
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nontobeko Eunice Mvubu | + 2281 word(s) | 2281 | 2021-09-08 13:10:00 | | | |
| 2 | Beatrix Zheng | + 99 word(s) | 2380 | 2021-09-13 05:39:29 | | |
Video Upload Options
Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), is a successful intracellular pathogen that is responsible for the highest mortality rate among diseases caused by bacterial infections. During early interaction with the host innate cells, M. tuberculosis cell surface antigens interact with Toll like receptor 4 (TLR4) to activate the nucleotide-binding domain, leucine-rich-repeat containing family, pyrin domain-containing 3 (NLRP3) canonical, and non-canonical inflammasome pathways. NLRP3 inflammasome activation in the alveoli has been reported to contribute to the early inflammatory response that is needed for an effective anti-TB response through production of pro-inflammatory cytokines, including those of the Interleukin 1 (IL1) family. However, overstimulation of the alveolar NLRP3 inflammasomes can induce excessive inflammation that is pathological to the host. Several studies have explored the use of medicinal plants and/or their active derivatives to inhibit excessive stimulation of the inflammasomes and its associated factors, thus reducing immunopathological response in the host. This review describes the molecular mechanism of the NLRP3 inflammasome activation in the alveoli during M. tuberculosis infection. Furthermore, the mechanisms of inflammasome inhibition using medicinal plant and their derivatives will also be explored, thus offering a novel perspective on the alternative control strategies of M. tuberculosis-induced immunopathology.
1. Introduction
Several studies have shown the effectiveness of medicinal plants and their bioactive derivatives as a promising approach to TB treatment since these are naturally sourced compounds with minimal side effects [1][2][3][4]. Medicinal plants and their respective active compounds exhibit their anti-inflammatory mechanisms through inhibition of the NLRP3 inflammasome-associated transcripts [5][6], inflammasome assembly [7][8] and production of inflammasome proteins, including IL1 cytokines [9] on in vitro and in vivo M. tuberculosis infection models. The current review reveals the molecular mechanism behind inflammasome activation on resident alveolar macrophages and pulmonary epithelial cells that results in IL1 cytokine production, which have been shown to contribute to the alveolar inflammatory response during M. tuberculosis infection. Furthermore, medicinal plants and their active derivatives that can be explored to prevent overstimulation of the alveolar NLRP3 inflammasome and their mechanisms of action will be reported as a novel host-directed therapy against M. tuberculosis .
2. M. tuberculosis Interaction with Alveolar Macrophages and Pulmonary Epithelial Cells
Previously, not much was known about specific receptors expressed by pulmonary epithelial cells during M. tuberculosis infection. Bermudez and Goodman [10] initially proposed that M. tuberculosis use microtubules and microfilament pathways to gain entry into pulmonary epithelial cells. This was shown by significant reduction of intracellular bacilli when these pathways were blocked [10]. Lee et al. [11] revealed an upregulation of Dectin-1 receptors in a TLR-2 dependent manner of A549 pulmonary epithelial cells during M. tuberculosis infection. Through the production of a Dectin-1 receptor protein, they showed that these non-phagocytic cells might use this receptor for recognition of M. tuberculosis. The M. tuberculosis strains of East-African Indian and Euro-American lineages both induced down-regulation of the NOD1 receptor of the A549 pulmonary epithelial cells at 72 h post-infection [12]. However, a time course analysis of the NOD-associated receptors is needed to establish their involvement in the recognition of M. tuberculosis . Mannose and DC-SIGN receptors have not been linked to M. tuberculosis invasion of pulmonary epithelial cells and this area remains to be investigated.
TLRs are involved in the recognition of different M. tuberculosis cell wall-associated structures, such as acylated lipoproteins (TLR1, TLR2, TLR6), ESAT-6, mycolic acid (TLR2), acylated lipomannan (TLR4), and trehalose dimycolates (TLR3, TLR4, and TLR9), respectively [13]. Sequeira et al. [14] showed the role of TLR2 of pulmonary epithelial cells in the production of pro-inflammatory cytokines during infection by M. tuberculosis. Inhibition of TLR2-mediated response by mycolic acid and mce1 operon mutant significantly reduce the production of both IL8 and MCP-1, which suggests that TLR2 is essential to effective inflammatory response of pulmonary epithelial cells to M. tuberculosis . Previously, we showed an increased expression of TLR2, TLR3, TLR5, and TLR8 in pulmonary epithelial cells at 48 h post-infection by genetically diverse M. tuberculosis clinical strains of East-Asian and Euro-American lineages, leading to production of a diverse range of inflammatory cytokines. Upregulation of TLR4 was only induced by the Beijing (East-Asian) and F11 (Euro-American) strains, while TLR1, TLR6, TLR7, and TLR9 were downregulated by all strains [15] .
A transcriptomic study performed by Hadifa et al. [12] revealed an increased expression of TLR1 and TLR3 by East-African Indian and Euro-American lineages of M. tuberculosis at 72 h post-infection of A549 pulmonary epithelial cells. Furthermore, the East-African Indian strain induced increased expression of TLR2, TLR5, and TLR9; while the Euro-American strain induced down-regulation of TLR4, TLR5, and TLR9. Both transcriptomic studies [12][15] indicate the TLRs are activated by different lineages of M. tuberculosis in pulmonary epithelial cells at 48 and 72 h post infection and these PRRs may be the main receptors used by this pathogen to gain entry into these cells.
Upon stimulation of the alveolar macrophages and pulmonary epithelial cells TLRs by M. tuberculosis PAMPs, the MyD88, TIR-domain-containing adapter-inducing interferon-β (TRIF), TRIF-related adapter molecule (TRAM), Toll-interleukin 1 receptor domain containing adaptor protein (TIRAP), B-cell adaptor for PI3K (BCAP), and sterile alpha and TIR motif containing (SARM) are adaptor molecules that are central components of the TLR signalling pathway [16]. Previously, we showed that genetically diverse strains of M. tuberculosis induced high enrichment of the TLR signalling pathways in pulmonary epithelial cells with increased expression of the MyD88 adaptor molecule [17]. Several studies have linked phagocytosis of M. tuberculosis by alveolar macrophages to the MyD88-signalling pathway [18][19] with a highly impaired inflammatory response in MyD88-knockout studies [20][21]. Thus, the activation of these adaptor molecules in pulmonary epithelial cells and alveolar macrophages results in downstream signalling cascade, leading to the activation transcriptional factors that are responsible for expression of inflammatory cytokines, including interferons and IL1 cytokine family [22]. Moreover, interaction between M. tuberculosis surface antigens with TLR4 triggers the NF-kB signalling pathway to induce expression of IL1β and IL18, whose maturation and production is dependent on the inflammasome assembly [23].
3. Activation of the Alveolar NLRP3 Inflammasome during M. tuberculosis Infection
Inflammasome is a multi-protein complex intracellular structure, which induces maturation of inflammatory cytokines, IL1β, and IL18 through the activation of caspase-1 and caspase-4/11 [24]. The activation of the inflammasome is triggered by signalling pathways that involves host PRRs, such as NOD-like receptors and TLRs [25]. Inflammasomes function in modulating host defence response, as well as pyroptosis, which is an inflammatory induced lytic mode of cell death that can be caspase-1 or caspase-11 dependent [26]. The assembly of an inflammasome is a coordinated signalling event, which is essential to producing an immune response after sensing M. tuberculosis [27][28]. The canonical NLRP3 inflammasomes is mediated by the activation of Caspase 1, while the non-canonical NLRP3 activation can be activated downstream of Caspase 4/11, respectively [25][22].
The second signal of the canonical inflammasome pathway involves the assembly of the NLRP3 inflammasome complex, which results in the recruitment of adaptor molecule, CARD and pro-caspase-1 in order to induce the processing and secretion of IL1β and IL18 cytokines. Pulmonary epithelial cells exhibit increased regulation of NLRP3 (by only F11 and H37Rv strains) PYCARD (ASC) and CASP1 during early infection by clinical strains of M. tuberculosis [15]. This upregulation may be time and lineage specific since the Euro-American lineage downregulated CASP1 at 72 h [12] in pulmonary epithelial cells. High levels of the mature IL18 were predominantly observed in M. tuberculosis-stimulated pulmonary epithelial cells, which indicated the upregulation of IL18 expression at both transcriptional and post-transcriptional levels, suggesting the involvement of CASP1 enzymatic activity [29]. There were no significant differences in the production of IL1β by pulmonary epithelial cells, which increased from 24, 48, and 72 h post-infection by wild type, Mycobacterium tuberculosis curli pili (MTP) mutant and complemented strains of M. tuberculosis [30], suggesting that IL1 cytokine production is not dependent on the MTP antigen. It should be noted that the activation of the canonical NLRP3 inflammasome pathway in pulmonary epithelial cells, if present, may be dependent on the lineage of the infecting strain, as well as the infection time as shown by the differences observed for the Euro-American and East-African Indian lineages [12][15]. Stimulation of the canonical inflammasome by Mycobacterium and it associated factors is well described and characterised in alveolar macrophages [28][31][32], as these cells have been shown to produce inflammatory IL1β and IL18 [31][32][33].
Non-canonical NLRP3 inflammasome mediated by CASP4/11 [34] has been shown to be activated by intestinal Gram-negative bacterial such as Citrobacter rodentium , Escherichia coli, Legionella pneumophila, Salmonella typhimurium, and Vibrio cholera [22] and other parasites such as Leishmania amazonensis [35]. The pathogen’s LPS and other surface antigens have been associated with non-canonical NLRP3 inflammasome activation through interaction with Guanylate binding proteins (GBPs). To our knowledge, there is no evidence of the non-canonical stimulation of the NLRP3 inflammasome by M. tuberculosis in pulmonary epithelial cells from transcriptomics and proteomics studies; and this area remains to be investigated. However, the NLRP3 CASP4/11-dependant inflammasome is activated in macrophages during M. tuberculosis infection [36]. Collectively, these studies indicate that M. tuberculosis stimulate alveolar macrophages canonical and non-canonical NLRP3 inflammasome, leading to production of inflammatory cytokines, IL-1β, and IL18 in a TLR4-NF-kB dependent manner. Furthermore, despite increased expression of the NLRP3 inflammasome transcripts and subsequence IL1 cytokine production in pulmonary epithelial cells, the presence of this complex and other types of inflammasomes such as NLRP6, NLRC4, and AIM2 remains to be investigated and confirmed in future studies. The activation of a canonical and non-canonical alveolar NLRP3 molecular mechanism during M. tuberculosis infection is depicted in Figure 1 .
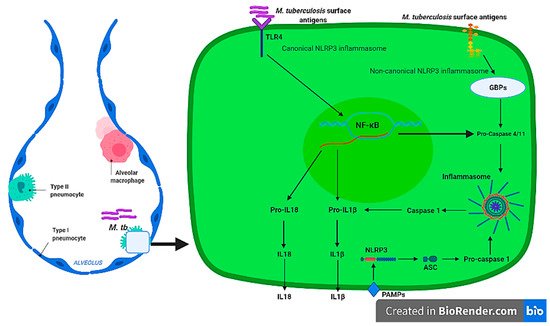
It is apparent that alveolar macrophages produce IL1 cytokines [28][29][30][36][37] through the activation of canonical and/or non-canonical NLRP3 inflammasome by the TLR-MYD88 signalling pathway, which results in stimulation of NF-κB transcriptional factors that transcribe pro-inflammatory cytokines during M. tuberculosis infections. However, this mechanism remains to be confirmed for pulmonary epithelial cells. IL1 are among the group of pro-inflammatory cytokines that are essential in host defence against M. tuberculosis [31]. Mice with IL1α and IL1β double knockouts and IL1R type I-deficient mice display a defective granuloma phenotype accompanied with increased mycobacterial growth [33]. IL18 is known as an interferon-γ inducing factor, which has a vital role in T helper1 (Th1) response [38][39] and has been shown to be responsible for the production of pro-inflammatory cytokines, chemokines, and transcriptional factors [40][41]. Moreover, IL18 defective mice were shown to be highly susceptible to M. tuberculosis and BCG strains [42]. Despite the critical role played by IL1 cytokines of the NLRP3 inflammasome, overproduction of pro-inflammatory cytokines without efficient anti-inflammatory response has been associated with increase disease severity and high bacterial burden [43] that can compromise effective host response during TB infection. The presence of IL1β was associated with caseous granulomatous inflammation during M. tuberculosis, while blocking IL1β production relieved pulmonary inflammation [44]. Significantly high IL1β and IL18 was identified in drug resistant compared to drug susceptible TB [45], while high cytokine concentrations were identified in patients with a severe disease [46]. Therefore, the ability of pulmonary epithelial cells and alveolar macrophages to produce IL1 (IL1β and IL18) cytokines through the activation of the inflammasome may contribute to the inflammatory response during early infection by M. tuberculosis that is pathological to the host, thus novel strategies must be exploited to regulate this response.
4. Medicinal Plants and Their Bioactive Derivatives as Regulators of Alveolar NLRP3 Inflammasome during M. tuberculosis Infection
The high prevalence of TB worldwide is partly due to development of drug resistant strains, rendering the current treatment regimen ineffective [47][48][49]. Several studies have proposed the use of medicinal plants or their bioactive compounds with antimycobacterial activity [3][7][50][51][52][53]. However, recent studies have also exploited the use of medicinal plants and/or their derivatives that are regulators of the immune system during M. tuberculosis infection [54][55][56]. Medicinal plants and their respective bioactive compounds that regulate the components of the immune system may be a promising strategy in controlling Mycobacterial infections because they are not directly targeting the bacillus, reducing the pathogen’s need to develop drug resistance and selective pressure [57]. Therefore, medicinal plants and their bioactive derivatives may provide a novel host-directed perspective in controlling immunopathology in the alveoli through inhibition of the NLRP3 inflammasomes pathways that are crucial in the production of IL1β and IL18 cytokines during M. tuberculosis infections. Medicinal plants and/or their bioactive derivatives that are used to inhibit NLRP3 inflammasome gene transcription, formation of the inflammasome complex and production of NLRP3 inflammasome proteins (including IL1 cytokines) are reviewed below.
There are many medicinal plant bioactive derivatives that act as NLRP3 inflammasome transcript inhibitors identified in non-M. tuberculosis models. These include Arctigenin [58], Silymarin [59], Rutin [60], Genistein [61], Aloe emodin [62], Anemoside B4 [63], epigallocathechin-3-gallate [64], Resveratrol [65], Silibinin [66], Artemisinin [67], Icariin [68], Polydatin [69], Cinnamaldehyde [70], Huangkui capsule [71] whose administration results in the inhibition of the inflammasome-associated transcription on both in vitro and in vivo models ( Figure 2 ). It is crucial that these medicinal plant compounds be tested in M. tuberculosis in vitro and in vivo models to identify their potential use as host-directed immunomodulatory plant derivatives that can be used to prevent lung pathology during infection.
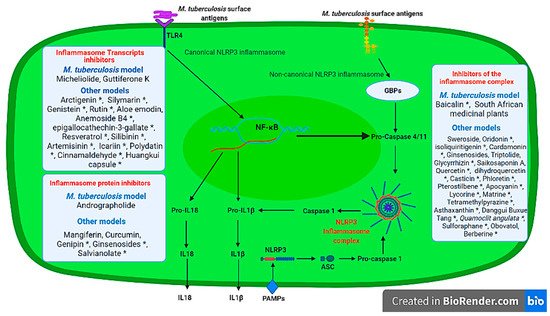
Several in vitro and in vivo models have identified other medicinal plant derivatives that have been found to inhibit NLRP3 inflammasome proteins and subsequent IL1 production; and these include Ginsenosides (Retinoblastoma and Compound K) [72][73], Curcumin [74], Genipin [75], Mangiferin [76], Salvianolate [77] that were derived from Panax ginseng , Curcuma longa , Gardenia jasminoides , Mangifera indica , Salvia miltiorrhiza medicinal plants, respectively.
There is growing appreciation of medicinal plants and their bioactive molecules in management of inflammatory response regulated by the NLRP3 inflammasome as excellently reviewed by several authors [78][79][80][81]. To date, only five studies [5][6][7][8][9] have explored the use of medicinal plants and/or their bioactive derivatives in the management of the inflammasome in M. tuberculosis infection models. There is a growing understanding that M. tuberculosis infection is a multifaceted condition that is also driven by the host inflammatory response. Thus, controlling the alveolar NLRP3 inflammasome seem to be one of the novel strategies for host directed control measures of pathological response induced by M. tuberculosis .
References
- Anochie, P.I.; Ndingkokhar, B.; Bueno, J.; Anyiam, F.E.; Ossai-Chidi, L.N.; Onyeneke, E.C.; Onyeozirila, A.C. African Medicinal Plants that Can Control or Cure Tuberculosis. Int. J. Pharm. Sci. Dev. Res. 2018, 4, 1–8.
- Rahman, F.; Hossan, S.; Mollik, A.; Islam, T.; Jahan, R.; Taufiq-Ur-Rahman, M.; Rahmatullah, M. Medicinal plants used against tuberculosis by traditional medicinal practitioners of Bogra district, Bangladesh. Planta Med. 2009, 75, PD64.
- Gupta, V.K.; Kumar, M.M.; Bisht, D.; Kaushik, A. Plants in our combating strategies against Mycobacterium tuberculosis: Progress made and obstacles met. Pharm. Biol. 2017, 55, 1536–1544.
- Semenya, S.S.; Maroyi, A. Medicinal plants used for the treatment of tuberculosis by Bapedi traditional healers in three districts of the Limpopo Province, South Africa. Afr. J. Tradit. Complement. Altern. Med. 2012, 10, 316–323.
- Zhang, Q.; Jiang, X.; He, W.; Wei, K.; Sun, J.; Qin, X.; Zheng, Y.; Jiang, X. MCL Plays an Anti-Inflammatory Role in Mycobacterium tuberculosis Induced Immune Response by Inhibiting NF-κB and NLRP3 Inflammasome Activation. Mediat. Inflamm. 2017, 2017, 2432904.
- Zhang, Q.; Sun, J.; Fu, Y.; He, W.; Li, Y.; Tan, H.; Xu, H.; Jiang, X. Guttiferone K Exerts the Anti-inflammatory Effect on Mycobacterium tuberculosis-(H37Ra-) Infected Macrophages by Targeting the TLR/IRAK-1 Mediated Akt and NF-κB Pathway. Mediat. Inflamm. 2020, 2020.
- Madikizela, B.; Ndhlala, A.; Finnie, J.; Van Staden, J. Antimycobacterial, anti-inflammatory and genotoxicity evaluation of plants used for the treatment of tuberculosis and related symptoms in South Africa. J. Ethnopharmacol. 2014, 153, 386–391.
- Zhang, Q.; Sun, J.; Wang, Y.; He, W.; Wang, L.; Zheng, Y.; Wu, J.; Zhang, Y.; Jiang, X. Antimycobacterial and Anti-inflammatory Mechanisms of Baicalin via Induced Autophagy in Macrophages Infected with Mycobacterium tuberculosis. Front. Microbiol. 2017, 8.
- He, W.; Sun, J.; Zhang, Q.; Li, Y.; Fu, Y.; Zheng, Y.; Jiang, X. Andrographolide exerts anti-inflammatory effects in Mycobacterium tuberculosis-infected macrophages by regulating the Notch1/Akt/NF-κB axis. J. Leukoc. Biol. 2020, 108, 1747–1764.
- Bermudez, L.E.; Goodman, J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect. Immun. 1996, 64, 1400–1406.
- Lee, H.M.; Yuk, J.M.; Shin, D.M.; Jo, E.K. Dectin-1 is inducible and plays an essential role for mycobacteria-induced innate immune responses in airway epithelial cells. J. Clin. Immunol. 2009, 29, 795–805.
- Hadifar, S.; Behrouzi, A.; Fateh, A.; Khatami, S.; Jamnani, F.R.; Siadat, S.D.; Vaziri, F. Interruption of signaling pathways in lung epithelial cell by Mycobacterium tuberculosis. bioRxiv 2018.
- Faridgohar, M.; Nikoueinejad, H. New findings of Toll-like receptors involved in Mycobacterium tuberculosis infection. Pathog. Glob. Health 2017, 111, 256–264.
- Sequeira, P.C.; Senaratne, R.H.; Riley, L.W. Inhibition of toll-like receptor 2 (TLR-2)-mediated response in human alveolar epithelial cells by mycolic acids and Mycobacterium tuberculosis mce1 operon mutant. Pathog. Dis. 2014, 70, 132–140.
- Mvubu, N.E.; Pillay, B.; McKinnon, L.R.; Pillay, M. Mycobacterium tuberculosis strains induce strain-specific cytokine and chemokine response in pulmonary epithelial cells. Cytokine 2018, 104, 53–64.
- Troutman, T.D.; Bazan, J.F.; Pasare, C. Toll-like receptors, signaling adapters and regulation of the pro-inflammatory response by PI3K. Cell Cycle 2012, 11, 3559–3567.
- Mvubu, N.E.; Pillay, B.; Gamieldien, J.; Bishai, W.; Pillay, M. Canonical pathways, networks and transcriptional factor regulation by clinical strains of Mycobacterium tuberculosis in pulmonary alveolar epithelial cells. Tuberculosis 2016, 97, 73–85.
- Kim, K.; Sohn, H.; Kim, J.-S.; Choi, H.-G.; Byun, E.-H.; Lee, K.-I.; Shin, S.J.; Song, C.-H.; Park, J.-K.; Kim, H.-J. Mycobacterium tuberculosis Rv0652 stimulates production of tumour necrosis factor and monocytes chemoattractant protein-1 in macrophages through the Toll-like receptor 4 pathway. Immunology 2012, 136, 231–240.
- Fremond, C.M.; Togbe, D.; Doz, E.; Rose, S.; Vasseur, V.; Maillet, I.; Jacobs, M.; Ryffel, B.; Quesniaux, V.F.J. IL-1 Receptor-Mediated Signal Is an Essential Component of MyD88-Dependent Innate Response to Mycobacterium tuberculosis Infection. J. Immunol. 2007, 179, 1178–1189.
- Scanga, C.A.; Bafica, A.; Feng, C.G.; Cheever, A.W.; Hieny, S.; Sher, A. MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect. Immun. 2004, 72, 2400.
- Sugawara, I.; Yamada, H.; Mizuno, S.; Takeda, K.; Akira, S. Mycobacterial infection in MyD88-deficient mice. Microbiol. Immunol. 2003, 47, 841–847.
- Pellegrini, C.; Antonioli, L.; Lopez-Castejon, G.; Blandizzi, C.; Fornai, M. Canonical and non-canonical activation of NLRP3 inflammasome at the crossroad between immune tolerance and intestinal inflammation. Front. Immunol. 2017, 8, 36.
- Wawrocki, S.; Druszczynska, M. Inflammasomes in Mycobacterium tuberculosis-Driven Immunity. Can. J. Infect. Dis. Med. Microbiol. 2017, 2017, 2309478.
- Hosseinian, N.; Cho, Y.; Lockey, R.F.; Kolliputi, N. The role of the NLRP3 inflammasome in pulmonary diseases. Ther. Adv. Respir. Dis. 2015, 9, 188–197.
- Diamond, C.E.; Khameneh, H.J.; Brough, D.; Mortellaro, A. Novel perspectives on non-canonical inflammasome activation. ImmunoTargets Ther. 2015, 4, 131.
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022.
- Sharma, D.; Kanneganti, T.-D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016, 213, 617–629.
- Amaral, E.P.; Riteau, N.; Moayeri, M.; Maier, N.; Mayer-Barber, K.D.; Pereira, R.M.; Lage, S.L.; Kubler, A.; Bishai, W.R.; D’Império-Lima, M.R.; et al. Lysosomal Cathepsin Release Is Required for NLRP3-Inflammasome Activation by Mycobacterium tuberculosis in Infected Macrophages. Front. Immunol. 2018, 9, 1427.
- Pechkovsky, D.V.; Goldmann, T.; Vollmer, E.; Müller-Quernheim, J.; Zissel, G. Interleukin-18 expression by alveolar epithelial cells type II in tuberculosis and sarcoidosis. FEMS Immunol. Med. Microbiol. 2006, 46, 30–38.
- Ramsugit, S.; Pillay, B.; Pillay, M. Evaluation of the role of Mycobacterium tuberculosis pili (MTP) as an adhesin, invasin, and cytokine inducer of epithelial cells. Braz. J. Infect. Dis. 2016, 20, 160–165.
- Juffermans, N.P.; Florquin, S.; Camoglio, L.; Verbon, A.; Kolk, A.H.; Speelman, P.; van Deventer, S.J.H.; van der Poll, T. Interleukin-1 Signaling Is Essential for Host Defense during Murine Pulmonary Tuberculosis. J. Infect. Dis. 2000, 182, 902–908.
- Jung, B.-G.; Vankayalapati, R.; Samten, B. Mycobacterium tuberculosis stimulates IL-1β production by macrophages in an ESAT-6 dependent manner with the involvement of serum amyloid A3. Mol. Immunol. 2021, 135, 285–293.
- Yamada, H.; Mizumo, S.; Horai, R.; Iwakura, Y.; Sugawara, I. Protective role of interleukin-1 in mycobacterial infection in IL-1 α/β double-knockout mice. Lab. Investig. 2000, 80, 759–767.
- Oh, C.; Verma, A.; Aachoui, Y. Caspase-11 Non-canonical Inflammasomes in the Lung. Front. Immunol. 2020, 11.
- Chaves, M.M.; Sinflorio, D.A.; Thorstenberg, M.L.; Martins, M.D.A.; Moreira-Souza, A.C.A.; Rangel, T.P.; Silva, C.L.; Bellio, M.; Canetti, C.; Coutinho-Silva, R. Non-canonical NLRP3 inflammasome activation and IL-1β signaling are necessary to L. amazonensis control mediated by P2X7 receptor and leukotriene B4. PLoS Pathog. 2019, 15, e1007887.
- Gutierrez, M.G.; Master, S.S.; Singh, S.B.; Taylor, G.A.; Colombo, M.I.; Deretic, V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 2004, 119, 753–766.
- Mishra, B.B.; Moura-Alves, P.; Sonawane, A.; Hacohen, N.; Griffiths, G.; Moita, L.F.; Anes, E. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell. Microbiol. 2010, 12, 1046–1063.
- Schneider, B.E.; Korbel, D.; Hagens, K.; Koch, M.; Raupach, B.; Enders, J.; Kaufmann, S.H.; Mittrücker, H.W.; Schaible, U.E. A role for IL-18 in protective immunity against Mycobacterium tuberculosis. Eur. J. Immunol. 2010, 40, 396–405.
- Nakanishi, K. Unique action of interleukin-18 on T cells and other immune cells. Front. Immunol. 2018, 9, 763.
- Netea, M.G.; Kullberg, B.J.; Verschueren, I.; Meer, J.W.V.d. Interleukin-18 induces production of proinflammatory cytokines in mice: No intermediate role for the cytokines of the tumor necrosis factor family and interleukin-1β. Eur. J. Immunol. 2000, 30, 3057–3060.
- Puren, A.J.; Fantuzzi, G.; Gu, Y.; Su, M.; Dinarello, C.A. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J. Clin. Investig. 1998, 101, 711–721.
- Sugawara, I.; Yamada, H.; Kaneko, H.; Mizuno, S.; Takeda, K.; Akira, S. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect. Immun. 1999, 67, 2585–2589.
- Kumar, N.P.; Moideen, K.; Banurekha, V.V.; Nair, D.; Babu, S. Plasma Proinflammatory Cytokines Are Markers of Disease Severity and Bacterial Burden in Pulmonary Tuberculosis. Open Forum Infect. Dis. 2019, 6, ofz257.
- Chao, W.-C.; Yen, C.-L.; Hsieh, C.-Y.; Huang, Y.-F.; Tseng, Y.-L.; Nigrovic, P.A.; Shieh, C.-C. Mycobacterial infection induces higher interleukin-1β and dysregulated lung inflammation in mice with defective leukocyte NADPH oxidase. PLoS ONE 2017, 12, e0189453.
- Wang, Y.; Hu, C.; Wang, Z.; Kong, H.; Xie, W.; Wang, H. Serum IL-1β and IL-18 correlate with ESR and CRP in multidrug-resistant tuberculosis patients. J. Biomed. Res. 2015, 29, 426–428.
- Santucci, N.; D’Attilio, L.; Besedovsky, H.; Del Rey, A.; Bay, M.L.; Bottasso, O. A clinical correlate of the dysregulated immunoendocrine response in human tuberculosis. Neuroimmunomodulation 2010, 17, 184–187.
- Boldrin, F.; Provvedi, R.; Cioetto Mazzabò, L.; Segafreddo, G.; Manganelli, R. Tolerance and Persistence to Drugs: A Main Challenge in the Fight Against Mycobacterium tuberculosis. Front. Microbiol. 2020, 11, 1924.
- Mandal, S.; Njikan, S.; Kumar, A.; Early, J.V.; Parish, T. The relevance of persisters in tuberculosis drug discovery. Microbiology 2019, 165, 492–499.
- Sarathy, J.P.; Via, L.E.; Weiner, D.; Blanc, L.; Boshoff, H.; Eugenin, E.A.; Barry III, C.E.; Dartois, V.A. Extreme drug tolerance of Mycobacterium tuberculosis in caseum. Antimicrob. Agents Chemother. 2018, 62, e02266-17.
- Kahaliw, W.; Aseffa, A.; Abebe, M.; Teferi, M.; Engidawork, E. Evaluation of the antimycobacterial activity of crude extracts and solvent fractions of selected Ethiopian medicinal plants. BMC Complement. Altern. Med. 2017, 17, 143.
- Gemechu, A.; Giday, M.; Worku, A.; Ameni, G. In vitro Anti-mycobacterial activity of selected medicinal plants against Mycobacterium tuberculosis and Mycobacterium bovis Strains. BMC Complement. Altern. Med. 2013, 13, 291.
- Assam, J.P.A.; Tcham, M.F.Y.; Moni, N.E.D.F.; Betote, D.P.H.; Fossi, T.C.; Penlap, B.V. Phytochemical screening, Antimycobacterial activity of three medicinal Cameroonians plants and Acute toxicity of hydroethanolic extract of Vitellaria paradoxa. J. Drug Deliv. Ther. 2020, 10, 96–104.
- Fyhrquist, P.; Laakso, I.; Marco, S.G.; Julkunen-Tiitto, R.; Hiltunen, R. Antimycobacterial activity of ellagitannin and ellagic acid derivate rich crude extracts and fractions of five selected species of Terminalia used for treatment of infectious diseases in African traditional medicine. S. Afr. J. Bot. 2014, 90, 1–16.
- Putri, D.U.; Rintiswati, N.; Soesatyo, M.H.; Haryana, S.M. Immune modulation properties of herbal plant leaves: Phyllanthus niruri aqueous extract on immune cells of tuberculosis patient—In vitro study. Nat. Prod. Res. 2018, 32, 463–467.
- Krug, S.; Parveen, S.; Bishai, W.R. Host-Directed Therapies: Modulating Inflammation to Treat Tuberculosis. Front. Immunol. 2021, 12.
- Zumla, A.; Rao, M.; Dodoo, E.; Maeurer, M. Potential of immunomodulatory agents as adjunct host-directed therapies for multidrug-resistant tuberculosis. BMC Med. 2016, 14, 89.
- Ahmed, S.; Raqib, R.; Guðmundsson, G.H.; Bergman, P.; Agerberth, B.; Rekha, R.S. Host-Directed Therapy as a Novel Treatment Strategy to Overcome Tuberculosis: Targeting Immune Modulation. Antibiotics 2020, 9, 21.
- Pu, Z.; Han, C.; Zhang, W.; Xu, M.; Wu, Z.; Liu, Y.; Wu, M.; Sun, H.; Xie, H. Systematic understanding of the mechanism and effects of Arctigenin attenuates inflammation in dextran sulfate sodium-induced acute colitis through suppression of NLRP3 inflammasome by SIRT1. Am. J. Transl. Res. 2019, 11, 3992.
- Yuan, R.; Fan, H.; Cheng, S.; Gao, W.; Xu, X.; Lv, S.; Ye, M.; Wu, M.; Zhu, X.; Zhang, Y. Silymarin prevents NLRP3 inflammasome activation and protects against intracerebral hemorrhage. Biomed. Pharmacother. 2017, 93, 308–315.
- Hu, Q.-H.; Zhang, X.; Pan, Y.; Li, Y.-C.; Kong, L.-D. Allopurinol, quercetin and rutin ameliorate renal NLRP3 inflammasome activation and lipid accumulation in fructose-fed rats. Biochem. Pharmacol. 2012, 84, 113–125.
- Wang, S.; Wang, J.; Wei, H.; Gu, T.; Wang, J.; Wu, Z.; Yang, Q. Genistein attenuates acute cerebral ischemic damage by inhibiting the NLRP3 inflammasome in reproductively senescent mice. Front. Aging Neurosci. 2020, 12, 153.
- Budai, M.M.; Varga, A.; Milesz, S.; Tőzsér, J.; Benkő, S. Aloe vera downregulates LPS-induced inflammatory cytokine production and expression of NLRP3 inflammasome in human macrophages. Mol. Immunol. 2013, 56, 471–479.
- Gong, Q.; He, L.-L.; Wang, M.-L.; Ouyang, H.; Gao, H.-W.; Feng, Y.-L.; Yang, S.-L.; Du, L.-J.; Li, J.; Luo, Y.-Y. Anemoside B4 protects rat kidney from adenine-induced injury by attenuating inflammation and fibrosis and enhancing podocin and nephrin expression. Evid. Based Complement. Altern. Med. 2019, 2019.
- Tsai, P.-Y.; Ka, S.-M.; Chang, J.-M.; Chen, H.-C.; Shui, H.-A.; Li, C.-Y.; Hua, K.-F.; Chang, W.-L.; Huang, J.-J.; Yang, S.-S. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic. Biol. Med. 2011, 51, 744–754.
- Ding, S.; Wang, H.; Wang, M.; Bai, L.; Yu, P.; Wu, W. Resveratrol alleviates chronic “real-world” ambient particulate matter-induced lung inflammation and fibrosis by inhibiting NLRP3 inflammasome activation in mice. Ecotoxicol. Environ. Saf. 2019, 182, 109425.
- Matias, M.L.; Gomes, V.J.; Romao-Veiga, M.; Ribeiro, V.R.; Nunes, P.R.; Romagnoli, G.G.; Peracoli, J.C.; Peracoli, M.T.S. Silibinin downregulates the NF-κB pathway and NLRP1/NLRP3 inflammasomes in monocytes from pregnant women with preeclampsia. Molecules 2019, 24, 1548.
- Wen, Y.; Pan, M.M.; Lv, L.L.; Tang, T.T.; Zhou, L.T.; Wang, B.; Liu, H.; Wang, F.M.; Ma, K.L.; Tang, R.N. Artemisinin attenuates tubulointerstitial inflammation and fibrosis via the NF-κB/NLRP3 pathway in rats with 5/6 subtotal nephrectomy. J. Cell. Biochem. 2019, 120, 4291–4300.
- Zhang, L.; Wang, X.Z.; Li, Y.S.; Zhang, L.; Hao, L.R. Icariin ameliorates IgA nephropathy by inhibition of nuclear factor kappa b/Nlrp3 pathway. FEBS Open Bio 2017, 7, 54–63.
- Tang, J.; Li, Y.; Wang, J.; Wu, Q.; Yan, H. Polydatin suppresses the development of lung inflammation and fibrosis by inhibiting activation of the NACHT domain-, leucine-rich repeat-, and pyd-containing protein 3 inflammasome and the nuclear factor-κB pathway after Mycoplasma pneumoniae infection. J. Cell. Biochem. 2019, 120, 10137–10144.
- Kang, L.-L.; Zhang, D.-M.; Ma, C.-H.; Zhang, J.-H.; Jia, K.-K.; Liu, J.-H.; Wang, R.; Kong, L.-D. Cinnamaldehyde and allopurinol reduce fructose-induced cardiac inflammation and fibrosis by attenuating CD36-mediated TLR4/6-IRAK4/1 signaling to suppress NLRP3 inflammasome activation. Sci. Rep. 2016, 6, 1–18.
- Han, W.; Ma, Q.; Liu, Y.; Wu, W.; Tu, Y.; Huang, L.; Long, Y.; Wang, W.; Yee, H.; Wan, Z.; et al. Huangkui capsule alleviates renal tubular epithelial-mesenchymal transition in diabetic nephropathy via inhibiting NLRP3 inflammasome activation and TLR4/NF-κB signaling. Phytomedicine 2019, 57, 203–214.
- Li, C.-W.; Deng, M.-Z.; Gao, Z.-J.; Dang, Y.-Y.; Zheng, G.-D.; Yang, X.-J.; Chao, Y.-X.; Cai, Y.-F.; Wu, X.-L. Effects of compound K, a metabolite of ginsenosides, on memory and cognitive dysfunction in db/db mice involve the inhibition of ER stress and the NLRP3 inflammasome pathway. Food Funct. 2020, 11, 4416–4427.
- Chen, W.; Wang, J.; Luo, Y.; Wang, T.; Li, X.; Li, A.; Li, J.; Liu, K.; Liu, B. Ginsenoside Rb1 and compound K improve insulin signaling and inhibit ER stress-associated NLRP3 inflammasome activation in adipose tissue. J. Ginseng Res. 2016, 40, 351–358.
- Gong, Z.; Zhou, J.; Li, H.; Gao, Y.; Xu, C.; Zhao, S.; Chen, Y.; Cai, W.; Wu, J. Curcumin suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Mol. Nutr. Food Res. 2015, 59, 2132–2142.
- Yu, S.-X.; Du, C.-T.; Chen, W.; Lei, Q.-Q.; Li, N.; Qi, S.; Zhang, X.-J.; Hu, G.-Q.; Deng, X.-M.; Han, W.-Y. Genipin inhibits NLRP3 and NLRC4 inflammasome activation via autophagy suppression. Sci. Rep. 2015, 5, 1–12.
- Pan, C.-W.; Pan, Z.-Z.; Hu, J.-J.; Chen, W.-L.; Zhou, G.-Y.; Lin, W.; Jin, L.-X.; Xu, C.-L. Mangiferin alleviates lipopolysaccharide and D-galactosamine-induced acute liver injury by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Eur. J. Pharmacol. 2016, 770, 85–91.
- Qiu, H.; Liu, W.; Lan, T.; Pan, W.; Chen, X.; Wu, H.; Xu, D. Salvianolate reduces atrial fibrillation through suppressing atrial interstitial fibrosis by inhibiting TGF-β1/Smad2/3 and TXNIP/NLRP3 inflammasome signaling pathways in post-MI rats. Phytomedicine 2018, 51, 255–265.
- Bagherniya, M.; Khedmatgozar, H.; Fakheran, O.; Xu, S.; Johnston, T.P.; Sahebkar, A. Medicinal plants and bioactive natural products as inhibitors of NLRP3 inflammasome. Phytother. Res. 2021.
- Liu, B.; Yu, J. Anti-NLRP3 Inflammasome Natural Compounds: An Update. Biomedicines 2021, 9, 136.
- Tőzsér, J.; Benkő, S. Natural compounds as regulators of NLRP3 inflammasome-mediated IL-1β production. Mediat. Inflamm. 2016, 2016.
- Ding, N.; Wei, B.; Fu, X.; Wang, C.; Wu, Y. Natural Products that Target the NLRP3 Inflammasome to Treat Fibrosis. Front. Pharmacol. 2020, 11, 2039.




