
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sergio Nogales | + 1673 word(s) | 1673 | 2021-08-30 11:16:00 | | | |
| 2 | Beatrix Zheng | + 477 word(s) | 2150 | 2021-09-10 12:25:41 | | |
Video Upload Options
Fresh-cut produce are quite popular among consumers due to their eating ease, high quality and functional content. However, some of the processing steps taking place during minimal processing (such as cutting, peeling, draining, etc.) might speed up decay, e.g., microbial growth, dehydration or browning. When it comes to the latter, polyphenol oxidase (PPO) plays an important role, being the center of many works focused on the understanding of its reaction mechanism and the application of conservative techniques. The aim of this review study was to compare recent research about the effect of PPO on minimally processed fruits and vegetables, trying to understand the way it acts, the measurement of its activity and current treatments, such as modified atmosphere packaging, washing treatments or edible coatings, among others. In conclusion, the combination of conservation techniques (that is, hurdle technology) is vital to guarantee global quality in minimally processed fruits and vegetables, including synergistic effects which will allow the use of mild treatment conditions to decrease PPO activity. However, further research is required to clearly understand PPO inhibition in trendy techniques such as irradiation.
1. Introduction
Minimally processed fruits and vegetables are highly demanded by consumers [1], growing their sales all over the world, as in the case of Europe [2]. This might be due to an increasing concern about health and lack of time to cook properly. Furthermore, minimally processed fruits and vegetables provide high functional and ready-to-eat food which are convenient products nowadays. Thus, there are a considerable amount of vegetables and fruits undergoing this technology, such as lettuce, cabbage, eggplant, apples, pears, peaches, plums, nectarines, etc. On account of the fact that fresh-cut produces come from whole vegetables and fruits, it is expected that these products have similar functional properties. Consequently, they contain high levels of fiber, minerals or antioxidants such as vitamins C and E, carotenoids, glucosinolates, polyphenols, etc. [3][4][5][6]. This fact has supposed an interesting research field for scientists, and its relevance has continuously increased. The trend in research about fresh-cut or minimally processed fruits and vegetables is shown in Figure 1.
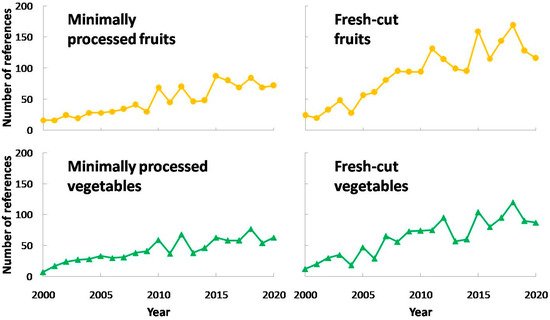
As observed in this figure, there was a steady increase in research works about fresh-cut or minimally processed fruits and vegetables, mainly starting in 2000 with a few works and surpassing 150 studies per year in the past five years. In addition, there were more research works about fresh-cut or minimally processed fruits compared to vegetables. In conclusion, these figures point out the increasing interest in minimally processing so far.
However, even though the processing of fresh-cut fruits and vegetables is minimal, some aspects such as cutting and peeling promote a faster deterioration [8], involving physiological, biochemical and microbiological changes [9]. Hence, there are a lot of enzymes that take part in these changes, such as pectin methylesterase, polygalacturonase (both of them affecting texture), lipooxidase (generating off-flavor compounds) and polyphenol oxidase (EC 1.14.18.1 or PPO), among others. The latter plays an important role on visual quality decay causing browning, as it will be discussed in following sections. This deterioration might change consumers’ choice as visual appearance is one of the highest rated aspects considered in order to purchase minimally processed fruits and vegetables. As a consequence, PPO has been widely studied (both in whole or minimally processed products) in order to characterize its content or to avoid its effect on quality of fruits or vegetables, (see Figure 2 ).
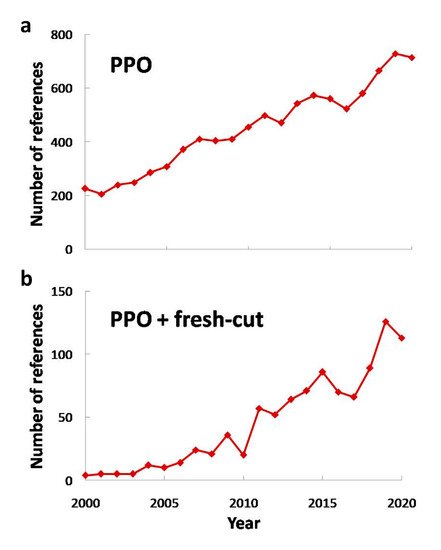
This way, PPO has been widely researched, and its interest has increased in the last two decades ( Figure 2 a). The same trend was observed for research about PPO and fresh-cut produce ( Figure 2 b), and if Figure 1 is taken into account, it can be noted that research about fresh-cut produce and its combination with PPO research are almost equivalent, pointing out the need of PPO characterization and the study of its consequences in fresh-cut fruits and vegetables in every research work in this field.
2. PPO Activity Assessment
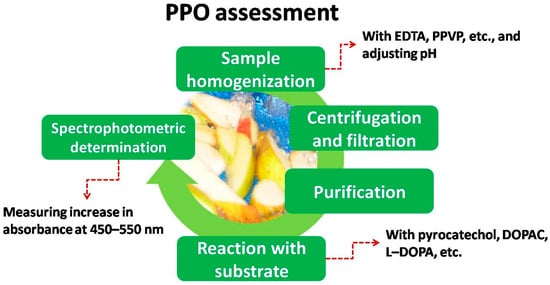
As explained earlier, there is a special need to assess PPO activity (among other enzymes) in order to assess its effect on browning. Even though there are different ways to assess PPO activity [10], the most common one used in fresh-cut produce was by spectrophotometry. In this case, whole produce and in-vitro assays follow similar procedures. In short, the steps are the ones gathered in Figure 3 . Additionally, Table 1 and Table 2 shows some details about PPO activity extraction and determination for vegetables and fruits, respectively.
| PPO Extraction | Spectrophotometric Determination | ||||
|---|---|---|---|---|---|
| Produce | Reference | Substrate Solution | pH | Wavelength (nm) | |
| Artichoke | [11] | PVP and acetate buffer (pH 5.6) | Chlorogenic acid | 6.0 | 410 |
| Artichoke | [12] | Benzamidine, ascorbic acid, PMSF, triton and phosphate buffer (pH 7.0) | MBTH | 4.5 | 400 |
| Carrot | [13] | PVPP and phosphate buffer (pH 6.5) | Catechol | 6.5 | 420 |
| Carrot | [14] | Phosphate buffer (pH 7.0) | Catechol | 7.0 | 410 |
| Eggplant | [15] | Citrate phosphate buffer (pH 7.0) | DOPAC, MBTH, methanol | 7.0 | 466 |
| Eggplant | [16] | PVP, PVPP, triton, ascorbic acid and phosphate buffer (pH 6.8) | 4-methylcatechol | 6.8 | 420 |
| Lettuce | [17] | PVPP and phosphate buffer (pH 6.5) | Catechol | 6.5 | 400 |
| Lettuce | [18] | PVPP and phosphate buffer (pH 7.0) | Catechol | 7.0 | 420 |
| Lettuce | [19] | Acetone, MBTH and citrate phosphate buffer (pH 7.5) | DOPAC | 7.0 | 505 |
| Lettuce | [20] | Phosphate buffer (pH 7.0) | MBTH | 7.0 | 467 |
| Lotus root | [21] | PVPP and phosphate buffer (pH 7.0) | Catechol | 7.0 | 410 |
| Mushroom | [22] | PVPP and NaCl (pH 6.5) | Catechol | 7.0 | 400 |
| Mushroom | [23] | PVPP, triton and phosphate buffer (pH 6.8) | Catechol | 6.8 | 420 |
| Potato | [24] | PVPP and phosphate buffer (pH 6.0) | 4-methylcatechol | 6.0 | 410 |
| Potato | [25] | Phosphate buffer (pH 6.5) | Catechol | 6.5 | 410 |
| Potato | [26] | PBS (pH 6.8) | Catechol | 5.5 | 405 |
| Potato | [27] | PVP, triton and phosphate buffer (pH 6.8) | Catechol | 7.0 | 475 |
| Red beet | [28] | NaCl, PVP and phosphate-citrate buffer (pH 6.5) | Pyrocatechol | 6.5 | 420 |
| Red beet | [29] | NaCl and phosphate buffer (pH 6.0) | Pyrocatechol | 7.0 | 420 |
| Sweet peppers | [30] | Acetone and citrate phosphate buffer (pH 7.5) | DOPAC | 7.0 | 505 |
| PPO Extraction | Spectrophotometric Determination | ||||
|---|---|---|---|---|---|
| Produce | Reference | Substrate Solution | pH | Wavelength (nm) | |
| Apple | [31] | PVPP and phosphate buffer (pH 7.0) | Cathecol | 5.8 | 420 |
| Apple | [32] | PVPP and phosphate buffer (pH 5.0) | 4-methylcatechol | 5.0 | 494 |
| Apple | [33] | Triton and phosphate buffer (pH 7.2) | Chlorogenic acid | 5.2 | 420 |
| Apple | [34] | PVPP, triton and Phosphate buffer (pH 7.0) | Citrate-phosphate | 5.0 | 420 |
| Apple | [35] | Potassium phosphate buffer (pH 7.0) | Sodium acetate | 5.5 | 405 |
| Banana | [36] | PVPP, triton and phosphate buffer (pH 6.5) | Catechol | 6.5 | 420 |
| Carambola | [37] | PVPP, KCl and phosphate buffer (pH 6.8) | Catechol | 7.2 | 410 |
| Carambola | [38] | PVPP, KCl and phosphate buffer (pH 6.8) | Catechol | 7.2 | 410 |
| Coconut water | [39] | Phosphate buffer (pH 5.5) | Pyrocatechol | 5.5 | 470 |
| Jicama | [40] | PVPP and phosphate buffer (pH 7.0) | Catechol | 7.0 | 420 |
| Papaya | [41] | Sodium phosphate buffer (pH 6.5) | Catechol | 6.5 | 420 |
| Peach | [42] | PMSF, PVPP, triton and phosphate buffer (pH 6.8) | L-Dopa | 6.8 | 475 |
| Peach | [43] | EDTA, MgCl2, PMSF, PVPP, triton and phosphate buffer (pH 7.0) | Resorcinol | 7.0 | 500 |
| Peach | [44] | EDTA, PVPP and phosphate buffer (pH 7.0) | -- | 7.0 | 420 |
| Pear | [45] | Dihydrogen phosphate buffer (pH 6.8) | Catechol | 6.8 | 398 |
| Pear | [46] | Triton and phosphate buffer (pH 7.2) | Chlorogenic acid | 4.2 | 420 |
| Strawberry | [47] | PVPP, triton and phosphate buffer (pH 6.5) | Catechol | 6.5 | 420 |
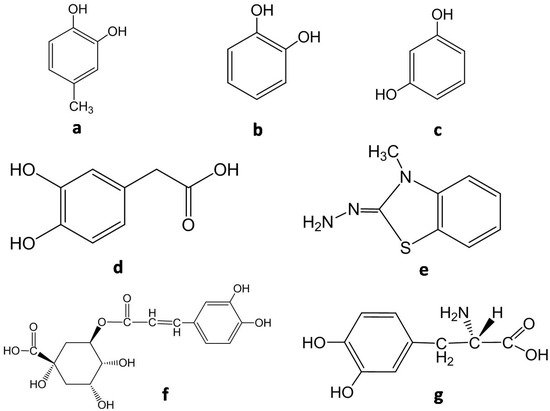
Thus, homogenization is required, mixing up the sample with some components such as EDTA, PMSF or PVPP and adjusting pH (generally between 6 and 8) with buffer [10]. After centrifugation and filtration, the spectrophotometric reaction takes place adding the extract to a reactive environment with suitable substrates and adjusting pH. Molecular structures of some of the most used substrates are shown in Figure 4 .
Finally, the increase in absorbance is measured at the beginning of the reaction (usually for 3 min), using wavelengths from 450 to 550. Figure 3 and Figure 4 show some of the most common extracts used in spectrophotometric determination of PPO activity applied to different fresh-cut vegetables and fruits, respectively.
3. Current Studies Focused on PPO Activity
According to many researchers, as included in Figure 5 , the main factors affecting PPO activity are temperature, pH, cell integrity, pre-harvest conditions and microbial activity, which will be explained in the following sections.
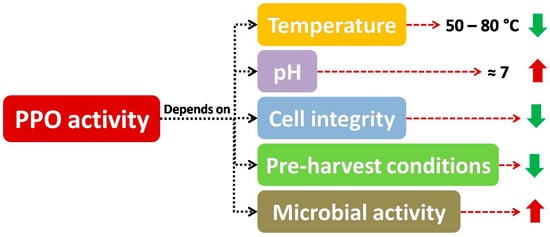
Apart from the activity of endogenous quality degrading enzymes, spoilage microorganism reaction considerably shortens the shelf life of fruit and vegetable products, both whole and fresh-cut produce [48][49]. Furthermore, microbial growth can be a matter of concern, with many studies considering microbial safety in minimally processed samples [50], as in the case of cantaloupe pieces [51].
As included in some studies, the effect of enzymes and microbial growth on shelf life of minimally processed fruits and vegetables could be synergistic, as microorganisms can worsen cell integrity of tissues, provoking cell lysis, which is highly related to the increase in enzyme activity, as enzymes and substrates are easily put in contact, accelerating decay in fruits and vegetables (both whole and fresh-cut samples) [52]. On the other hand, microbial growth and enzymatic reactions can present a common starting point (i.e., cell lysis) to put some components included in cell content (such as sugar or phenols for microbial counts or PPO activity, respectively), and consequently these two adverse effects can act in parallel. As a consequence, if some techniques promote cell reinforcement are used (such as the application of calcium treatments), a combined decrease in microbial and PPO activity can be observed, as outlined below.
This way, the effect of microbiological activity on some visual defects has been investigated in several papers that considered the relationship between psychrotrophic counts and visual decay [51]. As some authors have pointed out, the use of protective treatments seemed to be related to a direct decrease in browning, enzyme activity (such as PPO) and microbial growth [50][53], as in the case of edible coatings applied to fresh-cut fruits and vegetables [54]. Specifically, the reduction of microbial population may result in PPO activity inhibition, on account of the fact that pectinolytic micro-organisms could break down cell walls resulting in stress-related exposure of enzymes and substrates, which also could lead to enzymatic browning. Hence, some barriers such as modified atmosphere packaging or washing treatments might contribute (besides their direct effects on browning inhibition) to reduce microbial population and therefore reduce PPO activity, improving visual quality [55][56], as explained in many papers included in this review, although the decrease in microbial growth is not thoroughly covered in this work.
4. Current Treatments to Avoid or Reduce PPO Activity in Fresh-Cut Produce
In order to avoid the negative effects of PPO activity, or at least to reduce them, many treatments have been used in fresh-cut or minimally processed produces. Some of them can be exclusively used to reduce PPO activity, whereas others can show other beneficial effects on fresh-cut produce, such asantimicrobial effect, inhibition on other enzymes, etc. On the other hand, some of these treatments can be combined, such asthe use of washing with antioxidants or essential oils, the use of edible coatings containing antioxidants or the combined use of washing and modified atmosphere packaging, presenting additive or synergistic effects in many cases. In any case, some of the treatments that have drawn attention to researchers are included in Figure 6 . It should be pointed out that the search for mild treatments (by combining techniques or using the hurdle technology) seems to be one interesting and resourceful option for researchers, in order to avoid other undesirable effects on fresh-cut fruits and vegetables [57].
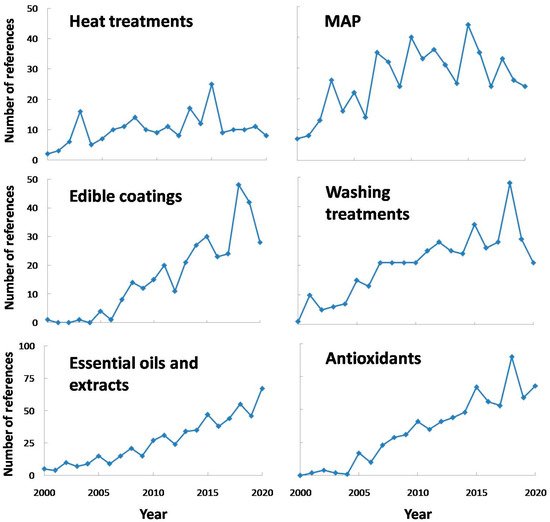
On the other hand, N -acetylcysteine is specifically used as a dietary supplement, being used in order to avoid cut browning. For instance, it is worth mentioning the optimization of a washing treatment including N -acetylcysteine in minimally processed apples. Thus, the use of 100 mM isoascorbic acid, 5 mM Caascorbate, 5 mM Capropionate and 5 mM N -acetylcysteine washing treatment on different cultivar apples was the optimum combination used in order to keep visual quality [58].
This product could be used included in washing treatments, as the literature showed promising results when it comes to PPO control. As some authors have appointed, the use of salicylic acid (SA) in washing treatments have showed good results concerning PPO inhibition in fresh-cut chestnut, and its effect was higher with concentration (up to 10 mM or at least 0.3 g/L, depending on the case). However, its inhibition properties are not clear yet, needing further studies [59][60].
Ultrasound (US) might cause enzyme inactivation by cell lysis due to vibration energy, which produces cavitation bubbles and temporarily generates spots of high pressure and temperature when imploded. Thus, its use might be suitable especially when combined with antioxidant treatments (normally included in washing treatments), just as in the case of fresh-cut apples. Specifically, PPO activity was reduced by combining US (40 kHz) and ascorbic acid (1%), pointing out the fact that the individual application of these treatments was not effective at inactivating enzyme activity [32]. Other recent study was focused on the suitability of this technique combined with ascorbic acid and citric acid for fresh-cut potatoes, showing around 40 % decrease in PPO activity in treated samples, compared to control samples [26].
References
- Giovenzana, V.; Casson, A.; Beghi, R.; Pampuri, A.; Fiorindo, I.; Tugnolo, A.; Guidetti, R. Evaluation of consumer domestic habits on the environmental impact of ready-to-eat and minimally processed fresh-cut lamb’s lettuce. Sustain. Prod. Consum. 2021, 28, 925–935.
- Baselice, A.; Colantuoni, F.; Lass, D.A.; Nardone, G.; Stasi, A. Trends in EU consumers’ attitude towards fresh-cut fruit and vegetables. Food Qual. Prefer. 2017, 59, 87–96.
- Nogales-Delgado, S.; Fuentes-Pérez, M.C.; Bohoyo-Gil, D. Cultivar characterization of stone fruits for their minimal processing. J. Food Sci. Technol. 2015, 52.
- Fuentes-Pérez, M.D.C.; Nogales-Delgado, S.; Ayuso, M.C.; Bohoyo-Gil, D. Different peach cultivars and their suitability for minimal processing. Czech J. Food Sci. 2014, 32, 2193–2201.
- Nogales-Delgado, S.; Fernández-León, A.M.; Delgado-Adámez, J.; Hernández-Méndez, M.T.; Bohoyo Gil, D. Effects of several sanitisers for improving quality attributes of minimally processed fragaria vesca strawberry. Czech J. Food Sci. 2013, 31, 49–54.
- Nogales-Delgado, S.; Fuentes-Pérez, M.C.; Ayuso-Yuste, C.; Bohoyo-Gil, D. Study of different nectarine cultivars and their suitability for fresh-cut processing. Int. J. Food Sci. Technol. 2014, 49, 114–120.
- SCOPUS Scopus Preview. Available online: https://www.scopus.com/home.uri (accessed on 30 July 2021).
- Rico, D.; Martín-Diana, A.B.; Barat, J.M.; Barry-Ryan, C. Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 373–386.
- Hodges, D.M.; Toivonen, P.M.A. Quality of fresh-cut fruits and vegetables as affected by exposure to abiotic stress. Postharvest Biol. Technol. 2008, 48, 155–162.
- Panadare, D.; Rathod, V.K. Extraction and purification of polyphenol oxidase: A review. Biocatal. Agric. BioTechnol. 2018, 14, 431–437.
- Cefola, M.; D’Antuono, I.; Pace, B.; Calabrese, N.; Carito, A.; Linsalata, V.; Cardinali, A. Biochemical relationships and browning index for assessing the storage suitability of artichoke genotypes. Food Res. Int. 2012, 48, 397–403.
- Cabezas-Serrano, A.B.; Amodio, M.L.; Colelli, G. Effect of solution pH of cysteine-based pre-treatments to prevent browning of fresh-cut artichokes. Postharvest Biol. Technol. 2013, 75, 17–23.
- Bi, X.; Wu, J.; Zhang, Y.; Xu, Z.; Liao, X. High pressure carbon dioxide treatment for fresh-cut carrot slices. Innov. Food Sci. Emerg. Technol. 2011, 12, 298–304.
- Chauhan, O.P.; Raju, P.S.; Ravi, N.; Singh, A.; Bawa, A.S. Effectiveness of ozone in combination with controlled atmosphere on quality characteristics including lignification of carrot sticks. J. Food Eng. 2011, 102, 43–48.
- Barbagallo, R.N.; Chisari, M.; Caputa, G. Effects of calcium citrate and ascorbate as inhibitors of browning and softening in minimally processed “Birgah” eggplants. Postharvest Biol. Technol. 2012, 73, 107–114.
- Mishra, B.B.; Gautam, S.; Sharma, A. Browning of fresh-cut eggplant: Impact of cutting and storage. Postharvest Biol. Technol. 2012, 67, 44–51.
- Martin-Diana, A.B.; Rico, D.; Barry-Ryan, C.; Frias, J.M.; Mulcahy, J.; Henehan, G.T.M. Calcium lactate washing treatments for salad-cut Iceberg lettuce: Effect of temperature and concentration on quality retention parameters. Food Res. Int. 2005, 38, 729–740.
- Chen, Z.; Zhu, C.; Zhang, Y.; Niu, D.; Du, J. Effects of aqueous chlorine dioxide treatment on enzymatic browning and shelf-life of fresh-cut asparagus lettuce (Lactuca sativa L.). Postharvest Biol. Technol. 2010, 58, 232–238.
- Chisari, M.; Todaro, A.; Barbagallo, R.N.; Spagna, G. Salinity effects on enzymatic browning and antioxidant capacity of fresh-cut baby Romaine lettuce (Lactuca sativa L. cv. Duende). Food Chem. 2010, 119, 1502–1506.
- Luna, M.C.; Tudela, J.A.; Martínez-Sánchez, A.; Allende, A.; Marín, A.; Gil, M.I. Long-term deficit and excess of irrigation influences quality and browning related enzymes and phenolic metabolism of fresh-cut iceberg lettuce (Lactuca sativa L.). Postharvest Biol. Technol. 2012, 73, 37–45.
- Du, J.; Fu, Y.; Wang, N. Effects of aqueous chlorine dioxide treatment on browning of fresh-cut lotus root. LWT Food Sci. Technol. 2009, 42, 654–659.
- Oms-Oliu, G.; Aguiló-Aguayo, I.; Martín-Belloso, O.; Soliva-Fortuny, R. Effects of pulsed light treatments on quality and antioxidant properties of fresh-cut mushrooms (Agaricus bisporus). Postharvest Biol. Technol. 2010, 56, 216–222.
- Tanhaş, E.; Martin, E.; Korucu, E.N.; Dirmenci, T. Effect of aqueous extract, hydrosol, and essential oil forms of some endemic Origanum L. (Lamiaceae) taxa on polyphenol oxidase activity in fresh-cut mushroom samples. J. Food Process. Preserv. 2020, 44, e14726.
- Cabezas-Serrano, A.B.; Amodio, M.L.; Cornacchia, R.; Rinaldi, R.; Colelli, G. Suitability of five different potato cultivars (Solanum tuberosum L.) to be processed as fresh-cut products. Postharvest Biol. Technol. 2009, 53, 138–144.
- Zhang, Z.; Yao, Y.; Shi, Q.; Zhao, J.; Fu, H.; Wang, Y. Effects of radio-frequency-assisted blanching on the polyphenol oxidase, microstructure, physical characteristics, and starch content of potato. LWT 2020, 125, 109357.
- Li, L.; Bai, J.; Wu, M.; Zhao, M.; Wang, R.; Guo, M.; Liu, H.; Liu, T. Studies on browning inhibition technology and mechanisms of fresh-cut potato. J. Food Process. Preserv. 2017, 41, 1–8.
- Li, J.; Wang, H.; Lu, Y.; Mao, T.F.; Xiong, J.; He, S.L.; Liu, H. Inhibitory effect of tartary buckwheat seedling extracts and associated flavonoid compounds on the polyphenol oxidase activity in potatoes (Solanum tuberosum L.). J. Integr. Agric. 2019, 18, 2173–2182.
- Preczenhak, A.P.; Orsi, B.; Lima, G.P.P.; Tezotto-Uliana, J.V.; Minatel, I.O.; Kluge, R.A. Cysteine enhances the content of betalains and polyphenols in fresh-cut red beet. Food Chem. 2019, 286, 600–607.
- Latorre, M.E.; Narvaiz, P.; Rojas, A.M.; Gerschenson, L.N. Effects of gamma irradiation on bio-chemical and physico-chemical parameters of fresh-cut red beet (Beta vulgaris L. var. conditiva) root. J. Food Eng. 2010, 98, 178–191.
- Barbagallo, R.N.; Chisari, M.; Patané, C. Polyphenol oxidase, total phenolics and ascorbic acid changes during storage of minimally processed “California Wonder” and “Quadrato d’Asti” sweet peppers. LWT Food Sci. Technol. 2012, 49, 192–196.
- Chung, H.S.; Moon, K.D. Browning characteristics of fresh-cut “Tsugaru” apples as affected by pre-slicing storage atmospheres. Food Chem. 2009, 114, 1433–1437.
- Jang, J.H.; Moon, K.D. Inhibition of polyphenol oxidase and peroxidase activities on fresh-cut apple by simultaneous treatment of ultrasound and ascorbic acid. Food Chem. 2011, 124, 444–449.
- Luo, Y.; Lu, S.; Zhou, B.; Feng, H. Dual effectiveness of sodium chlorite for enzymatic browning inhibition and microbial inactivation on fresh-cut apples. LWT Food Sci. Technol. 2011, 44, 1621–1625.
- Zambrano-Zaragoza, M.L.; Gutiérrez-Cortez, E.; Del Real, A.; González-Reza, R.M.; Galindo-Pérez, M.J.; Quintanar-Guerrero, D. Fresh-cut Red Delicious apples coating using tocopherol/mucilage nanoemulsion: Effect of coating on polyphenol oxidase and pectin methylesterase activities. Food Res. Int. 2014, 62, 974–983.
- Villamil-Galindo, E.; Van de Velde, F.; Piagentini, A.M. Extracts from strawberry by-products rich in phenolic compounds reduce the activity of apple polyphenol oxidase. LWT 2020, 133, 110097.
- Bico, S.L.S.; Raposo, M.F.J.; Morais, R.M.S.C.; Morais, A.M.M.B. Combined effects of chemical dip and/or carrageenan coating and/or controlled atmosphere on quality of fresh-cut banana. Food Control 2009, 20, 508–514.
- Teixeira, G.H.A.; Durigan, J.F.; Alves, R.E.; O’Hare, T.J. Use of modified atmosphere to extend shelf life of fresh-cut carambola (Averrhoa carambola L. cv. Fwang Tung). Postharvest Biol. Technol. 2007, 44, 80–85.
- Teixeira, G.H.A.; Durigan, J.F.; Ferraudo, A.S.; Alves, R.E.; O’Hare, T.J. Multivariate analysis of fresh-cut carambola slices stored under different temperatures. Postharvest Biol. Technol. 2012, 63, 91–97.
- Kanjanapongkul, K.; Baibua, V. Effects of ohmic pasteurization of coconut water on polyphenol oxidase and peroxidase inactivation and pink discoloration prevention. J. Food Eng. 2021, 292, 110268.
- Aquino-Bolaños, E.N.; Mercado-Silva, E. Effects of polyphenol oxidase and peroxidase activity, phenolics and lignin content on the browning of cut jicama. Postharvest Biol. Technol. 2004, 33, 275–283.
- Kuwar, U.; Sharma, S.; Tadapaneni, V.R.R. Aloe Vera Gel and Honey-Based Edible Coatings Combined with Chemical Dip as a Safe Means for Quality Maintenance and Shelf Life Extension of Fresh-Cut Papaya. J. Food Qual. 2015, 38, 347–358.
- Koukounaras, A.; Diamantidis, G.; Sfakiotakis, E. The effect of heat treatment on quality retention of fresh-cut peach. Postharvest Biol. Technol. 2008, 48, 30–36.
- Li-Qin, Z.; Jie, Z.; Shu-Hua, Z.; Lai-Hui, G. Inhibition of browning on the surface of peach slices by short-term exposure to nitric oxide and ascorbic acid. Food Chem. 2009, 114, 174–179.
- Serra, S.; Anthony, B.; Masia, A.; Giovannini, D.; Musacchi, S. Determination of Biochemical Composition in Peach by Di ff erent Flesh Color and Textural Typologies. Foods 2020, 9, 1452.
- Xiao, C.; Zhu, L.; Luo, W.; Song, X.; Deng, Y. Combined action of pure oxygen pretreatment and chitosan coating incorporated with rosemary extracts on the quality of fresh-cut pears. Food Chem. 2010, 121, 1003–1009.
- Xiao, Z.; Luo, Y.; Luo, Y.; Wang, Q. Combined effects of sodium chlorite dip treatment and chitosan coatings on the quality of fresh-cut d’Anjou pears. Postharvest Biol. Technol. 2011, 62, 319–326.
- Moreno, J.; Chiralt, A.; Escriche, I.; Serra, J.A. Effect of blanching/osmotic dehydration combined methods on quality and stability of minimally processed strawberries. Food Res. Int. 2000, 33, 609–616.
- Marques Silva, F.V.; Sulaiman, A. Polyphenoloxidase in Fruit and Vegetables: Inactivation by Thermal and Non-thermal Processes; Melton, L., Shahidi, F., Varelis, P.B.T.-E., Eds.; Academic Press: Oxford, UK, 2019; pp. 287–301. ISBN 978-0-12-814045-1.
- Mostafidi, M.; Sanjabi, M.R.; Shirkhan, F.; Zahedi, M.T. A review of recent trends in the development of the microbial safety of fruits and vegetables. Trends Food Sci. Technol. 2020, 103, 321–332.
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 2017, 64, 23–38.
- Ukuku, D.O.; Geveke, D.J.; Chau, L.; Niemira, B.A. Microbial safety and overall quality of cantaloupe fresh-cut pieces prepared from whole fruit after wet steam treatment. Int. J. Food Microbiol. 2016, 231, 86–92.
- Putnik, P.; Roohinejad, S.; Greiner, R.; Granato, D.; Bekhit, A.E.D.A.; Bursać Kovačević, D. Prediction and modeling of microbial growth in minimally processed fresh-cut apples packaged in a modified atmosphere: A review. Food Control 2017, 80, 411–419.
- Giannakourou, M.C.; Tsironi, T.N. Application of Processing and Packaging Hurdles for Fresh-Cut Fruits and Vegetables Preservation. Foods 2021, 10, 830.
- Maringgal, B.; Hashim, N.; Mohamed Amin Tawakkal, I.S.; Muda Mohamed, M.T. Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality. Trends Food Sci. Technol. 2020, 96, 253–267.
- Zhang, L.; Lu, Z.; Yu, Z.; Gao, X. Preservation of fresh-cut celery by treatment of ozonated water. Food Control 2005, 16, 279–283.
- Gómez-López, V.M.; Ragaert, P.; Ryckeboer, J.; Jeyachchandran, V.; Debevere, J.; Devlieghere, F. Shelf-life of minimally processed cabbage treated with neutral electrolysed oxidising water and stored under equilibrium modified atmosphere. Int. J. Food Microbiol. 2007, 117, 91–98.
- Tinello, F.; Lante, A. Recent advances in controlling polyphenol oxidase activity of fruit and vegetable products. Innov. Food Sci. Emerg. Technol. 2018, 50, 73–83.
- Abbott, J.A.; Saftner, R.A.; Gross, K.C.; Vinyard, B.T.; Janick, J. Consumer evaluation and quality measurement of fresh-cut slices of “Fuji,” “Golden Delicious,” “GoldRush,” and “Granny Smith” apples. Postharvest Biol. Technol. 2004, 33, 127–140.
- Peng, L.; Jiang, Y. Exogenous salicylic acid inhibits browning of fresh-cut Chinese water chestnut. Food Chem. 2006, 94, 535–540.
- Zhou, D.; Li, L.; Wu, Y.; Fan, J.; Ouyang, J. Salicylic acid inhibits enzymatic browning of fresh-cut Chinese chestnut (Castanea mollissima) by competitively inhibiting polyphenol oxidase. Food Chem. 2015, 171, 19–25.




