and interact with mesangial cells to regulate glomeruli function[59]. Mouse podocytes treated with TGF-β1 to induce transdifferentiation followed with RSV treatment resulted in significantly reduced albumin permeability across the podocyte monolayer, indicating reduced podocyte death and increased percentage of E-cadherin expressing cells[60]. Additionally, adhesion molecules P-cadherin, zonula occludens-1 (ZO-1), and kin of IRRE-like protein 1 (NEPH1) protein levels were significantly increased, while α-SMA protein levels were decreased with RSV treatment, indicating preserved podocyte function[60]. In conjunction, treatment of podocytes with RSV resulted in attenuation of the high glucose-induced mitochondrial stress, decreased mROS production and increased membrane potential, all involved in diabetic nephropathy development[61]. In addition, RSV treatment increased respiratory chain complex I and III activities, while release of pro-apoptotic proteins (cytochrome C and diablo) from the mitochondria was reduced, suggesting improved mitochondrial functioning and reduced podocyte damage. Additionally, SIRT1, PGC-1α, nuclear respiratory factor 1 (NRF-1), and TFAM mRNA and protein levels were increased with RSV treatment[61].
Overall, the studies suggest that treatment of cells of the renal corpuscle (podocytes) with RSV preserves membrane integrity and metabolic flux. RSV treatment reduces albumin permeability and α-SMA protein levels, suggesting preserved renal functioning. Increased mitochondria complex activities and decreased mROS production indicate increased metabolic flux and decreased oxidative stress with RSV treatment. These data show that treatment of cells of the renal corpuscle with RSV exhibit a kidney oxidative protective effect and improved function (Table 3).
|
Cell |
Resveratrol Concentration/Duration |
Effect |
Reference |
|---|---|---|---|
|
Mouse podocytes |
2–5 µM; 30 min |
↓Albumin permeability ↓Podocyte death ↑E-cadherin expression ↑P-cadherin, ZO-1, and NEPH1 protein ↓α-SMA protein |
[60] |
|
Immortalized podocytes |
10 μM; 48 h |
↓High glucose-induced
↑Complexes I and III activities ↑Mitochondrial membrane potential ↑SIRT1, PGC-1α, NRF1, TFAM mRNA and protein |
[61] |
ZO-1: zonula occludens-1; NEPH1: kin of IRRE-like protein 1; NRF1: nuclear respiratory factor 1.
2.4. In Vitro Studies: Effects of Resveratrol on Embryonic Kidney Cells
The development of the embryonic kidney begins with the invasion of the metanephric mesenchyme by the ureteric bud. Under a series of morphogenetic events that convert the mesenchyme to epithelium, the basis of the mature nephron is formed[62]. The human embryonic kidney (HEK) 293 cell line is commonly used in research as a model of kidney cell differentiation[63]. In a study by Rössler et al. (2015), treatment of HEK293 cells with RSV resulted in increased early growth response 1 (Egr-1) protein levels and the transcription of the Egr-1 responsive reporter gene, indicating increased activity[64]. In addition, RSV treatment increased ERK1/2 phosphorylation and Raf activation, while MAP kinase phosphatase-1 (MKP-1) activity was impaired[64]. ETS like-1 protein (Elk-1) transcriptional activity was significantly increased with RSV treatment. Importantly, inhibition of ERK or use of dominant negative Raf prevented the RSV induced increased Egr-1 levels. These data suggest that RSV induces the expression of Egr-1 by ERK and Raf activation and MKP-1 repression[64].
Ochratoxin A (OTA) is a nephrotoxin that results in the destruction of renal tubular epithelium resulting in progressive renal failure, effects associated with decreased antioxidant activity and increased ROS production[65]. Treatment of HEK293 cells with RSV resulted in significantly decreased intracellular ROS production; however, when co-treated with OTA, RSV was unable to mitigate the increased ROS production[66]. DNA damage was decreased in HEK293 cells treated with RSV alone and co-treated with OTA, suggesting improved epithelium preservation. Additionally, OTA-induced 8-oxoguanine glycosylase (OGG1) mRNA levels were significantly increased with RSV, indicating increased DNA repair. OTA-induced glutathione (GSH) levels were significantly increased in cells treated with RSV, compared to OTA treated cells[66]. Overall, these data indicate that RSV treatment protects against nephrotoxin-induced DNA damage through decreased ROS production and increased antioxidant GSH level.
Treatment of HEK293 cells with RSV resulted in significantly decreased high glucose-induced aging marker, β-galactosidase, mRNA levels, indicating reduced aging. RSV treatment also increased high glucose-induced SIRT1 and thioredoxin (Trx) mRNA levels while Trx interacting protein (TXNIP) mRNA levels were reduced indicating improved intracellular antioxidant expression[67].
Overall, these studies suggest that treatment of embryonic kidney cells with RSV reduced toxin or aging-induced DNA-damage and increased DNA-repair, indicative of improved cellular activity and longevity. In addition, RSV treatment reduced OTA- and high glucose-induced oxidative stress with increased GSH enzyme activity and decreased ROS production. These data show that RSV treatment protects embryonic kidney cells from DNA damage (Table 4).
|
Cell |
Resveratrol Concentration/Duration |
Effect |
Reference |
|---|---|---|---|
|
HEK293 cells |
20 μM; 24 h |
↑Egr-1 protein ↑Egr-1 reporter mRNA ↑Ph-ERK1/2 protein ↓MKP-1 activity ↑Elk-1 transcriptional activation potential |
[64] |
|
HEK293 cells |
25 μM; 24–48 h |
↓OTA-induced
↑OGG1 expression ↑GSH levels |
[66] |
|
HEK293 cells |
2.5, 5, and 10 μM; 12–48 h |
↓High glucose-induced Aging β-galactosidase mRNA TXNIP mRNA ↑SIRT1 mRNA ↑Trx mRNA |
[67] |
Egr-1: early growth response 1; MKP-1: MAP kinase phosphatase-1; Elk-1: ETS transcription factor; OTA: ochratoxin A; OGG1: OTA-induced 8-oxoguanine glycosylase; GSH: glutathione; Trx: thioredoxin; TXNIP: Trx interacting protein.
2.5. In Vitro Studies: Effects of Resveratrol on Kidney Fibroblasts
Kidney fibroblasts are found in the interstitium, are involved in the production of ECM components, such as fibronectin and collagen, and act to maintain ECM homeostasis by producing ECM-degrading proteases. With dysfunction, fibroblasts continue to produce ECM components resulting in tubulointerstitial fibrosis and renal failure[68]. Only one study exists (by He et al. (2016)) on the effects of RSV treatment on kidney fibroblast cells[69]. Treatment of NRF-49F fibroblasts with RSV resulted in the attenuation of the high glucose-induced cell proliferation and dose-dependently reduced ROS production. Additionally, RSV treatment increased phosphorylated AMPK and acetyl-CoA carboxylase (ACC) protein levels, while NOX4, α-SMA, and fibronectin protein levels were decreased back to levels similar to control cells (Table 5)[69]. These data suggest that RSV treatment increased phosphorylated AMPK and ACC reduces oxidative stress marker NOX4 activity and results in the reduction of ROS production.
|
Cell |
Resveratrol Concentration/Duration |
Effect |
Reference |
|---|---|---|---|
|
NRF-49F cells |
5, 10, and 20 µM; 1 h |
↓High glucose-induced
↑High glucose-induced
|
[69] |
ACC: acetyl-CoA carboxylase.
2.6. In Vitro Studies: Effects of Resveratrol on Renal Cancer Cells
Renal cancer accounts for more than 140,000 deaths/year, ranking as the 13th most common cause of cancer death worldwide[14][15]. Renal cancer is characterized by decreased kidney filtration, anemia, and increased blood pressure, resulting in impaired functioning and complete kidney failure[17]. Increased expression of vascular endothelial growth factor (VEGF) is associated with poor prognoses and increased metastasis[70]. Treatment of human renal cancer cells (786-0) with RSV resulted in reduced cell growth that was associated with reduced VEGF mRNA and protein levels[70]. Signal transducers and activators of transcription (STAT) proteins are upregulated in various malignancies, including renal cancer. Treatment of Caki-1 and 786-0 renal cancer cells with RSV promoted cell apoptosis and reduced cell survival as seen by the reduced colony formation[71]. RSV inhibited phospho-STAT3 (tyrosine 705 and serine 727), phospho-STAT5 (tyrosine 684 and tyrosine 699), and nuclear STAT3 and STAT5 protein levels, while protein tyrosine phosphatase (protein tyrosine phosphatase (PTP)ε and Src homology- 2 domain containing phosphatase (SHP-2)) mRNA and protein levels were increased[71]. Additionally, the protein levels of phosphorylated upstream kinases (Janus kinase (JAK)1, JAK2, and Src) were significantly inhibited by RSV. Bcl-2, bcl-xL, survivin, inhibitor of apoptosis (IAP)-1, and IAP-2 protein levels were reduced, while caspase-3 protein level and poly (ADP-ribose) polymerase (PARP) cleavage were increased by RSV treatment in both renal cancer cell lines[71].
Treatment of ACHN and A498 renal carcinoma cells with RSV resulted in significantly impaired cell growth, cell-to-cell contact, and migration[72]. RSV treatment inhibited the formation of filopodia, which are actin-rich microspikes that project out of the cell cytoplasm and are involved in migration. Additionally, RSV treatment reduced EMT markers (N-cadherin and vimentin), transcriptional repressor (Snail), tumor metastasis markers (MMP-2 and MMP-9), phosphorylated Akt, and ERK1/2 protein levels, while cell invasion suppressor marker (E-cadherin and tissue inhibitors of metalloproteinase 1 (TIMP-1)) protein levels were increased[72].
Overall, these studies suggest that treatment of renal carcinoma cells with RSV resulted in reduced cell proliferation, survival, and migration. RSV treatment promoted cell apoptosis and pro-apoptotic protein expression. These limited studies indicate protective effects of RSV against renal cancer (Table 6).|
Cell |
Resveratrol Concentration/Duration |
Effect |
Reference |
|---|---|---|---|
|
786-0 cells |
0, 10, 20 and 40 µM; 24, 48 and 72 h |
↓Cell growth ↓VEGF mRNA and protein |
[70] |
|
Caki-1 and 786-0 cells |
0, 10, 30 and 50 µM; 6 h |
↑Apoptosis ↓Survival ↓Migration ↓STAT3 and STAT5 activation ↑PTPε and SHP-2 protein ↓JAK1, JAK2, and c-Src protein ↓Bcl-2, bcl-xL, survivin, IAP-1, and IAP-2 protein ↑Caspase-3 protein |
[71] |
|
ACHN and A498 cells |
50 μM; 12 h |
↓Cell growth ↓Cell-to-cell contact ↓Migration ↓Filopodia formation ↓N-cadherin, vimentin, snail, MMP-2, MMP-9, ph-Akt and ph-ERK1/2 protein ↑E-cadherin and TIMP-1 protein |
[72] |
VEGF: vascular endothelial growth factor; STAT: Signal transducers and activators of transcription; PTP: protein tyrosine phosphatase; SHP-2: Src homology- 2 domain containing phosphatase; JAK: Janus kinase; IAP: inhibitor of apoptosis; TIMP: tissue inhibitors of metalloproteinase 1.
2.7. In Vivo Animal Studies: Effects of Resveratrol on Diabetic Nephropathy
Diabetic nephropathy is a major complication of T2DM, that results in glomeruli damage and an inability to correctly filter the blood[13]. Multiple models of diabetic nephropathy including genetic models db/db and C57BL/KsJ db/+ mice and chemical-induced streptozotocin (STZ) administered rats and mice were utilized to determine the effects of RSV treatment.
Overall, the studies suggest that treatment of animals suffering from diabetic nephropathy with RSV attenuates hyperglycemia, hyperlipidemia and improves kidney structural integrity and kidney function. RSV administration decreased urinary albumin and serum creatinine levels, indicating improved kidney functioning. In addition, renal oxidative stress, inflammatory cell infiltration, cytokine production, and MDA content were reduced with RSV administration, while antioxidant enzyme activity and SIRT1 expression were increased. These data show that RSV treatment has protective effects against diabetic nephropathy (Table 7).|
Animal |
Resveratrol Concentration/Duration |
Serum Effects |
Other Effects |
Reference |
|---|---|---|---|---|
|
db/db mice |
0.3% diet; 8 weeks |
↓Glucose levels ↓Insulin levels ↓Triglyceride levels ↓FFA levels |
↓Albuminuria ↓Mesangial expansion ↓Fibronectin accumulation ↓Macrophage infiltration ↑O2− scavenging ↑MnSOD activity ↓Mitochondrial biogenesis mRNA |
[73] |
|
Male Wistar rats |
5 mg/kg/day; 16 weeks |
↓Glucose levels ↓SOD activity ↓TBARS levels ↓TNF-α ↓IL-6 |
↓Apoptosis rate of kidney cells ↓NF-κB activity |
[74] |
|
Male Wistar rats |
20 mg/kg/day; 8 weeks |
↓Glucose levels ↓Creatinine levels |
↓Urinary protein excretion ↓Renal hypertrophy ↓Mesangial matrix expansion ↓Mesangial cell hyperplasia ↓GSTM expression |
[75] |
|
db/db mice |
20 mg/kg/day; 12 weeks |
No measured effects |
↓Kidney albuminuria ↓Kidney NEFA and triacylglycerol ↓Mesangial area ↓Oxidative stress ↓Type IV collagen ↓TGF-β1 ↓F4/80 positive cells ↑Ph-AMPK ↑SIRT1 protein ↓PI3K-Akt protein and activity ↓Ph-FOXO3a ↓BAX protein ↑BCL-2 production ↓Renal and Urinary 8-OHdG |
[76] |
|
FVB mice |
10 mg/kg/day; 12 weeks |
No measured effects |
↓Glomerular area ↓Extracellular matrix ↓Albumin levels ↓Ph-Akt protein ↓PAI-1 protein ↓ICAM-1 protein ↓PCNA mRNA |
[42] |
|
Sprague–Dawley rats |
200 mg/kg/day; 12 weeks |
No measured effects |
↓Glomerular area ↓Mesangial cell expansion ↓Glomerular basement membrane thickness ↓Collagen IV ↓Fibronectin ↑AdipoR1 expression ↓MDA production |
[41] |
|
Male Wistar rats |
10 mg/kg/day; 30 days |
↓Glucose levels ↓Urea nitrogen levels |
↓Glomeruli sclerotic changes ↓Epithelial desquamation ↓Tissue swelling ↓Intracytoplasmic vacuolization ↓Brush border loss ↓Kidney TGF-β1 ↑SOD and CAT activities ↓MDA levels |
[77] |
|
db/db mice |
40 mg/kg/day; 12 weeks |
↓BUN levels ↓Creatinine levels |
↓Glomerulosclerosis ↓Tubulointerstitial fibrosis ↓Albuminuria ↑Kidney SOD, Mn-SOD, Catalase protein ↓Renal MDA ↓α-SMA protein ↓E-cadherin protein ↓TGF-β, pSmad3, ph-Akt, ph-ERK ↓IGF-1R expression ↑HRD1 expression |
[78] |
|
db/db mice |
20 mg/kg/day; 12 weeks |
↓Triacylglycerol levels ↓NEFA levels ↑Adiponectin levels |
↓Glomerular matrix expansion ↓Albuminuria ↑AdipoR1 and AdipoR2 ↑Ph-AMPK, SIRT1, total FoxO1, total FoxO3a ↑PGC-1α, ERR-1α, ph-ACC ↓SREBP-1c ↓Bax ↑Bcl-2 ↓8-OHdG levels ↓8-isoprostane levels |
[79] |
|
db/db mice |
40 mg/kg/day; 12 weeks |
No measured effects |
↓Mesangial area ↓Albuminuria ↓Collagen deposition ↓FSP-1, α-SMA, and fibronectin protein ↓NOX4 protein ↑Ph-AMPK, ph-ACC |
[69] |
|
Sprague-Dawley rats |
5 mg/kg/day; 4 months |
↓Glucose levels ↓Cholesterol levels ↓Triglyceride levels ↓HbA1c levels ↓Creatinine levels ↓Urea nitrogen levels ↓Cycstatin C levels ↓TNF-a, IL-6, IL-1B, and IL-10 levels |
↓Albuminuria ↓Renal 8-OHdG ↑SIRT1 mRNA and protein ↑Atg5 and Atg7 mRNA |
[80] |
|
Male Wistar rats |
30 mg/kg/day; 16 weeks |
↓Creatinine levels |
↑Renal function ↓Kidney weight ↑Kidney SOD activity ↓Kidney MDA content ↑CAT protein ↓SIRT1 protein ↑SIRT1 activity ↓Acetylated-FOXO3a |
[56] |
|
Sprague-Dawley rats |
20 mg/kg/day; 4 weeks |
↓Glucose levels ↓Creatinine levels |
↓Kidney weight ↓Glomerular thickening ↓Interstitial fibrosis ↓Epithelial cellular vacuolar degeneration ↓Hyaline casts ↓Arteriolopathy ↓Ph-p38 and p38 protein ↓TGF-β1 protein ↓Fibronectin protein ↓Urinary albumin |
[43] |
|
Male Wistar rats |
5 mg/kg/day; 45 days |
No measured effects |
↓Renal hypertrophy ↓Mesangial expansion ↓Fibrosis ↓Oxidative damage ↓Kidney AGE accumulation ↓DNA damage ↓4-HNE protein ↓Caspase-3 protein ↓Cleaved caspase-3 protein |
[81] |
|
C57BL/KsJ db/+ mice |
10 mg/kg/day; 8 weeks |
↓Glucose levels ↓Insulin levels ↓IL-1β, IL-17, IL-10 and TNF-α levels ↑IL-6 and VEGF levels |
↓Renal cell apoptosis ↓Apaf-1, caspase-3, caspase-8 and caspase-9 mRNA ↓Ph-AMPK ↓Total thiol level ↑GSH level |
[82] |
|
CD-1 mice |
30 mg/kg/day; 12 weeks |
↓Glucose levels ↓Cholesterol levels ↓Urea nitrogen levels |
↓Glomerular thickening ↓Mesangial area ↑Podocyte mitochondria ↓Renal cell apoptosis ↑Nephrin, SIRT1, PGC-1α, NRF1, TFAM protein ↓Kidney MDA content ↓Kidney Mn-SOD activity |
[61] |
FFA: free-fatty acid; TBARS: thiobarbituric acid reactive substances; GSTM: glutathione S-transferase Mu; NEFA: non-esterified fatty acid; 8-OHdG: 8-hydroxydeoxyguanosine; PAI: plasminogen activator inhibitor; ICAM: intercellular adhesion molecule; PCNA: proliferating cell nuclear antigen; SOD: superoxide dismutase; Mn-SOD: manganese superoxide dismutase; BUN; blood urea nitrogen; IGF-1R: insulin-like growth factor 1 receptor; HRD1: 3-hydroxy-3-methylglutaryl reductase degradation; ERR: estrogen-related receptor; SREBP: sterol regulatory element-binding protein; FSP: fibroblast-specific protein; HbA1c: hemoglobin A1c; Atg: autophagy related; AGE: advanced glycation end production; 4-HNE: 4-Hydroxynonenal; Apaf: Apoptotic protease activating factor.
2.8. In Vivo Animal Studies: Effects of Resveratrol on Renal Fibrosis
Renal fibrosis is often characterized by glomerulosclerosis and tubulointerstitium damage and is the final symptom manifestation of CKDs. Additionally, renal fibrosis can be pathologically described with inflammatory infiltration, loss of renal parenchyma due to tubular atrophy, capillary loss, and podocyte depletion[83].
Overall, the studies suggest that administration of RSV to animal models of renal fibrosis reduced extracellular matrix protein deposition, reduced tubulointerstitium damage, and mesangial cell proliferation. RSV reduced serum creatinine levels and kidney oxidative stress, while kidney antioxidant enzymes (SOD, CAT, GPx, and GSH) were increased. In addition, RSV treatment improved mitochondrial biogenesis, mitochondrial complex I and III activities, and electron transport protein expression, while mPTP opening and fission protein expression were reduced. RSV treatment also exerted anti-inflammatory effects, by reducing mRNA and protein expression of pro-inflammatory signaling molecules and cytokines. These data demonstrate that RSV treatment exerts protective effects against renal fibrosis (Table 8).
|
Animal |
Resveratrol Concentration/Duration |
Serum Effects |
Other Effects |
Reference |
|---|---|---|---|---|
|
Sprague–Dawley rats |
10 mg/kg/day; 21 days |
↓MDA levels |
↓Urine calcium oxalate crystals ↓Hyaluronan protein ↓Osteopontin protein ↑GPx protein ↑CAT protein ↑SOD protein |
[48] |
|
Male Wistar rats |
8 mg/kg/alternating days; 8 days |
↓Creatinine levels ↓Urea nitrogen levels |
↓Oxidative stress ↓Renal tubular epithelial cell necrosis ↓MDA, BUN, CRE, and ROS levels ↑SOD and GPx levels ↑Selenium content |
[84] |
|
C57BL/6J mice |
20 mg/kg/day; 14 days |
No measured effects |
↓Extracellular matrix deposition ↓Tubulointerstitium damage ↓Oxidative stress ↓ICAM-1 mRNA ↓TNF-α mRNA ↓TGF-β mRNA ↓Acetyl-Smad3 ↓Fibronectin |
[85] |
|
UUO-Sprague-Dawley rats |
20 mg/kg/day; 7–14 days |
↓Creatinine levels |
↓Renal interstitial damage ↓Tubular dilation and atrophy ↓Collagen deposition ↓Inflammation cell infiltration ↓α-SMA and type III collagen mRNA and protein ↑E-cadherin protein and mRNA ↓TGF-β1 expression |
[50] |
|
I/R and UUO C57BL/6 mice |
20 mg/kg/day; 6 weeks |
↓Creatinine levels ↓BUN levels |
↑α-SMA protein ↑COL1A1 protein |
[52] |
|
Sprague–Dawley rats |
50 mg/kg; 8 h |
↓Creatinine levels ↓Urea nitrogen levels |
↓Apoptosis ↑SIRT1 activity and protein ↑SIRT3 activity and protein ↑SOD2 protein ↓Acetyl-SOD2 ↑GSH and ATP content ↑GSH/GSSG ratio ↑CAT activity ↓mPTP opening |
[86] |
|
Male cystic (Cy/+) rats |
200 mg/kg/day; 5 weeks |
↓BUN levels ↓Creatinine levels |
↓Cyst density ↓Macrophage infiltration ↓MCP-1 ↓TNF-α ↓CFB ↓Ph-p65, ph-S6K and p50 |
[54] |
|
Sprague–Dawley rats |
3 and 10 mg/kg/injection; 70 h |
↓BUN levels ↓Creatinine levels ↓Nitrogen levels |
↑Survival ↓Cystatin C ↓KIM-1 ↓TNF-α ↓IL-1B ↓IL-6 ↓Renal injury index |
[87] |
|
Kunming mice |
10 mg/kg/day; 1 week |
↓BUN levels ↓Creatinine levels |
↓Apoptosis ↓Caspase-3 activity ↓Bax protein ↓ERK1/2 protein |
[57] |
|
Male AKI rats |
30 mg/kg; 12 h |
↓Creatinine levels ↓Urea nitrogen levels ↓TNF-α, IL-1β, IL-6 levels |
↑Renal function ↓Tubular epithelial cell injury ↑Survival ↓p-65 positive cells ↓Renal TNF-α, IL-1β, IL-6 mRNA ↓IRE1 protein |
[55] |
|
5/6 Nephrectomized Sprague–Dawley rats |
20 mg/kg/day; 4 weeks |
No measured effects |
↓Mesangial cell proliferation ↓Glomeruli matrix expansion ↓TGF-β ↑ATP production ↓ROS production ↑Activities of complex I and III ↑ATP synthase B ↑COX I, Opa1, Mfn2 ↓Drp1 |
[44] |
|
C57BL/6 mice |
25 and 100 mg/kg/day; 2 weeks |
↓Creatinine levels |
25 mg/kg RSV: ↓Renal fibrosis ↓Tubular lesion score ↓Interstitial collagen deposition ↓α-SMA protein ↓Snail protein ↓Fibronectin protein ↑SIRT1 ↓Phospho-Smad3 100 mg/kg RSV: ↑Renal fibrosis ↑α-SMA and TFAM |
[58] |
CRE: creatinine; GPx: glutathione peroxidase; mPTP: mitochondrial permeability transition pore; KIM-1: kidney injury molecule 1; IRE-1: Inositol-requiring enzyme 1; Opa1: optic atrophy 1; Mfn2: mitofusin 2; Drp1: dynamin related protein 1.
3. Effects of Resveratrol on Human Kidneys
Only two clinical studies exist measuring the effects of RSV in humans with kidney disease. In a randomized, double-blinded pilot study by Saldanha et al. (2016), administration of RSV (500 mg/day) for 4 weeks to non-dialyzed chronic kidney disease (CKD) patients (GFR between 15 and 60 mL/min/m2) resulted in no significant effects. Antioxidant and anti-inflammatory marker levels were the same in RSV and placebo supplemented participants[88]. It should be emphasized that administration of RSV (500 mg/day) for 4 weeks had low toxicity.
In another randomized, double-blinded study by Lin et al., low-dose (150 mg/day) or high-dose (450 mg/day) of RSV intake for 12 weeks by peritoneal dialysis (PD) patients resulted in significant improvements in mean net ultrafiltration (UF) volume and rate[89]. In addition, angiogenesis markers, VEGF, fetal liver kinase-1 (Flk-1), and angiopoietin (Ang)-2 levels in peritoneal dialysate effluent (PDE) were significantly reduced in the high-dose RSV group. The levels of angiopoietin receptor (Tie-2) and thrombospondin-1 (Tsp-1) in the PDE were increased with RSV treatment[89]. These data suggest that RSV treatment has angiogenesis-ameliorating effects in PD patients and improves ultrafiltration kidney function. It should be mentioned that in the study by Saldanha et al.[88] administration of 500 mg RSV/day for 4 weeks resulted in no significant effects, while in the study by Lin et al.[89] administration of 450 mg RSV/day for 12 weeks resulted in significant improvements and health benefits, suggesting that longer duration of administration of a specific dose of RSV (450 or 500 mg/day) may be required to see/elicit beneficial effects.
Other clinical studies exist showing beneficial effects of RSV administration in cardiovascular disease, diabetes mellitus and cancer, however, the effect of RSV supplementation in kidney disease patients has not been extensively studied[90][91]. In a randomized, double-blinded study by Brasnyo et al. (2011), oral administration of RSV (10 mg/day) in type 2 diabetic (T2DM) individuals (following the WHO diagnostic guidelines), significantly increased insulin sensitivity and reduced serum glucose and cholesterol levels[92]. In addition, RSV treatment significantly reduced serum creatinine levels and maintained GFR, suggesting improved kidney function[92]. In a similar randomized, open-label, controlled study, administration of RSV (250 mg/day) for 4 months in T2DM patients (3 year duration of T2DM and minimum 6 months oral hypoglycemic treatment) resulted in significantly improved lipid profile, with reduced total cholesterol and triglyceride levels[93] [117]. Serum creatinine, urea nitrogen levels, and total protein excretion were reduced with RSV treatment, suggesting improved kidney function[93]. These studies[92][93] show that treatment of individuals with T2DM and impaired kidney function with RSV resulted in improved glucose, insulin, and lipid homeostasis and better kidney function.
Although there are numerous studies measuring the effects of RSV in diabetes, the studies mentioned above were performed in individuals with established CKD and diabetic nephropathy and show a kidney-protective effect of RSV administration. These data highlight the importance of future clinical trials required to investigate the exact effects of RSV in individuals with kidney disease (Table 9).|
Patients |
Resveratrol Concentration/Duration |
Effect |
Reference |
|---|---|---|---|
|
Nondialyzed CKD patients |
500 mg/day; 4 weeks |
No significant effects |
[88] |
|
PD patients |
150 and 450 mg/day; 12 weeks |
↓UF volume and rate ↓PDE VEGF, Flk-1 and Ang-2 ↑PDE Tie-2 and Tsp-1 |
[89] |
|
T2DM patients |
10 mg/day; 4 weeks |
↑Kidney filtration ↑Insulin sensitivity ↓Glucose levels ↓Lipid levels ↓Serum creatinine |
[92] |
|
T2DM patients |
250 mg/day; 4 months |
↑Kidney function ↓Cholesterol levels ↓Triglyceride levels ↓Serum creatinine ↓Total protein excretion ↓Urea nitrogen levels |
[93] |
CKD: chronic kidney disease; PD: peritoneal dialysis; UF: ultrafiltration; PDE: peritoneal dialysate effluent; Flk-1: fetal liver kinase 1; Ang: angiopoietin; Tie-2: angiopoietin receptor; Tsp-1: thrombospondin-1; T2DM: type 2 diabetes mellitus.
4. Effects of RSV at the Cellular/Molecular Level
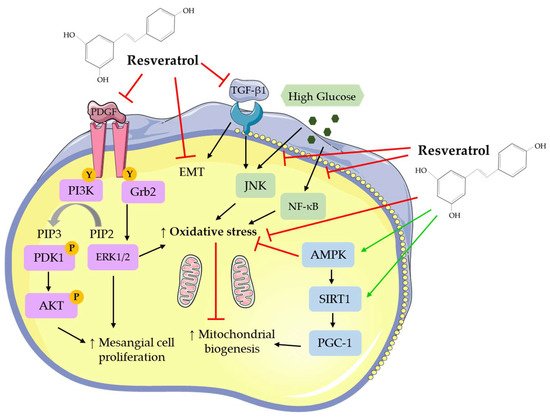
5. Conclusions and Future Directions
Overall, all available in vitro and in vivo animal and human studies examining the effects of RSV in kidney disease indicate that it can reduce fibrosis, mesangial expansion, oxidative stress, and inflammatory cytokine levels, while improving kidney structure and function. Treatment of renal mesangial, epithelial, and corpuscle cells with RSV resulted in reduced structural changes and ROS production, while antioxidant and mitochondrial activities were improved. In addition, RSV treatment reduced fibroblast proliferation and activation to improve kidney structural maintenance. Renal cancer cells treated with RSV had reduced cell growth, cell-to-cell contact, and migration, and increased apoptosis.
In in vivo animal models of diabetic nephropathy treatment with RSV showed improved glucose homeostasis, reduced inflammation and increased antioxidant activity and kidney function. Animals with renal fibrosis administered RSV had reduced structural changes and inflammatory cell infiltration, cytokine expression, and decreased tubulointerstitium damage and oxidative stress.
The limited human studies indicate a protective effect of RSV administration on chronic kidney disease with increased kidney filtration rates and volume. The health benefits of RSV are widespread, and the low toxicity of the molecule makes it a prime candidate for medicinal use against kidney disease. However, more research and clinical studies are required to fully understand the effects of RSV on kidney disease.
Further investigation and clarification are required in the following areas: (1) dosage and bioavailability, (2) metabolism, tissue distribution, and biological effects of RSV analogs and metabolites, and (3) signaling mechanisms involved.
Only limited number of studies exist examining RSV administration in humans. More studies should be performed to determine the optimal dosage and route of administration of RSV and analogs with higher biological activity. RSV analogs (methylated and with other novel derivatives) may have great biological activity [54]. Most in vitro studies and evidence have used RSV and not its metabolites. The potential biological activity of RSV metabolites should be considered in future investigations.
Furthermore, future research should be conducted examining the exact signaling/cellular mechanisms affected by RSV and contributing to the attenuation of kidney disease.References
- Priya Vart; Morgan E. Grams; Measuring and Assessing Kidney Function. Seminars in Nephrology 2016, 36, 262-272, 10.1016/j.semnephrol.2016.05.003.
- Martin R. Pollak; Susan E. Quaggin; Melanie P. Hoenig; Lance D. Dworkin; The Glomerulus: The Sphere of Influence. Clinical Journal of the American Society of Nephrology 2014, 9, 1461-1469, 10.2215/cjn.09400913.
- Asimina Mitrakou; Kidney: Its impact on glucose homeostasis and hormonal regulation. Diabetes Research and Clinical Practice 2011, 93, S66-S72, 10.1016/s0168-8227(11)70016-x.
- Dalal, R; Sehdev, J.S.. Physiology, renal, blow flow and filtration; StatPearls Publishing: Treasure Island, FL, USA, 2018; pp. 1.
- Adeera Levin; Brenda Hemmelgarn; Bruce Culleton; Sheldon Tobe; Philip McFarlane; Marcel Ruzicka; Kevin Burns; Braden Manns; Colin White; Francoise Madore; et al.Louise MoistScott KlarenbachBrendan BarrettRobert FoleyKailash JindalPeter SeniorNeesh PannuSabin ShurrawAyub AkbariAdam CohnMartina ReslerovaVinay DevedDavid MendelssohnGihad NesrallahJoanne KappelMarcello TonelliFor The Canadian Society Of Nephrology Guidelines for the management of chronic kidney disease. Canadian Medical Association Journal 2008, 179, 1154-1162, 10.1503/cmaj.080351.
- Prathibha Reddy Gajjala; Maryam Sanati; Joachim Jankowski; Cellular and Molecular Mechanisms of Chronic Kidney Disease with Diabetes Mellitus and Cardiovascular Diseases as Its Comorbidities. Frontiers in Immunology 2015, 6, 340, 10.3389/fimmu.2015.00340.
- Leslie Gewin; Roy Zent; Ambra Pozzi; Progression of chronic kidney disease: too much cellular talk causes damage. Kidney International 2016, 91, 552-560, 10.1016/j.kint.2016.08.025.
- Carlamaria Zoja; Mauro Abbate; Giuseppe Remuzzi; Progression of renal injury toward interstitial inflammation and glomerular sclerosis is dependent on abnormal protein filtration. Nephrology Dialysis Transplantation 2014, 30, 706-712, 10.1093/ndt/gfu261.
- Jeffrey M. Turner; Carolyn Bauer; Matthew K. Abramowitz; Michal L. Melamed; Thomas H. Hostetter; Treatment of chronic kidney disease. Kidney International 2012, 81, 351-362, 10.1038/ki.2011.380.
- Simon Ds Fraser; Thomas Blakeman; Chronic kidney disease: identification and management in primary care. Pragmatic and Observational Research 2016, ume 7, 21-32, 10.2147/por.s97310.
- Pui Yuen Lee; Liyanne F.M. Van De Laarschot; Jesus Banales; Joost P.H. Drenth; Genetics of polycystic liver diseases. Current Opinion in Gastroenterology 2019, 35, 65-72, 10.1097/mog.0000000000000514.
- Sj Chadban; Rc Atkins; Glomerulonephritis. The Lancet 2005, 365, 1797-1806, 10.1016/s0140-6736(05)66583-x.
- Heshmatollah Shahbazian; Diabetic kidney disease; review of the current knowledge. Journal of Renal Injury Prevention 2013, 2, 73-80, 10.12861/JRIP.2013.24.
- Jacques Ferlay; Isabelle Soerjomataram; Rajesh Dikshit; Sultan Eser; Colin Mathers; Marise Rebelo; Donald Maxwell Parkin; David Forman; Freddie Bray; Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2014, 136, E359-E386, 10.1002/ijc.29210.
- Rebecca L. Siegel; Kimberly D. Miller Mph; Ahmedin Jemal; Cancer statistics, 2018. CA: A Cancer Journal for Clinicians 2018, 68, 7-30, 10.3322/caac.21442.
- Shahzaib Nabi; Elizabeth R. Kessler; Brandon Bernard; Thomas W. Flaig; Elaine T. Lam; Renal cell carcinoma: a review of biology and pathophysiology. F1000Research 2018, 7, 307, 10.12688/f1000research.13179.1.
- Umberto Capitanio; Karim Bensalah; Axel Bex; Stephen A. Boorjian; Freddie Bray; Jonathan Coleman; John L. Gore; Maxine Sun; Christopher Wood; Paul Russo; et al. Epidemiology of Renal Cell Carcinoma. European Urology 2018, 75, 74-84, 10.1016/j.eururo.2018.08.036.
- Pedro C. Barata; Brian I. Rini; Treatment of renal cell carcinoma: Current status and future directions. CA: A Cancer Journal for Clinicians 2017, 67, 507-524, 10.3322/caac.21411.
- Kanti Bhooshan Pandey; Syed Ibrahim Rizvi; Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Medicine and Cellular Longevity 2008, 2, 270-278, 10.4161/oxim.2.5.9498.
- Jennifer Burns; Takao Yokota; Hiroshi Ashihara; Michael E. J. Lean; Alan Crozier; Plant Foods and Herbal Sources of Resveratrol. Journal of Agricultural and Food Chemistry 2002, 50, 3337-3340, 10.1021/jf0112973.
- Cláudia K. Sautter; Sandra Denardin; Audrei O. Alves; Carlos A. Mallmann; Neidi G. Penna; Luisa H. Hecktheuer; Determinação de resveratrol em sucos de uva no Brasil. Food Science and Technology 2005, 25, 437-442, 10.1590/s0101-20612005000300008.
- Ulrik Stervbo; Ole Vang; Christine Bonnesen; A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine. Food Chemistry 2007, 101, 449-457, 10.1016/j.foodchem.2006.01.047.
- Kailash Prasad; Resveratrol, Wine, and Atherosclerosis. International Journal of Angiology 2012, 21, 007-018, 10.1055/s-0032-1306417.
- S. Bo; Giovannino Ciccone; A. Castiglione; R. Gambino; F. De Michieli; P. Villois; M. Durazzo; P. Cavallo-Perin; M. Cassader; Anti-Inflammatory and Antioxidant Effects of Resveratrol in Healthy Smokers A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Trial. Current Medicinal Chemistry 2013, 20, 1323-1331, 10.2174/0929867311320100009.
- Albino Carrizzo; Maurizio Forte; Antonio Damato; Valentina Trimarco; Francesco Salzano; Michelangelo Bartolo; Anna Maciag; Annibale A. Puca; Carmine Vecchione; Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food and Chemical Toxicology 2013, 61, 215-226, 10.1016/j.fct.2013.07.021.
- Lindsay G Carter; John A D'orazio; Kevin J Pearson; Resveratrol and cancer: focus on in vivo evidence. Endocrine-Related Cancer 2014, 21, R209-R225, 10.1530/erc-13-0171.
- Ying Wang; Yiming Jiang; Xiaomei Fan; Huasen Tan; Hang Zeng; Yongtao Wang; Pan Chen; Min Huang; Huichang Bi; Hepato-protective effect of resveratrol against acetaminophen-induced liver injury is associated with inhibition of CYP-mediated bioactivation and regulation of SIRT1–p53 signaling pathways. Toxicology Letters 2015, 236, 82-89, 10.1016/j.toxlet.2015.05.001.
- He Peiyuan; Hou Zhiping; Song Chengjun; Wang Chunqing; Li Bingqing; Mustapha Umar Imam; Resveratrol Ameliorates Experimental Alcoholic Liver Disease by Modulating Oxidative Stress. Evidence-Based Complementary and Alternative Medicine 2017, 2017, 1-10, 10.1155/2017/4287890.
- Elisabeth Wenzel; Veronika Somoza; Metabolism and bioavailability oftrans-resveratrol. Molecular Nutrition & Food Research 2005, 49, 472-481, 10.1002/mnfr.200500010.
- Charles-Henry Cottart; Valérie Nivet-Antoine; Christelle Laguillier-Morizot; Jean-Louis Beaudeux; Resveratrol bioavailability and toxicity in humans. Molecular Nutrition & Food Research 2009, 54, 7-16, 10.1002/mnfr.200900437.
- Thomas Walle; Bioavailability of resveratrol. Annals of the New York Academy of Sciences 2011, 1215, 9-15, 10.1111/j.1749-6632.2010.05842.x.
- David Boocock; Guy E.S. Faust; Ketan R. Patel; Anna M. Schinas; Victoria A. Brown; Murray P. Ducharme; Tristan D. Booth; James A. Crowell; Marjorie Perloff; Andreas J. Gescher; et al.William P. StewardDean E. Brenner Phase I Dose Escalation Pharmacokinetic Study in Healthy Volunteers of Resveratrol, a Potential Cancer Chemopreventive Agent. Cancer Epidemiology Biomarkers & Prevention 2007, 16, 1246-1252, 10.1158/1055-9965.epi-07-0022.
- Luis Almeida; Manuel Vaz Silva; Amílcar Falcão; Eva Soares; Raquel Costa; Ana I. Loureiro; Carlos Fernandes-Lopes; José-Francisco Rocha; Teresa Nunes; Lyndon Wright; et al.Patricio Soares-Da-Silva Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Molecular Nutrition & Food Research 2009, 53, S7-S15, 10.1002/mnfr.200800177.
- Detlef Schlondorff; The glomerular mesangial cell: an expanding role for a specialized pericyte. The FASEB Journal 1987, 1, 272-281, 10.1096/fasebj.1.4.3308611.
- A. Richard Kitching; Holly L. Hutton; The Players: Cells Involved in Glomerular Disease. Clinical Journal of the American Society of Nephrology 2016, 11, 1664-1674, 10.2215/CJN.13791215.
- Y. Uchida; H. Yamazaki; S. Watanabe; K. Hayakawa; Y. Meng; N. Hiramatsu; A. Kasai; K. Yamauchi; Jian Yao; M. Kitamura; et al. Enhancement of NF-kappaB activity by resveratrol in cytokine-exposed mesangial cells. Clinical & Experimental Immunology 2005, 142, 76-83, 10.1111/j.1365-2249.2005.02895.x.
- Ana I. Morales; Alicia Rodríguez-Barbero; Cesáreo Vicente-Sánchez; Paula Mayoral; José M. Lopez-Novoa; Fernando Pérez-Barriocanal; Resveratrol inhibits gentamicin-induced mesangial cell contraction. Life Sciences 2006, 78, 2373-2377, 10.1016/j.lfs.2005.09.045.
- Balachandar Venkatesan; Nandini Ghosh‐Choudhury; Falguni Das; Lenin Mahimainathan; Amrita Kamat; Balakuntalam S. Kasinath; Hanna E. Abboud; Goutam Ghosh Choudhury; Resveratrol inhibits PDGF receptor mitogenic signaling in mesangial cells: role of PTP1B. The FASEB Journal 2008, 22, 3469-3482, 10.1096/fj.08-109488.
- Ying Xu; Ling Nie; Yang-Guang Yin; Jian-Lin Tang; Ji-Yin Zhou; Dan-Dan Li; Shi-Wen Zhou; Resveratrol protects against hyperglycemia-induced oxidative damage to mitochondria by activating SIRT1 in rat mesangial cells. Toxicology and Applied Pharmacology 2012, 259, 395-401, 10.1016/j.taap.2011.09.028.
- Lanyu Zhang; Shanshan Pang; Bo Deng; Lihua Qian; Juan Chen; Junjie Zou; Jiaoyang Zheng; Linghui Yang; Chunyang Zhang; Xiangfang Chen; et al.Zhimin LiuYingying Le High glucose induces renal mesangial cell proliferation and fibronectin expression through JNK/NF-κB/NADPH oxidase/ROS pathway, which is inhibited by resveratrol. The International Journal of Biochemistry & Cell Biology 2012, 44, 629-638, 10.1016/j.biocel.2012.01.001.
- Hongfei Ji; Lina Wu; Xiaokun Ma; Guijun Qin; The effect of resveratrol on the expression of AdipoR1 in kidneys of diabetic nephropathy. Molecular Biology Reports 2014, 41, 2151-2159, 10.1007/s11033-014-3064-2.
- Feng Xu; Yuehui Wang; Wenpeng Cui; Hang Yuan; Jing Sun; Man Wu; Qiaoyan Guo; Lili Kong; Hao Wu; Lining Miao; et al. Resveratrol Prevention of Diabetic Nephropathy Is Associated with the Suppression of Renal Inflammation and Mesangial Cell Proliferation: Possible Roles of Akt/NF-κB Pathway. International Journal of Endocrinology 2014, 2014, 1-9, 10.1155/2014/289327.
- Yuan Qiao; Ke Gao; Yangwei Wang; Xueliang Wang; Bo Cui; Resveratrol ameliorates diabetic nephropathy in rats through negative regulation of the p38 MAPK/TGF-β1 pathway. Experimental and Therapeutic Medicine 2017, 13, 3223-3230, 10.3892/etm.2017.4420.
- Yan Hui; Miaomiao Lu; Yarong Han; Hongli Zhou; Wei Liu; Lijing Li; Ruixia Jin; Resveratrol improves mitochondrial function in the remnant kidney from 5/6 nephrectomized rats. Acta Histochemica 2017, 119, 392-399, 10.1016/j.acthis.2017.04.002.
- 12. Smith, P.L.; Buffington, D.A.; Humes, H.D.. Kidney epithelial cells; Klimanskaya, I., Lanza, R., Eds.; Academic Press: London, UK, 2006; pp. 194-207.
- Myung-Ja Lee; Denis Feliers; Kavithalakshmi Sataranatarajan; Meenalakshmi M. Mariappan; Manli Li; Jeffrey L. Barnes; Goutam Ghosh Choudhury; Balakuntalam S. Kasinath; Resveratrol ameliorates high glucose-induced protein synthesis in glomerular epithelial cells. Cellular Signalling 2010, 22, 65-70, 10.1016/j.cellsig.2009.09.011.
- Duk Hoon Kim; Yu Jin Jung; Jung Eun Lee; Ae Sin Lee; Kyung Pyo Kang; Sik Lee; Sung Kwang Park; Myung Kwan Han; Sang Yong Lee; Kunga Mohan Ramkumar; et al.Mi Jeong SungWon Kim SIRT1 activation by resveratrol ameliorates cisplatin-induced renal injury through deacetylation of p53. American Journal of Physiology-Renal Physiology 2011, 301, F427-F435, 10.1152/ajprenal.00258.2010.
- Sang Hyuk Hong; Hyo-Jung Lee; Eun Jung Sohn; Hyun-Suk Ko; Bum Sang Shim; Kyoo Seok Ahn; Sunghoon Kim; Anti-nephrolithic potential of resveratrol via inhibition of ROS, MCP-1, hyaluronan and osteopontin in vitro and in vivo. Pharmacological Reports 2013, 65, 970-979, 10.1016/s1734-1140(13)71078-8.
- Kelly M. Weixel; Allison Marciszyn; Rodrigo Alzamora; Hui Li; Oliver Fischer; Robert S. Edinger; Kenneth R. Hallows; John P. Johnson; Resveratrol Inhibits the Epithelial Sodium Channel via Phopshoinositides and AMP-Activated Protein Kinase in Kidney Collecting Duct Cells. PLOS ONE 2013, 8, e78019, 10.1371/journal.pone.0078019.
- Yongheng Bai; Hong Lu; Cunzao Wu; Yong Liang; Silu Wang; Chengcheng Lin; Bicheng Chen; Peng Xia; Resveratrol inhibits epithelial-mesenchymal transition and renal fibrosis by antagonizing the hedgehog signaling pathway. Biochemical Pharmacology 2014, 92, 484-493, 10.1016/j.bcp.2014.09.002.
- Ting He; Xu Guan; Song Wang; Tangli Xiao; Ke Yang; Xinli Xu; Junping Wang; Jinghong Zhao; Resveratrol prevents high glucose-induced epithelial–mesenchymal transition in renal tubular epithelial cells by inhibiting NADPH oxidase/ROS/ERK pathway. Molecular and Cellular Endocrinology 2014, 402, 13-20, 10.1016/j.mce.2014.12.010.
- Zhou Xiao; Chen Chen; Ting Meng; Wenzheng Zhang; Qiaoling Zhou; Resveratrol attenuates renal injury and fibrosis by inhibiting transforming growth factor-β pathway on matrix metalloproteinase 7. Experimental Biology and Medicine 2015, 241, 140-146, 10.1177/1535370215598401.
- Yen Ta Huang; Yi Ya Chen; Yu-Hsien Lai; Chuan Chu Cheng; Tzu Chun Lin; Ying Shih Su; Chin-Hung Liu; Pei Chun Lai; Resveratrol alleviates the cytotoxicity induced by the radiocontrast agent, ioxitalamate, by reducing the production of reactive oxygen species in HK-2 human renal proximal tubule epithelial cells in vitro. International Journal of Molecular Medicine 2015, 37, 83-91, 10.3892/ijmm.2015.2404.
- Ming Wu; Junhui Gu; Shuqin Mei; Dechao Xu; Ying Jing; Qing Yao; Meihan Chen; Ming Yang; Sixiu Chen; Bo Yang; et al.Na QiHuimin HuRudolf P. WüthrichChanglin Mei Resveratrol delays polycystic kidney disease progression through attenuation of nuclear factor κB-induced inflammation. Nephrology Dialysis Transplantation 2016, 31, 1826-1834, 10.1093/ndt/gfw058.
- Nian Wang; Li Mao; Liu Yang; Jiang Zou; Ke Liu; Meidong Liu; Huali Zhang; Xianzhong Xiao; Kangkai Wang; Resveratrol protects against early polymicrobial sepsis-induced acute kidney injury through inhibiting endoplasmic reticulum stress-activated NF-κB pathway. Oncotarget 2017, 8, 36449-36461, 10.18632/oncotarget.16860.
- Xueling Wang; Linghang Meng; Long Zhao; Zengfu Wang; Haiying Liu; Gang Liu; Guangju Guan; Resveratrol ameliorates hyperglycemia-induced renal tubular oxidative stress damage via modulating the SIRT1/FOXO3a pathway. Diabetes Research and Clinical Practice 2017, 126, 172-181, 10.1016/j.diabres.2016.12.005.
- Beibei Fu; Jiamin Zhao; Wei Peng; Haibo Wu; Yong Zhang; Resveratrol rescues cadmium-induced mitochondrial injury by enhancing transcriptional regulation of PGC-1α and SOD2 via the Sirt3/FoxO3a pathway in TCMK-1 cells. Biochemical and Biophysical Research Communications 2017, 486, 198-204, 10.1016/j.bbrc.2017.03.027.
- Shuyun Liu; Meng Zhao; Yijie Zhou; Chengshi Wang; Yujia Yuan; Lan Li; William Bresette; Younan Chen; Jingqiu Cheng; Yanrong Lu; et al.Jingping Liu Resveratrol exerts dose-dependent anti-fibrotic or pro-fibrotic effects in kidneys: A potential risk to individuals with impaired kidney function. Phytomedicine 2018, 57, 223-235, 10.1016/j.phymed.2018.12.024.
- Hermann Pavenstädt; Roles of the podocyte in glomerular function. American Journal of Physiology-Renal Physiology 2000, 278, F173-F179, 10.1152/ajprenal.2000.278.2.f173.
- Ru-Chun Yang; Xiao-Ling Zhu; Hua-Qin Zhang; Wei-Dong Li; [Study of resveratrol suppressing TGF-beta1 induced transdifferentiation of podocytes].. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi = Chinese journal of integrated traditional and Western medicine 2013, 33, 1677-1682.
- Tao Zhang; Yanqing Chi; Yingli Kang; Hua Lu; Honglin Niu; Wei Liu; Ying Li; Resveratrol ameliorates podocyte damage in diabetic mice via SIRT1/PGC‐1α mediated attenuation of mitochondrial oxidative stress. Journal of Cellular Physiology 2018, 234, 5033-5043, 10.1002/jcp.27306.
- Mark S Lechner; Gregory R Dressler; The molecular basis of embryonic kidney development. Mechanisms of Development 1997, 62, 105-120, 10.1016/s0925-4773(97)00667-9.
- Yao-Cheng Lin; Morgane Boone; Leander Meuris; Irma Lemmens; Nadine Van Roy; Arne Soete; Joke Reumers; Matthieu Moisse; Stephane Plaisance; Radoje T Drmanac; et al.Jason ChenFranki SpelemanDiether LambrechtsYves Van de PeerJan TavernierNico Callewaert Genome dynamics of the human embryonic kidney 293 lineage in response to cell biology manipulations. Nature Communications 2014, 5, 4767, 10.1038/ncomms5767.
- Oliver G. Rössler; Daniel Glatzel; Gerald Thiel; Resveratrol upregulates Egr-1 expression and activity involving extracellular signal-regulated protein kinase and ternary complex factors. Experimental Cell Research 2015, 332, 116-127, 10.1016/j.yexcr.2015.01.013.
- Travis R. Bui-Klimke; Felicia Wu; Ochratoxin A and Human Health Risk: A Review of the Evidence. Critical Reviews in Food Science and Nutrition 2013, 55, 1860-1869, 10.1080/10408398.2012.724480.
- Shanel Raghubeer; Savania Nagiah; Alisa Phulukdaree; Anil Chuturgoon; The Phytoalexin Resveratrol Ameliorates Ochratoxin A Toxicity in Human Embryonic Kidney (HEK293) Cells. Journal of Cellular Biochemistry 2015, 116, 2947-2955, 10.1002/jcb.25242.
- Mina Hemmati; Fatemeh Abharzanjani; Mohammad Afshar; Maryam Moossavi; Short-term high dose of quercetin and resveratrol alters aging markers in human kidney cells. International Journal of Preventive Medicine 2016, 8, 64, 10.4103/ijpvm.ijpvm_139_17.
- Frank Strutz; Michael Zeisberg; Renal Fibroblasts and Myofibroblasts in Chronic Kidney Disease. Journal of the American Society of Nephrology 2006, 17, 2992-2998, 10.1681/asn.2006050420.
- Ting He; Jiachuan Xiong; Ling Nie; Yanlin Yu; Xu Guan; Xinli Xu; Tangli Xiao; Ke Yang; Liang Liu; Daohai Zhang; et al.Yunjian HuangJingbo ZhangJunping WangKumar SharmaJinghong Zhao Resveratrol inhibits renal interstitial fibrosis in diabetic nephropathy by regulating AMPK/NOX4/ROS pathway. Journal of Molecular Medicine 2016, 94, 1359-1371, 10.1007/s00109-016-1451-y.
- Huizhen Zhang; Rui Yang; Lihong Zhu; Inhibitory effect of resveratrol on the expression of the VEGF gene and proliferation in renal cancer cells. Molecular Medicine Reports 2011, 4, 981-983, 10.3892/mmr.2011.511.
- Chulwon Kim; Sang Hyun Baek; Jae-Young Um; Bum Sang Shim; Kwang Seok Ahn; Resveratrol attenuates constitutive STAT3 and STAT5 activation through induction of PTPε and SHP-2 tyrosine phosphatases and potentiates sorafenib-induced apoptosis in renal cell carcinoma. BMC Nephrology 2016, 17, 1-13, 10.1186/s12882-016-0233-7.
- Yuwan Zhao; Huancheng Tang; Xin Zeng; Dongcai Ye; Jianjun Liu; Resveratrol inhibits proliferation, migration and invasion via Akt and ERK1/2 signaling pathways in renal cell carcinoma cells. Biomedicine & Pharmacotherapy 2018, 98, 36-44, 10.1016/j.biopha.2017.12.029.
- Munehiro Kitada; Shinji Kume; Noriko Imaizumi; Daisuke Koya; Resveratrol Improves Oxidative Stress and Protects Against Diabetic Nephropathy Through Normalization of Mn-SOD Dysfunction in AMPK/SIRT1-Independent Pathway. Diabetes 2011, 60, 634-643, 10.2337/db10-0386.
- Farhad Ghadiri Soufi; Mina Vardyani; Roghayeh Sheervalilou; Mustafa Mohammadi; Mohammad Hussein Somi; Long-term treatment with resveratrol attenuates oxidative stress pro-inflammatory mediators and apoptosis in streptozotocin-nicotinamide-induced diabetic rats. General physiology and biophysics 2011, 31, 431-438, 10.4149/gpb_2012_039.
- Bei Jiang; Ling Guo; Bao-Ying Li; Jun-Hui Zhen; Jian Song; Tao Peng; Xiang-Dong Yang; Zhao Hu; Hai-Qing Gao; Resveratrol Attenuates Early Diabetic Nephropathy by Down-Regulating Glutathione S-Transferases Mu in Diabetic Rats. Journal of Medicinal Food 2013, 16, 481-486, 10.1089/jmf.2012.2686.
- M. Y. Kim; J. H. Lim; H. H. Youn; Y. A. Hong; K. S. Yang; H. S. Park; S. Chung; S. H. Koh; S. J. Shin; B. S. Choi; et al.H. W. KimY. S. KimJ. H. LeeY. S. ChangC. W. Park Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK–SIRT1–PGC1α axis in db/db mice. Diabetologia 2012, 56, 204-217, 10.1007/s00125-012-2747-2.
- H Elbe; N Vardi; M Esrefoglu; Burhan Ates; S Yologlu; C Taskapan; Amelioration of streptozotocin-induced diabetic nephropathy by melatonin, quercetin, and resveratrol in rats. Human & Experimental Toxicology 2014, 34, 100-113, 10.1177/0960327114531995.
- Caifeng Yan; Weifeng Xu; Yujie Huang; Min Li; Yachen Shen; Hui You; Xiubin Liang; HRD1-Mediated IGF-1R Ubiquitination Contributes to Renal Protection of Resveratrol in db/db Mice. Molecular Endocrinology 2016, 30, 600-613, 10.1210/me.2015-1277.
- Hoon Suk Park; Ji Hee Lim; Min Young Kim; Yaeni Kim; You Ah Hong; Sun Ryoung Choi; Sungjin Chung; Hyung Wook Kim; Bum Soon Choi; Yong Soo Kim; et al.Yoon Sik ChangCheol Whee Park Resveratrol increases AdipoR1 and AdipoR2 expression in type 2 diabetic nephropathy. Journal of Translational Medicine 2016, 14, 1-13, 10.1186/s12967-016-0922-9.
- Liqun Ma; Rongguo Fu; Zhaoyang Duan; Jiamei Lu; Jie Gao; Lifang Tian; Zhian Lv; Zhao Chen; Jin Han; Lining Jia; et al.Li Wang Sirt1 is essential for resveratrol enhancement of hypoxia-induced autophagy in the type 2 diabetic nephropathy rat. Pathology - Research and Practice 2016, 212, 310-318, 10.1016/j.prp.2016.02.001.
- Heba Al-Hussaini; Narayana Kilarkaje; Trans-resveratrol mitigates type 1 diabetes-induced oxidative DNA damage and accumulation of advanced glycation end products in glomeruli and tubules of rat kidneys. Toxicology and Applied Pharmacology 2017, 339, 97-109, 10.1016/j.taap.2017.11.025.
- Haiyan Guo; Linyun Zhang; Resveratrol provides benefits in mice with type II diabetes‑induced chronic renal failure through AMPK signaling pathway. Experimental and Therapeutic Medicine 2018, 16, 333-341, 10.3892/etm.2018.6178.
- Youhua Liu; Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney International 2005, 69, 213-217, 10.1038/sj.ki.5000054.
- Weiqian Zhang; Yan Liu; Ming Ge; Jiang Jing; Yan Chen; Huijie Jiang; Hongxiang Yu; Ning Li; Zhigang Zhang; Protective effect of resveratrol on arsenic trioxide-induced nephrotoxicity in rats. Nutrition Research and Practice 2013, 8, 220-226, 10.4162/nrp.2014.8.1.220.
- Jin Liang; Shoufu Tian; Junxia Han; Peihua Xiong; Resveratrol as a therapeutic agent for renal fibrosis induced by unilateral ureteral obstruction. Renal Failure 2013, 36, 285-291, 10.3109/0886022x.2013.844644.
- Siqi Xu; Youguang Gao; Qin Zhang; Siwei Wei; Zhongqing Chen; Xingui Dai; Zhenhua Zeng; Ke-Seng Zhao; SIRT1/3 Activation by Resveratrol Attenuates Acute Kidney Injury in a Septic Rat Model. Oxidative Medicine and Cellular Longevity 2016, 2016, 1-12, 10.1155/2016/7296092.
- Y Gan; S Tao; D Cao; H Xie; Q Zeng; Protection of resveratrol on acute kidney injury in septic rats. Human & Experimental Toxicology 2016, 36, 1015-1022, 10.1177/0960327116678298.
- Juliana F. Saldanha; Viviane O. Leal; Felipe Rizzetto; Gustavo H. Grimmer; Marcelo Ribeiro-Alves; Julio Daleprane; José C. Carraro-Eduardo; Denise Mafra; Effects of Resveratrol Supplementation in Nrf2 and NF-κB Expressions in Nondialyzed Chronic Kidney Disease Patients: A Randomized, Double-Blind, Placebo-Controlled, Crossover Clinical Trial. Journal of Renal Nutrition 2016, 26, 401-406, 10.1053/j.jrn.2016.06.005.
- Chong-Ting Lin; Xiao-Yan Sun; Ai-Xia Lin; Supplementation with high-dose trans-resveratrol improves ultrafiltration in peritoneal dialysis patients: a prospective, randomized, double-blind study. Renal Failure 2015, 38, 214-221, 10.3109/0886022x.2015.1128236.
- Juliana F. Saldanha; Viviane De O. Leal; Peter Stenvinkel; José Carlos Carraro-Eduardo; Denise Mafra; Resveratrol: Why Is It a Promising Therapy for Chronic Kidney Disease Patients?. Oxidative Medicine and Cellular Longevity 2013, 2013, 1-6, 10.1155/2013/963217.
- Akhand Pratap Singh; Rachna Singh; Sumit Singh Verma; Vipin Rai; Catherine H. Kaschula; Pralay Maiti; Subash Chandra Gupta; Health benefits of resveratrol: Evidence from clinical studies. Medicinal Research Reviews 2019, 39, 1851-1891, 10.1002/med.21565.
- Pál Brasnyó; Gergo A. Molnar; Márton Mohás; Lajos Markó; Boglárka Laczy; Judit Cseh; Esztella Mikolás; István András Szijártó; Ákos Mérei; Richárd Halmai; et al.László G. MészárosBalázs SümegiIstván Wittmann Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. British Journal of Nutrition 2011, 106, 383-389, 10.1017/s0007114511000316.
- Jayesh Kumar Bhatt; Sabin Thomas; Moola Joghee Nanjan; Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutrition Research 2012, 32, 537-541, 10.1016/j.nutres.2012.06.003.




