Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gabriel G. Malouf | + 1536 word(s) | 1536 | 2021-09-01 08:30:51 | | | |
| 2 | Jessie Wu | Meta information modification | 1536 | 2021-09-10 10:48:17 | | | | |
| 3 | Felix Wu | Meta information modification | 1536 | 2021-11-02 04:15:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Malouf, G.G. Efficacy of Immune Checkpoint Inhibitors. Encyclopedia. Available online: https://encyclopedia.pub/entry/14067 (accessed on 05 March 2026).
Malouf GG. Efficacy of Immune Checkpoint Inhibitors. Encyclopedia. Available at: https://encyclopedia.pub/entry/14067. Accessed March 05, 2026.
Malouf, Gabriel G.. "Efficacy of Immune Checkpoint Inhibitors" Encyclopedia, https://encyclopedia.pub/entry/14067 (accessed March 05, 2026).
Malouf, G.G. (2021, September 10). Efficacy of Immune Checkpoint Inhibitors. In Encyclopedia. https://encyclopedia.pub/entry/14067
Malouf, Gabriel G.. "Efficacy of Immune Checkpoint Inhibitors." Encyclopedia. Web. 10 September, 2021.
Copy Citation
Upper tract urothelial carcinoma (UTUC) represents 5 to 10% of urothelial carcinoma. Their mutational profile is different as compared to bladder urothelial carcinoma (UC). While immune checkpoint inhibitors are now part of the therapeutic landscape of urothelial carcinoma, data concerning their use in UTUC patient’s treatment remain scarce. We reviewed the latest molecular characterization data and proposed an insight for future therapeutic strategies based on molecular alteration profiles.
immune checkpoint inhibitors
UTUC
1. Introduction
Urothelial carcinoma (UC) represents the fourth most common malignancy worldwide, with an urgent need for tailored approaches in the management of the metastatic disease [1]. Depending on the level of muscle invasion seen on the pathological exam, UC is divided into muscle-invasive (MI) and non-muscle invasive (NMI) disease. MIUC of the bladder represents 25% of tumors [2] as compared to 60% in upper tract urothelial carcinoma (UTUC), explaining their increased aggressiveness [3][4][5][6]. The 5-year extravesical recurrence and overall survival rates are 28% and 23% for UTUC and bladder UC, respectively [7]. While bladder origin represents 90–95% of UCs, UTUC is less common. It represents 5–10% of UCs and can arise within the renal pelvis or ureter, which are derived from a different embryologic origin as compared to the bladder [1][8].
There is a strong relationship between UC of the bladder and UTUC since approximately 50% of patients with UTUC will have urinary bladder urothelial carcinomas either at presentation or subsequently, justifying the need to perform annual cystoscopy in the follow-up of these patients [1].
For high-risk localized disease, nephroureterectomy along with peri-operative chemotherapy is the standard of care management approach [1]. In the metastatic setting, platinum-based chemotherapy regimen remains the first-line recommended treatment [1]. However, there is a growing body of evidence concerning the use of immune checkpoint inhibitors (ICI) in the treatment of urothelial carcinoma [1][9] with the approval of several compounds in the first and second-line settings of advanced UCs. However, given their rarity, patients with UTUC represent a minority of patients included in clinical trials, and there is a paucity of data concerning ICI use in this setting.
UTUC has a different behavior as compared to bladder UC [10], and while molecular alterations of urothelial bladder carcinoma have been widely studied by The Cancer Genome Atlas, data about such alterations in UTUC remain scarce [4][11][12]. However, the novel molecular insights provided by these studies led to a better understanding of this aggressive disease and provided a rationale for new therapeutic approaches.
This review summarizes the available literature regarding the use of ICIs and the biological rationale underlying their use in high-grade urothelial upper tract carcinoma management.
2. Immune Checkpoint Inhibition in UTUC
Given their relative rarity, there are no studies specifically focusing on UTUC. Therefore, data related to ICI efficacy are extracted from a larger cohort of patients with UC that included a small subgroup of UTUC.
2.1. Immune Checkpoint Inhibitors in the Perioperative Setting
In the adjuvant setting, ICI-based therapy has been widely tested since the role of adjuvant treatment in high-risk muscle-invasive urothelial carcinoma after radical surgery was not clear.
Since the first promising results of adjuvant pembrolizumab in the management of UC [13][14], several studies have now included UTUC patients in ICI-based adjuvant treatment (Table 1).
Table 1. Adjuvant and neoadjuvant systemic treatment for UTUC patients.
| Trial | Drug | Study Design | Line | Overall pts n, UTUC pts n. (%) | Outcomes (Primary Endpoint) |
|---|---|---|---|---|---|
| IMvigor 010 [15] | Atezolizumab | Phase 3 RCT | Adjuvant | 809; 54 (6.7%) | Median disease-free survival, 19.4 months (95% CI 15.9–24.8) |
| Checkmate 274 [16] | Nivolumab | Phase 3 RCT | Adjuvant | 709; 149 (21%) | Median disease-free survival 20.8 months (95% confidence interval [CI], 16.5 to 27.6) |
| NCT02690558 [17] | Cisplatin, gemcitabine, pembrolizumab | Phase 2 | Neoadjuvant | 39; na | pCR:36% |
| POUT [18] | Cisplatin or carboplatin + gemcitabine | Phase 3 RCT | Adjuvant | 261 | Disease-free survival (hazard ratio 0.45, 95% CI 0.30–0.68; p = 0.0001) |
Abbreviations: RCT: randomized controlled trial, pts: patients; na: non available; pCR: pathologic complete response.
The IMvigor 010 study enrolled 809 high-risk UC patients to be randomized between adjuvant atezolizumab versus placebo. There were only 7% of UTUC patients in the atezolizumab arm as compared to 6% in the placebo arm (Table 1). There was no statistical difference in terms of median disease-free survival, 19.4 months (95% CI 15.9–24.8) with atezolizumab and 16.6 months (11.2–24.8) with observation (stratified hazard ratio 0.89 [95% CI 0.74–1.08]; p = 0.24) [15]. More recently, the data of the Checkmate 274 trial were reported. It was a phase 3 trial, including 709 patients randomized between adjuvant nivolumab versus placebo. A total of 21% of enrolled patients were patients with UTUC (Table 1). However, based on the results of the POUT trial, the inclusion of UTUC patients was prematurely interrupted. In the intention-to-treat population, median disease-free survival was 20.8 months (95% CI, 16.5 to 27.6) with nivolumab and 10.8 months (95% CI, 8.3 to 13.9) with placebo; (HR, 0.70; 98.22% CI, 0.55 to 0.90; p < 0.001). For the UTUC subgroup, the HR for disease recurrence or death were 1.23 (CI 95% 0.67–2.23) and 1.56 (CI 95% 0.7–3.48) for UTUC arising in renal pelvis and ureter, respectively. The percentage of patients was 74.5% and 55.7%, respectively (hazard ratio, 0.55; 98.72% CI, 0.35 to 0.85; p < 0.001), for those expressing PD-L1 more than 1% [16]. Moreover, several neoadjuvant trials combining chemotherapy with ICI are actively recruiting, but available data are currently limited [17][19][20].
2.2. Immune Checkpoint Inhibitors in the Metastatic Setting
In the metastatic setting, ICI are widely used in the management of UC (Table 2). Indeed, avelumab as maintenance therapy after platinum-based chemotherapy is currently the standard of care according to the results of the JAVELIN-100 trial [21]. For cisplatin-ineligible UC patients, based on phase 2 trials IMvigor 210 and KEYNOTE 052 provided interesting results for the use of ICI in this frail population (Table 2) [22][23]. The overall response rate for UTUC patients was 39% with atezolizumab [22] and 22% with pembrolizumab in monotherapy in this setting [23].
Table 2. Studies assessing ICI in patients with locally advanced or metastatic UC (only trials reporting data of UTUC patients were selected).
| Trial | Drug/Control Arm | Study Design |
Line | Overall pts n, UTUC pts n. (%) | Outcomes (Primary Endpoint) |
|---|---|---|---|---|---|
| JAVELIN-100 [21] | Avelumab/BSC | Phase 3 RCT | 1L | 700, 187 (27%) | median OS: 21.4 months vs. 14.3 months; hazard ratio for death, 0.69; 95% confidence interval [CI], 0.56 to 0.86; p = 0.001 |
| KEYNOTE 052 [23] | Pembrolizumab | Phase 2 | 1L | 370, 69 (19%) | ORR: 24%, 95% CI 20–29) |
| IMvigor 130 [24] | Atezolizumab + platinum-based chemotherapy (A)/Atezolizumab (B)/Platinum-based chemotherapy | Phase 3 RCT | 1L | 1213, 312 (26%) | median PFS: 8.2 months (95% CI 6.5–8.3) in group A and 6.3 months (6.2–7.0) in group C (stratified hazard ratio [HR] 0.82, 95% CI 0.70–0.96; one-sided p = 0.007). median OS: 16.0 months (13.9–18.9) in group A and 13.4 months (12.0–15.2) in group C (0.83, 0.69–1.00; one-sided p = 0.027). Median overall survival was 15.7 months (13.1–17.8) for group B and 13.1 months (11.7–15.1) for group C (1.02, 0.83–1.24) |
| KEYNOTE 361 [25] | Cisplatin or Carboplatin + Gemcitabine + Pembrolizumab/Pembrolizumab/Cisplatin or Carboplatin + Gemcitabine | Phase 3 RCT | 1L | 1010, 211 (21%) | median OS: 17·0 months (14.5–19.5) in the pembrolizumab plus chemotherapy group versus 14.3 months (12.3–16.7) in the chemotherapy group (0.86, 0.72–1.02; p = 0.0407) median PFS: 8.3 months (95% CI 7.5–8.5) in the pembrolizumab plus chemotherapy group versus 7.1 months (6.4–7.9) in the chemotherapy group (hazard ratio [HR] 0.78, 95% CI 0.65–0.93; p = 0.0033) |
| KEYNOTE-045 [26] | Pembrolizumab/Paclitaxel or Docetaxel or Vinflunine | Phase 3 RCT | 2L | 748, 75 (10%) | median OS: 10.3 months (95% CI 8.0 to 11.8) vs. 7.4 months (95% CI, 6.1 to 8.3) (hazard ratio for death, 0.73; 95% CI, 0.59 to 0.91; p = 0.002) median PFS: 2.1 months (95% CI, 2.0 to 2.2) vs. 3.3 months (95% CI, 2.3 to 3.5) (HR 0.98; 95% CI, 0.81 to 1.19; p = 0.42) |
| IMvigor 211 [27] | Atezolizumab/Paclitaxel or Docetaxel or Vinflunine | Phase 3 RCT | 2L | 931, 236 (25%) | median OS: 11.1 (95% CI 8.6–15.5) vs. 10.6 months (95% CI 8.4–12.2) p = 0.41 |
| IMvigor 210 [22] | Atezolizumab | Phase 2 | 2L | 119, 33 (28%) | ORR: 23% (95% CI 16–31) |
Abbreviations: BSC: best supportive care; RCT: randomized controlled trial; OS: overall survival; PFS: progression free survival; ORR: objective response rate.
The IMvigor 130 trial, a randomized phase 3 trial, showed significant PFS improvement of the addition of atezolizumab to platinum-based chemotherapy [24] (Table 2). Specific outcomes of UTUC patients were not assessed. However, unfortunately, atezolizumab in monotherapy failed to improve overall survival compared to chemotherapy in pretreated metastatic UC [27]. In the same manner, the addition of pembrolizumab to first-line platinum-based chemotherapy was not associated with a survival benefit compared to chemotherapy alone (Table 2) [25].
In the second or later line, atezolizumab, durvalumab, avelumab, nivolumab, and pembrolizumab have been demonstrated safe and efficient in platinum pretreated UC population. However, UTUC patients’ data remained scarce (Table 2).
3. Perspectives in UTUC Management
According to the recent advances in the molecular characterization of UTUC, there is a rationale to develop new treatment combinations. Indeed, given the high prevalence of FGFR3 mutations and their association with a T-cell depleted phenotype in UTUC, there is a rationale for combining ICI with FGFR3 inhibitors (Figure 1). Erdafitinib, a pan-FGFR inhibitor, is now approved based on the results of the phase 2 trial in metastatic bladder cancer, with a 40% of response rate in patients with FGFR actionable alterations [28]. Moreover, Ding et al. reported the case of a 67 years old metastatic, chemo-refractory UTUC’s patient having a dramatic response to pembrolizumab in association with erdafitinib [29]. However, reliable response biomarkers are still needed to improve precision medicine in urothelial carcinoma. The ongoing trials assessing immune checkpoint inhibitors-based combinations therapies in UTUC metastatic setting are reported in Table 3. They often include backbone ICI in combination with chemotherapy, antibody-drug conjugates, and tyrosine kinase inhibitors.
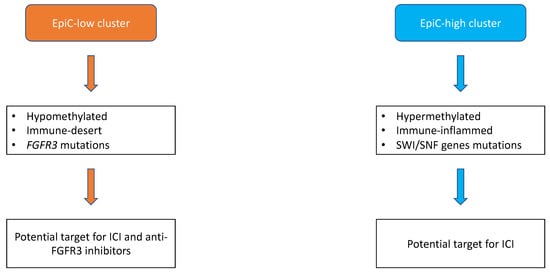
Figure 1. Proposal of molecular subtypes classification of upper-tract urothelial carcinomas adapted from Su et al. [12]. Broadly, upper-tract urothelial carcinomas can be divided into two subtypes, namely EpiC-high and EpiC-low. Epic-low subtype is hypomethylated, immune-desert, and characterized by FGFR3 somatic mutations with potential efficacy of the combination of FGFR3 immunotherapy and immune checkpoint inhibitors (ICI). Conversely, EpiC-high subtype is hypermethylated, immune-inflamed, and enriched with somatic mutations of SWI/SNF genes with potential benefit for ICI.
Table 3. Ongoing trials assessing immune checkpoint inhibitors-based combinations therapies in the metastatic setting.
| Trial Identification |
Drugs | Comparative Arm | Administration | Study Design | Line | Primary Endpoint |
|---|---|---|---|---|---|---|
| NCT03513952 | Atezolizumab/CYT107 | Atezolizumab | IV | Phase 2 | ≥2 | ORR |
| NCT03237780 | Atezolizumab/eribulin | Eribulin | IV | Phase 2 | >2 | ORR |
| NCT02496208 | Cabozantinib/Nivolumab ± Ipilimumab | NA | PO/IV | Phase 1 | >1 | RP2D/safety |
| NCT04940299 | Tocilizumab/Ipilimumab/Nivolumab | NA | IV | Phase 2 | 1 | Safety/DLT |
| NCT03606174 | Sitravatinib/Nivolumab and Sitravatinib/Pembrolizumab/Enfortumab vedotin | NA | PO/IV and PO/IV/IV | Phase 2 | 1, ≥2 | ORR |
| NCT04602078 | Atezolizumab/Gemcitabine/Cisplatin | NA | IV | Phase 2 | 1 | ORR |
References
- Rouprêt, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Mostafid, A.H.; et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur. Urol. 2020, 79, 62–79.
- Sylvester, R.J.; Rodríguez, O.; Hernández, V.; Turturica, D.; Bauerová, L.; Bruins, H.M.; Bründl, J.; van der Kwast, T.H.; Brisuda, A.; Rubio-Briones, J.; et al. European association of urology (EAU) prognostic factor risk groups for non–muscle-invasive bladder cancer (NMIBC) incorporating the WHO 2004/2016 and WHO 1973 classification systems for grade: An update from the EAU NMIBC guidelines panel. Eur. Urol. 2021, 79, 480–488.
- Bersanelli, M.; Buti, S.; Giannatempo, P.; Raggi, D.; Necchi, A.; Leonetti, A.; Banna, G.L.; Petrelli, F. Outcome of patients with advanced upper tract urothelial carcinoma treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Crit. Rev. Oncol. 2021, 159, 103241.
- Moss, T.J.; Qi, Y.; Xi, L.; Peng, B.; Kim, T.-B.; Ezzedine, N.E.; Mosqueda, M.E.; Guo, C.C.; Czerniak, B.A.; Ittmann, M.; et al. Comprehensive genomic characterization of upper tract urothelial carcinoma. Eur. Urol. 2017, 72, 641–649.
- Sfakianos, J.P.; Cha, E.K.; Iyer, G.; Scott, S.N.; Zabor, E.C.; Shah, R.; Ren, Q.; Bagrodia, A.; Kim, P.H.; Hakimi, A.A.; et al. Genomic characterization of upper tract urothelial carcinoma. Eur. Urol. 2015, 68, 970–977.
- Lee, J.Y.; Kim, K.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; Choi, H.-Y.; Kwon, G.-Y.; et al. Molecular characterization of urothelial carcinoma of the bladder and upper urinary tract. Transl. Oncol. 2017, 11, 37–42.
- Margulis, V.; Shariat, S.F.; Matin, S.F.; Kamat, A.M.; Zigeuner, R.; Kikuchi, E.; Lotan, Y.; Weizer, A.; Raman, J.; Wood, C.G.; et al. Outcomes of radical nephroureterectomy: A series from the upper tract urothelial carcinoma collaboration. Cancer 2009, 115, 1224–1233.
- Leow, J.J.; Chong, K.T.; Chang, S.L.; Bellmunt, J. Upper tract urothelial carcinoma: A different disease entity in terms of management. ESMO Open 2016, 1, e000126.
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Espinós, E.L.; Lorch, A.; Neuzillet, Y.; et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2020 guidelines. Eur. Urol. 2020, 79, 82–104.
- Green, D.A.; Rink, M.; Xylinas, E.; Matin, S.F.; Stenzl, A.; Roupret, M.; Karakiewicz, P.I.; Scherr, D.; Shariat, S.F. Urothelial carcinoma of the bladder and the upper tract: Disparate twins. J. Urol. 2012, 189, 1214–1221.
- Robinson, B.D.; Vlachostergios, P.; Bhinder, B.; Liu, W.; Li, K.; Moss, T.J.; Bareja, R.; Park, K.; Tavassoli, P.; Cyrta, J.; et al. Upper tract urothelial carcinoma has a luminal-papillary T-cell depleted contexture and activated FGFR3 signaling. Nat. Commun. 2019, 10, 1–11.
- Su, X.; Lu, X.; Bazai, S.K.; Compérat, E.; Mouawad, R.; Yao, H.; Rouprêt, M.; Spano, J.-P.; Khayat, D.; Davidson, I.; et al. Comprehensive integrative profiling of upper tract urothelial carcinomas. Genome Biol. 2021, 22, 1–25.
- Necchi, A.; Anichini, A.; Raggi, D.; Briganti, A.; Massa, S.; Lucianò, R.; Colecchia, M.; Giannatempo, P.; Mortarini, R.; Bianchi, M.; et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): An open-label, single-arm, phase II study. J. Clin. Oncol. 2018, 36, 3353–3360.
- Necchi, A.; Raggi, D.; Gallina, A.; Madison, R.; Colecchia, M.; Lucianò, R.; Montironi, R.; Giannatempo, P.; Farè, E.; Pederzoli, F.; et al. Updated results of PURE-01 with preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma with variant histologies. Eur. Urol. 2020, 77, 439–446.
- Bellmunt, J.; Hussain, M.; Gschwend, J.E.; Albers, P.; Oudard, S.; Castellano, D.; Daneshmand, S.; Nishiyama, H.; Majchrowicz, M.; Degaonkar, V.; et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 525–537.
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114.
- Rose, T.L.; Harrison, M.R.; Deal, A.M.; Osterman, C.K.; Ramalingam, S.; Whang, Y.E.; Brower, B.Y.; Bjurlin, M.; Smith, A.B.; Nielsen, M.E.; et al. Phase II study of gemcitabine and split-dose cisplatin plus pembrolizumab as neoadjuvant therapy prior to radical cystectomy (RC) in patients with muscle-invasive bladder cancer (MIBC). J. Clin. Oncol. 2021, 39, 396.
- Birtle, A.; Johnson, M.; Chester, J.; Jones, R.; Dolling, D.; Bryan, R.; Harris, C.; Winterbottom, A.; Blacker, A.; Catto, J.W.F.; et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomised controlled trial. Lancet 2020, 395, 1268–1277.
- Lopez-Beltran, A.; Cimadamore, A.; Blanca, A.; Massari, F.; Vau, N.; Scarpelli, M.; Cheng, L.; Montironi, R. Immune checkpoint inhibitors for the treatment of bladder cancer. Cancers 2021, 13, 131.
- Califano, G.; Ouzaid, I.; Verze, P.; Hermieu, J.-F.; Mirone, V.; Xylinas, E. Immune checkpoint inhibition in upper tract urothelial carcinoma. World J. Urol. 2020, 39, 1357–1367.
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230.
- Balar, A.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2016, 389, 67–76.
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492.
- Galsky, M.D.; Arija, J.A.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-Del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izumi, K.; et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1547–1557.
- Powles, T.; Csőszi, T.; Özgüroğlu, M.; Matsubara, N.; Géczi, L.; Cheng, S.Y.-S.; Fradet, Y.; Oudard, S.; Vulsteke, C.; Barrera, R.M.; et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 931–945.
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026.
- Powles, T.; Durán, I.; van der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757.
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 2019, 381, 338–348.
- Ding, X.; Zong, J.; Li, X.; Bai, X.; Tan, B.; Sun, W.; Wang, R.; Ding, Y. Dramatic responses of recurrent upper urinary tract urothelial carcinoma harboring FGFR3 and TP53 activating mutations to pembrolizumab in combination with erdafitinib: A case report. OncoTargets Ther. 2021, 14, 2177–2183.
More
Information
Subjects:
Urology & Nephrology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
767
Revisions:
3 times
(View History)
Update Date:
02 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





