
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mandla Chabalala | + 3498 word(s) | 3498 | 2021-09-08 04:11:25 | | | |
| 2 | Peter Tang | Meta information modification | 3498 | 2021-09-10 03:55:42 | | |
Video Upload Options
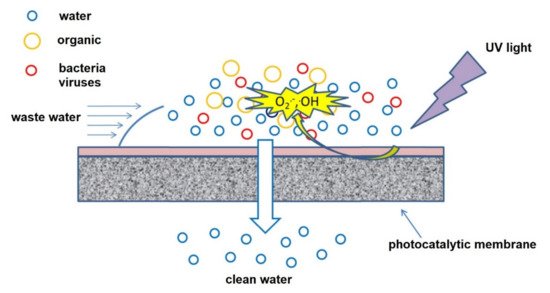
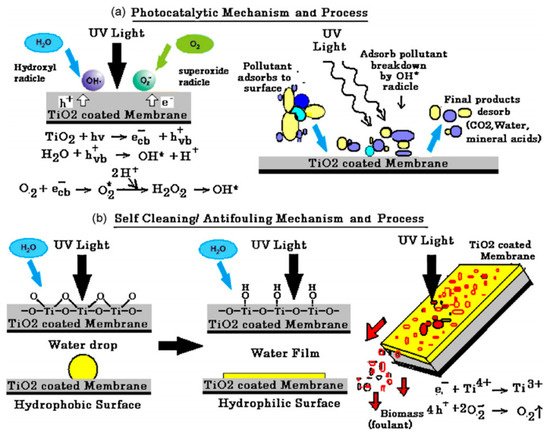
Photocatalytic nanofiber membranes are nanofiber membranes infused with photocatalytic nanoparticles. The performance of photocatalytic membranes is attributed to the photogenerated reactive oxygen species such as hydroxyl radicals, singlet oxygen, and superoxide anion radicals produced from reactions with photogenerated electrons and holes introduced by catalytic nanoparticles such as TiO2 and ZnO upon light irradiation. Hydroxyl radicals are the most reactive species responsible for most of the unselective photodegradation of unwanted pollutants.
1. Introduction
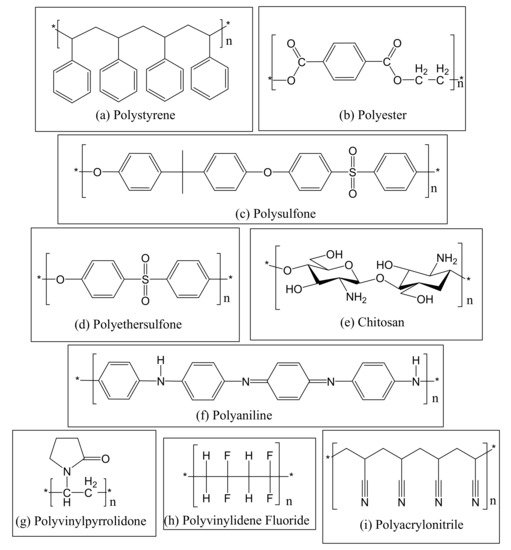
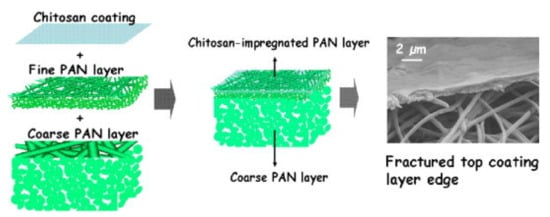
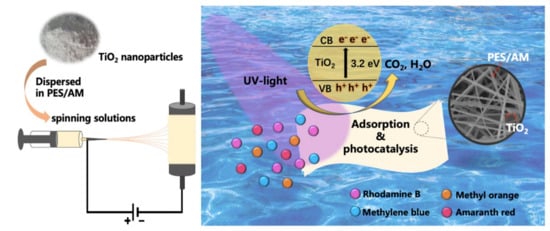
2. Photocatalytic Electrospun Nanofiber Membranes
3. Antimicrobial Membranes
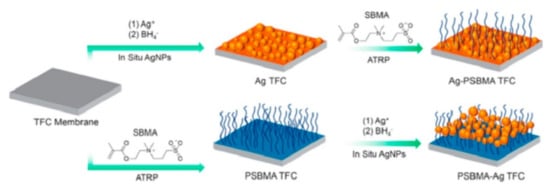
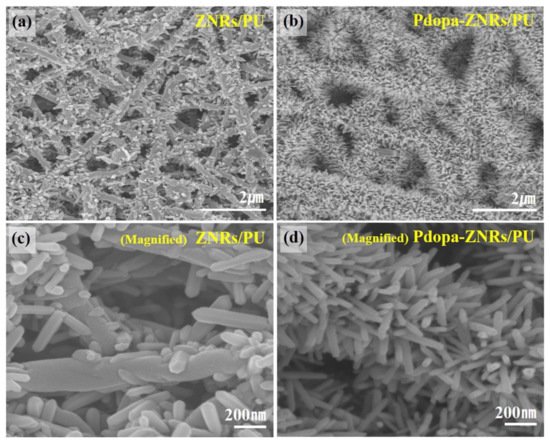
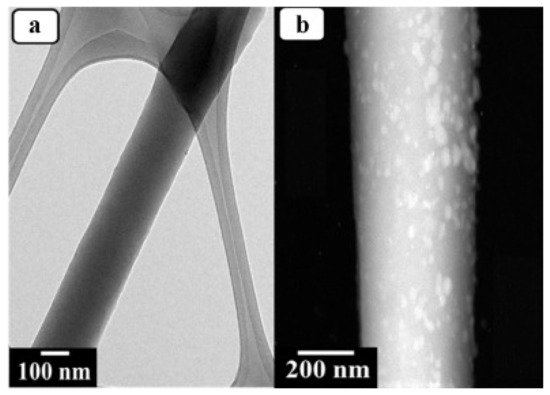
|
Polymer |
Antimicrobial Agent |
Method |
Application |
Antimicrobial Activity |
Ref. |
|---|---|---|---|---|---|
|
Polyurethane |
Polydopamine-ZnO |
Electrospinning |
Antimicrobial (E. coli) Photodegradation (Methylene blue) |
Active |
[61] |
|
Polyacrylonitrile |
Ag3PO4 |
Electrospinning |
Antimicrobial (E. coli and S. aureus) Photodegradation (Methylene blue) |
Active |
[62] |
|
Polyacrylonitrile |
Ag nanoparticles |
Electrospinning |
Antimicrobial (E. coli and S. aureus) |
Active |
[69] |
|
Polyacrylonitrile |
Ag nanoparticles |
Electrospinning |
Antimicrobial (E. coli and S. aureus) |
Active |
[70] |
|
Polyacrylonitrile |
Ag nanoparticles |
Electrospinning |
Antimicrobial (E. coli and S. aureus) Forward osmosis |
Active |
[71] |
|
Chitosan |
Ag nanoparticles |
Centrifugal spinning |
Antimicrobial (S. aureus) Wound healing |
Active |
[72] |
|
Polysulfone |
CNT/Ag |
Radical solution polymerization and wet-phase inversion |
Antimicrobial (E. coli and B. subtilis) |
Active |
[73] |
|
3D woven fabric filters |
Ag nanoparticles |
Electrospinning |
Antimicrobial (S. aureus) Water treatment |
Active |
[74] |
|
Polyvinyl alcohol |
Polyimide-Ag |
Electrospinning |
Antimicrobial (E. coli and S. aureus) Oily wastewater treatment |
Active |
[75] |
|
Polyacrylonitrile |
CuO |
Electrospinning |
Antimicrobial (E. coli and B. subtilis) for breath masks Drug release |
Active |
[76] |
|
Chitosan/poly(ethylene oxide) |
Poly(hexamethylene biguanide) hydrochloride |
Electrospinning |
Antimicrobial (E. coli and S. aureus) |
Active |
[77] |
|
Poly(ε-caprolactone) and gelatine |
Octadecyldimethyl[3 -(trimethoxysilyl)propyl]ammonium chloride |
Electrospinning |
Antimicrobial (S. aureus and P. aeruginosa) Wound dressing |
Active |
[78] |
|
Polylactic acid |
Fe3O4-COOH |
Electrospinning |
Antimicrobial (E. coli and S. aureus) Drug delivery |
Active |
[79] |
|
Triaxial |
Nisin |
Electrospinning |
Antimicrobial (S. aureus) |
Active |
[80] |
|
Cellulose acetate/polyester urethane |
Polyhexamethylene biguanide |
Electrospinning |
Antimicrobial (E. coli) Cytotoxicity Wound healing |
Active |
[81] |
|
Polycaprolactone/gelatine |
Metronidazole |
Electrospinning |
Antimicrobial (F. nucleatum) Cytotoxicity (L929 Cells) Drug delivery |
Active |
[82] |
|
Silk fibroin |
Peptide motif |
Electrospinning |
Antimicrobial (S. aureus, E. coli, S. epidermidis and P. aeruginosa) Wound dressing |
Active |
[83] |
|
Polycaprolactone |
2-(Methacryloyloxy) ethyl trimethylammonium/polycaprolactone |
Cross-linking polymerization and electrospinning |
Antimicrobial (E. coli and S. aureus) Wound dressing |
Active |
[84] |
|
Nylon 6 |
N-Halamine |
Electrospinning |
Antimicrobial (E. coli and S. aureus) |
Active |
[85] |
|
Polycaprolactone |
Peptide dissolved micro needles |
Coaxial electrospinning and electrospray deposition |
Antimicrobial (S. aureus, K. pneumoniae, A. baumannii, and P. aeruginosa) Chronic wound dressing |
Active |
[86] |
4. Other Applications of Hybrid Photocatalytic Membrane Processes
References
- Lipczynska-kochany, E. Humic substances, their microbial interactions and effects on biological transformations of organic pollutants in water and soil: A review. Chemosphere 2018, 202, 420–437.
- Mart, C.A.; Rodrigo, M.A.; Sire, I.; Scialdone, O. Single and Coupled Electrochemical Processes and Reactors for the Abatement of Organic Water Pollutants: A Critical Review. Chem. Rev. 2015, 115, 13362–13407.
- Bi, O.; Charles, L.; Casado, C.; Marugán, J.; Septien, S.; Ndlovu, T.; Nsikayezwe, L. Photocatalytic degradation of atrazine in aqueous solution using hyperbranched polyethyleneimine templated morphologies of BiVO4 fused. J. Environ. Chem. Eng. 2020, 8, 104215.
- Nieto-delgado, C.; Partida-gutierrez, D.; Rangel-mendez, J.R. Preparation of activated carbon cloths from renewable natural fabrics and their performance during the adsorption of model organic and inorganic pollutants in water. J. Clean. Prod. 2019, 213, 650–658.
- Cai, W.; Yu, J.; Jaroniec, M. Template-free synthesis of hierarchical spindle-like g-Al2O3 materials and their adsorption affinity towards organic and inorganic pollutants in water. J. Mater. Chem. 2010, 20, 4587–4594.
- Rivas, B.L.; Urbano, B.F.; Sánchez, J. Water-Soluble and Insoluble Polymers, Nanoparticles, Nanocomposites and Hybrids With Ability to Remove Hazardous Inorganic Pollutants in Water. Front. Chem. 2018, 6, 1–13.
- Naidoo, S.; Olaniran, A.O. Treated wastewater effluent as a source of microbial pollution of surface water resources. Int. J. Environ. Res. Public Health 2014, 11, 249–270.
- Elliott, M. Biological pollutants and biological pollution—An increasing cause for concern. Mar. Pollut. Bull. 2003, 46, 275–280.
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135.
- Tisa, F.; Raman, A.A.A.; Wan Daud, W.M.A. Applicability of fluidized bed reactor in recalcitrant compound degradation through advanced oxidation processes: A review. J. Environ. Manag. 2014, 146, 260–275.
- Chabalala, M.B.; Seshabela, B.C.; Van Hulle, S.W.H.; Mamba, B.B.; Mhlanga, S.D.; Nxumalo, E.N. Cyclodextrin-Based Nanofibers and Membranes: Fabrication, Properties and Applications. In Cyclodextrin: A Versatile Ingredient; IntechOpen: London, UK, 2018; p. 29. ISBN 978-953-51-0246-5.
- Lin, T.; Wang, H.; Wang, H.; Wang, X. The charge effect of cationic surfactants on the elimination of fibre beads in the electrospinning of polystyrene. Nanotechnology 2004, 15, 1375–1381.
- Kamiyama, M.; Soeda, T.; Nagajima, S.; Tanaka, K. Development and application of high-strength polyester nanofibers. Polym. J. 2012, 44, 987–994.
- Fan, Z.; Wang, Z.; Sun, N.; Wang, J.; Wang, S. Performance improvement of polysulfone ultrafiltration membrane by blending with polyaniline nanofibers. J. Memb. Sci. 2008, 320, 363–371.
- Liao, K.; Li, S. Interfacial characteristics of a carbon nanotube-polystyrene composite system. Appl. Phys. Lett. 2001, 79, 4225–4227.
- An, H.; Shin, C.; Chase, G.G. Ion exchanger using electrospun polystyrene nanofibers. J. Memb. Sci. 2006, 283, 84–87.
- Lu, X.; Zhou, J.; Zhao, Y.; Qiu, Y.; Li, J. Room temperature ionic liquid based polystyrene nanofibers with superhydrophobicity and conductivity produced by electrospinning. Chem. Mater. 2008, 20, 3420–3424.
- Bordes, P.; Pollet, E.; Avérous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155.
- Dhakal, H.N.; Zhang, Z.Y.; Richardson, M.O.W. Effect of water absorption on the mechanical properties of hemp fibre reinforced unsaturated polyester composites. Compos. Sci. Technol. 2007, 67, 1674–1683.
- Mishra, S.; Mohanty, A.K.; Drzal, L.T.; Misra, M.; Parija, S.; Nayak, S.K.; Tripathy, S.S. Studies on mechanical performance of biofibre/glass reinforced polyester hybrid composites. Compos. Sci. Technol. 2003, 63, 1377–1385.
- Gopal, R.; Kaur, S.; Feng, C.Y.; Chan, C.; Ramakrishna, S.; Tabe, S.; Matsuura, T. Electrospun nanofibrous polysulfone membranes as pre-filters: Particulate removal. J. Memb. Sci. 2007, 289, 210–219.
- Babaeijandaghi, F.; Shabani, I.; Seyedjafari, E.; Naraghi, Z.S.; Vasei, M.; Haddadi-Asl, V.; Hesari, K.K.; Soleimani, M. Accelerated epidermal regeneration and improved dermal reconstruction achieved by polyethersulfone nanofibers. Tissue Eng. Part A 2010, 16, 3527–3536.
- Hu, H.; Huang, X.; Deng, C.; Chen, X.; Qian, Y. Hydrothermal synthesis of ZnO nanowires and nanobelts on a large scale. Mater. Chem. Phys. 2007, 106, 58–62.
- Li, J.; Zhang, Q.; Zhang, Y.; Wei, Q.; Sun, S.; Zhao, C. Polyethersulfone nanofibers for the removal of endocrine disruptors. Desalin. Water Treat. 2012, 29, 158–163.
- Ahmadiannamini, P.; Li, X.; Goyens, W.; Joseph, N.; Meesschaert, B.; Vankelecom, I.F.J. Multilayered polyelectrolyte complex based solvent resistant nanofiltration membranes prepared from weak polyacids. J. Memb. Sci. 2012, 394–395, 98–106.
- Feng, C.; Xu, J.; Li, M.; Tang, Y.; Gao, C. Studies on a novel nanofiltration membrane prepared by cross-linking of polyethyleneimine on polyacrylonitrile substrate. J. Memb. Sci. 2014, 451, 103–110.
- Tripathi, B.P.; Dubey, N.C.; Subair, R.; Choudhury, S.; Stamm, M. Enhanced hydrophilic and antifouling polyacrylonitrile membrane with polydopamine modified silica nanoparticles. RSC Adv. 2016, 6, 4448–4457.
- Wu, Q.Y.; Wan, L.S.; Xu, Z.K. Structure and performance of polyacrylonitrile membranes prepared via thermally induced phase separation. J. Memb. Sci. 2012, 409–410, 355–364.
- Zhi, S.H.; Deng, R.; Xu, J.; Wan, L.S.; Xu, Z.K. Composite membranes from polyacrylonitrile with poly(N,N-dimethylaminoethyl methacrylate)-grafted silica nanoparticles as additives. React. Funct. Polym. 2015, 86, 184–190.
- Arshad, S.N.; Naraghi, M.; Chasiotis, I. Strong carbon nanofibers from electrospun polyacrylonitrile. Carbon 2011, 49, 1710–1719.
- Wang, T.; Kumar, S. Electrospinning of polyacrylonitrile nanofibers. J. Appl. Polym. Sci. 2006, 102, 1023–1029.
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432.
- Ghaee, A.; Shariaty-Niassar, M.; Barzin, J.; Matsuura, T. Effects of chitosan membrane morphology on copper ion adsorption. Chem. Eng. J. 2010, 165, 46–55.
- Miao, Y.E.; Fan, W.; Chen, D.; Liu, T. High-performance supercapacitors based on hollow polyaniline nanofibers by electrospinning. ACS Appl. Mater. Interfaces 2013, 5, 4423–4428.
- Lubasova, D.; Niu, H.; Zhao, X.; Lin, T. Hydrogel properties of electrospun polyvinylpyrrolidone and polyvinylpyrrolidone/poly(acrylic acid) blend nanofibers. RSC Adv. 2015, 5, 54481–54487.
- Vazquez, B.; Vasquez, H.; Lozano, K. Preparation and Characterization of Polyvinylidene Fluoride Nanofibrous Membranes by Forcespinning. Polym. Eng. Sci. 2012, 52, 1–10.
- Sinha, M.K.; Purkait, M.K. Use of CS-PAA nanoparticles as an alternative to metal oxide nanoparticles and their effect on fouling mitigation of a PSF ultrafiltration membrane. RSC Adv. 2015, 5, 66109–66121.
- Adout, A.; Kang, S.; Asatekin, A.; Mayes, A.M.; Elimelech, M. Ultrafiltration membranes incorporating amphiphilic comb copolymer additives prevent irreversible adhesion of bacteria. Environ. Sci. Technol. 2010, 44, 2406–2411.
- Yar, A.; Haspulat, B.; Ustun, T.; Eskizeybek, V.; Avcı, A.; Kamış, H.; Achour, S. Electrospun TiO2/ZnO/PAN hybrid nanofiber membranes with efficient photocatalytic activity. RSC Adv. 2017, 7, 29806–29814.
- Reddy, A.V.R.; Patel, H.R. Chemically treated polyethersulfone/polyacrylonitrile blend ultrafiltration membranes for better fouling resistance. Desalination 2008, 221, 318–323.
- Mandal, T.; Maity, S.; Dasgupta, D.; Datta, S. Advanced oxidation process and biotreatment: Their roles in combined industrial wastewater treatment. Desalination 2010, 250, 87–94.
- Yoon, K.; Kim, K.; Wang, X.; Fang, D.; Hsiao, B.S.; Chu, B. High flux ultrafiltration membranes based on electrospun nanofibrous PAN scaffolds and chitosan coating. Polymer 2006, 47, 2434–2441.
- Tijing, L.D.; Woo, Y.C.; Yao, M.; Ren, J. Electrospinning for Membrane Fabrication: Strategies and Applications. In Comprehensive Membrane Science and Engineering; Elsevier: Oxford, UK, 2017; ISBN 9780124095472.
- Xu, Y.; Lin, W.; Wang, H.; Guo, J.; Yuan, D.; Bao, J.; Sun, S.; Zhao, W.; Zhao, C. Dual-functional polyethersulfone composite nanofibrous membranes with synergistic adsorption and photocatalytic degradation for organic dyes. Compos. Sci. Technol. 2020, 199, 108353–108363.
- Anwar, S.; Nabeela, A.; Sundarrajan, S.; Abdulrahim, S.; Nizar, S.; Balamurugan, R.; Ramakrishna, S. Advancement in electrospun nanofibrous membranes modification and their application in water treatment. Membranes 2013, 3, 266–284.
- Fox, M.A.; Dulay, M.T. Heterogeneous Photocatalysis. Chem. Rev. 1993, 93, 341–357.
- Molinari, R.; Lavorato, C.; Argurio, P. Recent progress of photocatalytic membrane reactors in water treatment and in synthesis of organic compounds: A review. Catal. Today 2017, 281, 144–164.
- Kuvarega, A.T.; Khumalo, N.; Dlamini, D.; Mamba, B.B. Polysulfone/N, Pd co-doped TiO2 composite membranes for photocatalytic dye degradation. Sep. Purif. Technol. 2018, 191, 122–133.
- Fernández-ramos, C.; Šatínský, D.; Šmídová, B.; Solich, P. Trends in Analytical Chemistry Analysis of trace organic compounds in environmental, food and biological matrices using large-volume sample injection in column- switching liquid chromatography. Trends Anal. Chem. 2014, 62, 69–85.
- Zhang, X.; Wang, D.K.; Diniz, J.C. Recent progresses on fabrication of photocatalytic membranes for water treatment. Catal. Today 2014, 230, 47–54.
- Foster, H.A.; Ditta, I.B.; Varghese, S. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 1847–1868.
- Pachepsky, Y.; Shelton, Ã.D.R.; Mclain, Ã.J.E.T.; Patel, J.; Mandrell, R.E. Irrigation Waters as a Source of Pathogenic Microorganisms in Produce: A Review; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 113, ISBN 9780123864734.
- Leclerc, H.; Schwartzbrod, L.; Leclerc, H.; Schwartzbrod, L. Microbial agents associated with waterborne diseases. Crit. Rev. Microbiol. 2008, 7828.
- Hunter, P.R.; Colford, J.M.; Lechevallier, M.W.; Binder, S.; Berger, P.S.; Chapman, L. Waterborne Diseases Xenotransplantation: Benefits and Risks. Emerg. Infecious Dis. 2001, 7, 544–545.
- Thomas, V.; Mcdonnell, G.; Denyer, S.P.; Maillard, J.Y. Free-living amoebae and their intracellular pathogenicmicroorganisms: Risks for water quality. FEMS Microbiol. Rev. 2010, 34, 231–259.
- Chen, Q.; Yu, Z.; Pan, Y.; Zeng, G.; Shi, H. Enhancing the photocatalytic and antibacterial property of polyvinylidene fluoride membrane by blending Ag–TiO2 nanocomposites. J. Mater. Sci. Mater. Electron. 2017, 28, 3865–3874.
- Zhu, J.; Hou, J.; Zhang, Y.; Tian, M.; He, T.; Liu, J.; Chen, V. Polymeric antimicrobial membranes enabled by nanomaterials for water treatment. J. Memb. Sci. 2018, 550, 173–197.
- Celebioglu, A.; Uyar, T. Electrospinning of nanofibers from non-polymeric systems: Polymer-free nanofibers from cyclodextrin derivatives. Nanoscale 2012, 4, 621–631.
- Liu, Y.; Wang, X.; Yang, F.; Yang, X. Excellent antimicrobial properties of mesoporous anatase TiO2 and Ag/TiO2 composite films. Microporous Mesoporous Mater. 2008, 114, 431–439.
- Zhang, T.; Li, Z.; Wang, W.; Wang, Y.; Gao, B.; Wang, Z. Enhanced antifouling and antimicrobial thin film nanocomposite membranes with incorporation of Palygorskite/titanium dioxide hybrid material. J. Colloid Interface Sci. 2019, 537, 1–10.
- Hee, J.; Kumar, M.; Lee, J.; Hee, C.; Sang, C. Polydopamine-assisted immobilization of hierarchical zinc oxide nanostructures on electrospun nanofibrous membrane for photocatalysis and antimicrobial activity. J. Colloid Interface Sci. 2018, 513, 566–574.
- Panthi, G.; Park, S.; Chae, S.; Kim, T.; Chung, H.; Hong, S.; Park, M.; Kim, H. Immobilization of Ag3PO4 nanoparticles on electrospun PAN nano fi bers via surface oximation: Bifunctional composite membrane with enhanced photocatalytic and antimicrobial activities. J. Ind. Eng. Chem. 2017, 45, 277–286.
- Xu, Z.; Ye, S.; Zhang, G.; Li, W.; Gao, C.; Shen, C. Antimicrobial polysulfone blended ultra fi ltration membranes prepared with Ag/Cu2O hybrid nanowires. J. Membr. Sci. 2016, 509, 83–93.
- Damodar, R.A.; You, S.; Chou, H. Study the self cleaning, antibacterial and photocatalytic properties of TiO2 entrapped PVDF membranes. J. Hazard. Mater. 2009, 172, 1321–1328.
- Jalvo, B.; Faraldos, M.; Bahamonde, A.; Rosal, R. Antimicrobial and antibiofilm efficacy of self-cleaning surfaces functionalized by TiO2 photocatalytic nanoparticles against Staphylococcus aureus and Pseudomonas putida. J. Hazard. Mater. 2017, 340, 160–170.
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107.
- Bodaghi, H.; Mosto, Y.; Oromiehie, A.; Zamani, Z.; Ghanbarzadeh, B.; Costa, C.; Conte, A.; Alessandro, M.; Nobile, D. LWT-Food Science and Technology Evaluation of the photocatalytic antimicrobial effects of a TiO2 nanocomposite food packaging film by in vitro and in vivo tests. LWT-Food Sci. Technol. 2013, 50, 702–706.
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Alvarez, P.J.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009, 43, 715–723.
- Zhang, L.; Luo, J.; Menkhaus, T.J.; Varadaraju, H.; Sun, Y.; Fong, H. Antimicrobial nano-fibrous membranes developed from electrospun polyacrylonitrile nanofibers. J. Memb. Sci. 2011, 369, 499–505.
- Chaudhary, A.; Gupta, A.; Mathur, R.B.; Dhakate, S.R. Effective antimicrobial filter from electrospun polyacrylonitrile-silver composite nanofibers membrane for conducive environment. Adv. Mater. 2014, 10, 562–568.
- Pan, S.; Ke, X.; Wang, T.; Liu, Q.; Zhong, L. Synthesis of Silver Nanoparticles Embedded Electrospun PAN Nanofiber Thin-Film Composite Forward Osmosis Membrane to Enhance Performance and Antimicrobial Activity. Ind. Eng. Chem. Res. 2019, 58, 984–993.
- Cremar, L.; Gutierrez, J.; Martinez, J.; Materon, L.A.; Gilkerson, R.; Xu, F.; Lozano, K. Development of antimicrobial chitosan based nanofiber dressings for wound healing applications. Nanomed. J. 2020, 5, 6–14.
- Mocanu, A.; Rusen, E.; Diacon, A.; Isopencu, G.; Must, G.; Raluca, Ş.; Dinescu, A. Antimicrobial properties of polysulfone membranes modified with carbon nanofibers and silver nanoparticles. Mater. Chem. Phys. 2019, 223, 39–45.
- Zhao, F.; Chen, S.; Hu, Q.; Xue, G.; Ni, Q.; Jiang, Q.; Qiu, Y. Antimicrobial three dimensional woven filters containing silver nanoparticle doped nanofibers in a membrane bioreactor for wastewater treatment. Sep. Purif. Technol. 2017, 175, 130–139.
- Kwon, H.; Cha, J.; Lee, C.W. Preparation and Characterization of Antimicrobial Bilayer Electrospun Nanofiber Membrane for Oily Wastewater Treatment. J. Korean Phys. Soc. 2020, 76, 34–43.
- Hashmi, M.; Ullah, S.; Kim, I.S. Copper oxide (CuO) loaded polyacrylonitrile (PAN) nano fi ber membranes for antimicrobial breath mask applications. Curr. Res. Biotechnol. 2019, 1, 1–10.
- Dilamian, M.; Montazer, M.; Masoumi, J. Antimicrobial electrospun membranes of chitosan/poly (ethylene oxide) incorporating poly (hexamethylene biguanide) hydrochloride. Carbohydr. Polym. 2013, 94, 364–371.
- Shi, R.; Geng, H.; Gong, M.; Ye, J.; Wu, C.; Hu, X.; Zhang, L. Long-acting and broad-spectrum antimicrobial electrospun poly(e-caprolactone)/gelatin micro/anofibers for wound dressing. J. Colloid Interface Sci. 2018, 509, 275–284.
- Han, C.; Cai, N.; Chan, V.; Liu, M.; Feng, X.; Yu, F. Enhanced drug delivery, mechanical properties and antimicrobial activities in poly (lactic acid) nanofiber with mesoporous Fe3O4-COOH nanoparticles. Colloids Surfaces A 2018, 559, 104–114.
- Han, D.; Sherman, S.; Filocamo, S.; Steckl, A.J. Long-term antimicrobial effect of nisin released from electrospun triaxial fiber membranes. Acta Biomater. 2017, 53, 242–249.
- Liu, X.; Lin, T.; Gao, Y.; Xu, Z.; Huang, C.; Yao, G.; Jiang, L.; Tang, Y.; Wang, X. Antimicrobial electrospun nanofibers of cellulose acetate and polyester urethane composite for wound dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100 B, 1556–1565.
- Xue, J.; Niu, Y.; Gong, M.; Shi, R.; Chen, D.; Zhang, L.; Lvov, Y.; Materials, B.; Composites, I.; Technology, C.; et al. Electrospun Microfiber Membranes Embedded with Drug-Loaded Clay Nanotubes for Sustained Antimicrobial. ACS Nano 2015, 9, 1600–1612.
- Woong, D.; Hwan, S.; Hwan, H.; Hoon, K.; Seok, C.; Hwan, Y. Multi-biofunction of antimicrobial peptide-immobilized silk fibroin nanofiber membrane: Implications for wound healing. Acta Biomater. 2016, 39, 146–155.
- Huang, Y.; Dan, N.; Dan, W.; Zhao, W.; Bai, Z.; Chen, Y.; Yang, C. Bilayered Antimicrobial Nano fiber Membranes for Wound Dressings via in Situ Cross-Linking Polymerization and Electrospinning. Ind. Eng. Chem. Res. 2018, 57, 17048–17057.
- Tan, K.; Obendorf, S.K. Fabrication and evaluation of electrospun nanofibrous antimicrobial nylon 6 membranes. J. Memb. Sci. 2007, 305, 287–298.
- Su, Y.; Mainardi, V.L.; Wang, H.; Mccarthy, A.; Zhang, Y.S.; Chen, S.; John, J.V.; Wong, S.L.; Hollins, R.R.; Wang, G.; et al. Dissolvable microneedles coupled with nanfiber dressings eradicate biofilms via effectively delivering a database designed antimicrobial peptite. ACS Nano 2020, 14, 11775–11786.
- Kaur, S.; Sundarrajan, S.; Rana, D. Review: The characterization of electrospun nanofibrous liquid filtration membranes. J. Mater. Sci. 2014, 49, 6143–6159.




