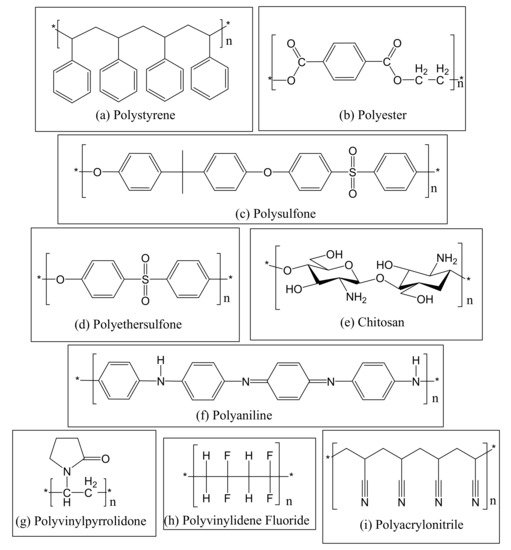
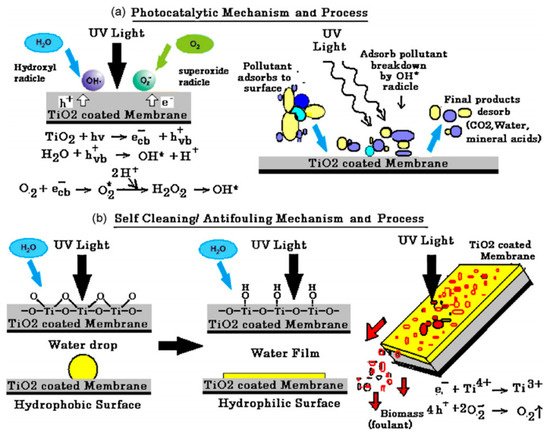
3. Antimicrobial Membranes
Microorganisms such as bacteria, fungi, archaea, algae, protozoa and viruses [
109] form part of the water pollution system and are the cause of various types of waterborne diseases such as polio, malaria, cholera, hepatitis, diarrhea, ascariasis, malnutrition, ringworm and lymphatic filariasis, among others [
119,
120]. Due to the high levels of water pollution, the high ratio of water demand to water availability, as well as inefficient water treatment facilities; human beings, animals and aquatic biota have to bear the burden of waterborne diseases and infections [
121,
122,
123].
Researchers are introducing the use of antimicrobial material in current water treatment methods. Nanocomposites are being produced with the addition of materials that have antimicrobial properties to use in hybrid water treatment processes [
124]. Photocatalysis is one of the processes that has been reported to have the ability to “kill” a wide range of bacteria, viruses, algae, endospores, protozoa and fungi, and has also demonstrated the ability to inactivate prions and to destroy microbial toxins [
109]. Membrane technology is another process that has such properties depending on the materials used to fabricate the membrane; however, they are well known to be prone to biofouling. Membrane biofouling is a challenging aspect to control using chemical, biological, or physical methods due to its compact nature, strong adaptive resistant microbes, and the cost of post-treatment, hence the need for modification [
123].
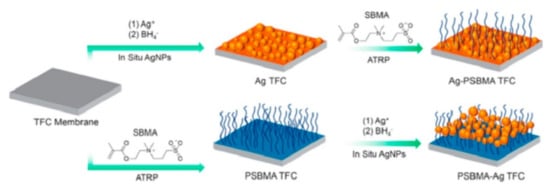
Figure 5 shows two different ways of fabricating a thin film composite membrane with antimicrobial properties that was used for water treatment, as reported by Zhu et al. In the first method, the thin film composite (TFC) membrane is chemically modified with Ag nanoparticles followed by coating with SBMA. In the second method, the TFC membrane is co-polymerized with SBMA followed by coating with Ag nanoparticles [
124].
Figure 5. The fabrication process of antimicrobial TFC membranes used for water treatment. Reprinted from [
124] with permission from Elsevier.
The modification of photocatalytic materials and membrane materials with Ag, Cu or graphene material enhances the antimicrobial properties of the materials which also suggests that such modified materials have self-cleaning/sterilising properties.
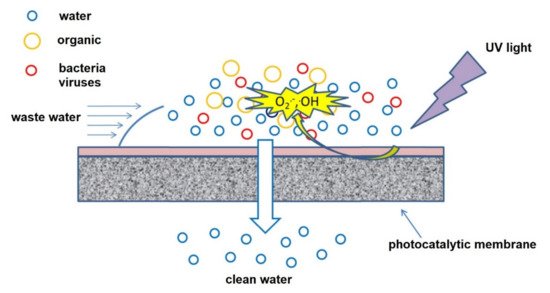
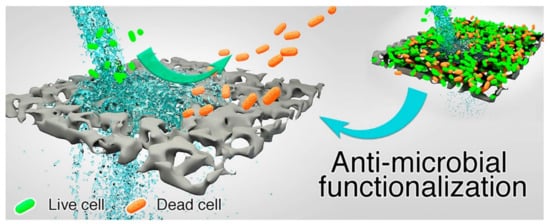
Figure 6 illustrates how an antimicrobial membrane with cleaning properties operates [
124]. The use of photocatalytic and/or antimicrobial membranes in water treatment results in improved water quality, enhanced efficiency, and prolonged life span of the membrane by reducing/eliminating fouling [
124,
125,
126]. The properties induced by antimicrobial agents have paved a way for the successful production of antimicrobial photocatalysts, antimicrobial membranes, and antimicrobial-photocatalytic membranes as evidenced by numerous research studies.
Figure 6. Illustration of antimicrobial activity on a surface of an antimicrobial membrane during water filtration. Reprinted from [
124] with permission from Elsevier.
Zhang et al. fabricated thin-film composite membranes with enhanced antifouling and antimicrobial properties by the incorporation of palygorskite/TiO
2 hybrid material. Palygorskite and palygorskite/TiO
2 were embedded on a reverse osmosis polyamide membrane through interfacial polymerisation. The tubular structure of palygorskite played a role in the facilitation of water molecules through the thin-film membrane. The palygorskite-incorporated membranes had a 1.6-fold increase (up to 40 L/m
2 h) in water flux compared to the bare membranes. The palygorskite/TiO
2-containing membranes showed a 1.4-fold increase compared to the bare membranes. Antifouling properties were observed with increasing flux against humic acid and bovine serum albumin while antimicrobial properties were also successful against
Escherichia coli [
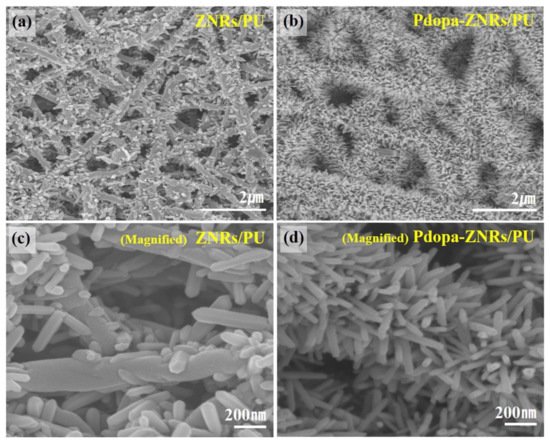
127]. Hee et al. studied the photocatalytic and antimicrobial activity of ZnO-incorporated electrospun nanofibrous membranes. Polyurethane nanofibers were fabricated by electrospinning followed by coating with polydopamine using the dip-coating method. For the incorporation of ZnO nanoparticles, the polydopamine-coated nanofibers were soaked in a ZnO aqueous solution followed by hydrothermal treatment to grow ZnO nanorods on the surface of the nanofibers. Characterization confirmed that the ZnO nanorods were grown and adhered to the polydopamine-coated polyurethane nanofibers as shown on
Figure 7. The resulting material successfully degraded methylene blue within 180 min and showed positive antimicrobial properties against
Escherichia coli [
128].
Figure 7. Field emission–scanning electron microscope (FE-SEM) images of (
a,
c) ZnO-nanorods/polyurethane, (
b,
d) Polydopamine-ZnO-nanorods/polyurethane. Reprinted from [
128] with permission from Elsevier.
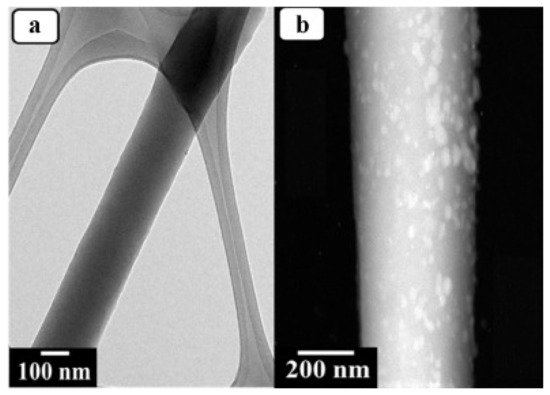
Panthi et al. produced a photocatalytic and antimicrobial bifunctional composite membrane immobilised with Ag
3PO
4 nanoparticles (
Figure 8). PAN nanofibers were produced by electrospinning and then modified with amidoxime to use as anchoring sites for Ag
+ ions; AgNO
3 was used as the source of Ag. The composite was then reacted with Na
2HPO
4 to produce the final bifunctional composite membrane Ag
3PO
4/PAN. Antimicrobial activity was confirmed by testing against Gram-negative
Escherichia coli and Gram-positive
Staphylococcus aureus, respectively. The composite (150 mg) degraded up to 90% of methylene blue in a solution containing 50 mL of the dye (10 mg/L) within 60 min using a 200 W mercury lamp [
129]. Xu et al. fabricated a hybrid antimicrobial nanofiltration membrane. The Ag-Cu
2O nanowires were prepared by grafting L-dopa on the surface of Cu
2O nanowires via in situ polymerisation which resulted in a zwitterionic surface suitable for the attachment of Ag
+ ions. The final Ag-Cu
2O-PSF composite was fabricated using the in-situ phase inversion method. Antimicrobial studies against
Escherichia coli and
Staphylococcus aureus using the composite revealed enhanced antimicrobial activity compared to that of the bare PSF membrane. Bovine serum albumin was used for protein rejection studies whereby the modified and pure PSF membranes rejected up to 94.70 and 86.81%, respectively. The modified membrane also achieved higher water flux (up to 164.1 L/m
2·h) compared to the bare membrane (40.4 L/m
2·h). The modified membranes also demonstrated better flux recovery than the pure PSF membrane [
130].
Figure 8. Transmission electron microscopy (TEM) images of (
a) polyacrylonitrile (PAN) and (
b) Ag
3PO
4/PAN composite membranes used for photodegradation and antimicrobial studies by Panthi et al. Reprinted from [
129] with permission from Elsevier.
Damodar et al. studied the self-cleaning, antibacterial, and photocatalytic properties of polyvinylidene fluoride (PVDF) membranes embedded with TiO
2 (
Figure 9). The PVDF/TiO
2 membranes were prepared via the phase inversion method where the TiO
2 loading was varied from 0–4%. Generally, PVDF/TiO
2 membranes showed higher antimicrobial properties compared to the pristine PVDF membrane with 4% TiO
2 having the highest activity against
Escherichia coli. Over 95% degradation of Reactive Black 5 was achieved within 60 min using the 2% PVDF/TiO
2 membrane while the pristine membrane showed no photocatalytic activity. The PVDF/TiO
2 membranes showed good antifouling and self-cleaning properties under UV irradiation with increased water flux and excellent flux recovery compared to pristine PVDF membranes. The photodegradation and self-cleaning processes on the membrane are as demonstrated in
Figure 9 [
131]. Jalvo et al. studied the antimicrobial and anti-biofilm efficacy of a TiO
2 coated glass surface with self-cleaning properties. The material was prepared by coating a glass side with the TiO
2 suspension. The coated glass slide showed cell reduction viability of over 99% during antimicrobial studies against biofilm-forming bacteria
Staphylococcus aureus and
Pseudomonas putida. Self-cleaning properties were tested against the degradation of adsorbed methylene blue. The material achieved 85% degradation confirming that the material has both self-cleaning properties and anti-biofilm efficacy added to their photocatalytic and antimicrobial properties [
132].
Figure 9. Demonstration of the (
a) photocatalysis mechanism and process as well as (
b) Self-cleaning/antifouling mechanism and process of polyvinylidene fluoride (PVDF)/TiO
2 membrane. Reprinted from [
131] with permission from Elsevier.
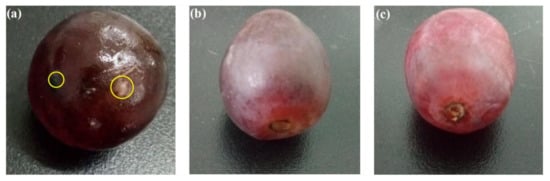
Zhang et al. prepared a chitosan-based antimicrobial film against foodborne pathogens to use in food packaging under visible light. The material was prepared by coating a plastic film-covered glass plate with chitosan-TiO
2 emulsion crosslinked by epichlorohydrin, followed by drying naturally overnight. The obtained composite film was tested against
Escherichia coli, Staphylococcus aureus, Candida albicans, and
Aspergillus niger, and achieved 100% sterilisation within 12 h. Positive results were also obtained in terms of the prevention of microbial growth in packaged red grapes with an extended life span as shown in
Figure 10 [
133]. Bodaghi et al. studied the photocatalytic antimicrobial effects of TiO
2 coated packaging film by conducting in vivo and in vitro tests. The TiO
2-polyethylene film was prepared using the melt blending method whereby modified TiO
2 powder, polyethylene granules, and glycerol were mixed and blended for an hour. The resulting nanocomposite film was used for all studies. Under in vitro studies, the film reduced the number of surviving cells for
Pseudomonas spp. and
Rhodotorula mucilaginosa by 4 and 2 logs compared to 1.35 and 0.64 log reduction of polyethylene, respectively. In vivo studies were conducted on packaged fresh pears under fluorescent light irradiation for 17 days where a significant decrease in mesophilic bacteria and yeast cells was observed for TiO
2-polyethylene [
134].
Figure 10. Preservation of red grape packed in different materials at 37 °C for 6 days: (
a) plastic wrap; (
b) pure chitosan film; (
c) chitosan-TiO
2 film. Reprinted from [
133] with permission from Elsevier.
Antimicrobial activity is one of the most important properties in membrane technology. As indicated, antimicrobial activity prevents microbial membrane fouling which prolongs the lifespan of the membrane [
135].
Table 1 shows some of the nanomaterials with antimicrobial activity that can be blended with polymer nanofibers for various applications. Silver nanoparticles are well documented for their excellent antimicrobial activity and it is no surprise that the table shows that pristine and doped-Ag nanoparticles are mostly used for the preparation of antimicrobial nanofiber composite membranes [
129,
136,
137,
138,
139,
140,
141,
142].
Table 1 further shows that there are other antimicrobial materials such as ZnO, CuO, poly(hexamethylene biguanide) hydrochloride, octadecyldimethyl[3-(trimethoxysilyl)propyl]ammonium chloride, Fe
3O
4-COOH, nisin, and metronidazole which can be further explored to reduce the demand of Ag nanoparticles for antimicrobial applications [
128,
143,
144,
145,
146,
147]. The application section of
Table 1 indicates that the application of antimicrobial membranes is not limited to water treatment but extends to areas such as cytotoxicity [
148,
149], drug release [
143,
146,
149], and wound dressing [
139,
145,
148,
150,
151].
Table 1. Antimicrobial nanofiber membranes and their various applications.
|
Polymer
|
Antimicrobial Agent
|
Method
|
Application
|
Antimicrobial Activity
|
Ref.
|
|
Polyurethane
|
Polydopamine-ZnO
|
Electrospinning
|
Antimicrobial (E. coli)
Photodegradation (Methylene blue)
|
Active
|
[128]
|
|
Polyacrylonitrile
|
Ag3PO4
|
Electrospinning
|
Antimicrobial (E. coli and S. aureus)
Photodegradation (Methylene blue)
|
Active
|
[129]
|
|
Polyacrylonitrile
|
Ag nanoparticles
|
Electrospinning
|
Antimicrobial (E. coli and S. aureus)
|
Active
|
[136]
|
|
Polyacrylonitrile
|
Ag nanoparticles
|
Electrospinning
|
Antimicrobial (E. coli and S. aureus)
|
Active
|
[137]
|
|
Polyacrylonitrile
|
Ag nanoparticles
|
Electrospinning
|
Antimicrobial (E. coli and S. aureus)
Forward osmosis
|
Active
|
[138]
|
|
Chitosan
|
Ag nanoparticles
|
Centrifugal spinning
|
Antimicrobial (S. aureus)
Wound healing
|
Active
|
[139]
|
|
Polysulfone
|
CNT/Ag
|
Radical solution polymerization and wet-phase inversion
|
Antimicrobial (E. coli and B. subtilis)
|
Active
|
[140]
|
|
3D woven fabric filters
|
Ag nanoparticles
|
Electrospinning
|
Antimicrobial (S. aureus)
Water treatment
|
Active
|
[141]
|
|
Polyvinyl alcohol
|
Polyimide-Ag
|
Electrospinning
|
Antimicrobial (E. coli and S. aureus)
Oily wastewater treatment
|
Active
|
[142]
|
|
Polyacrylonitrile
|
CuO
|
Electrospinning
|
Antimicrobial (E. coli and B. subtilis) for breath masks
Drug release
|
Active
|
[143]
|
|
Chitosan/poly(ethylene oxide)
|
Poly(hexamethylene biguanide) hydrochloride
|
Electrospinning
|
Antimicrobial (E. coli and S. aureus)
|
Active
|
[144]
|
|
Poly(ε-caprolactone) and gelatine
|
Octadecyldimethyl[3 -(trimethoxysilyl)propyl]ammonium chloride
|
Electrospinning
|
Antimicrobial (S. aureus and P. aeruginosa)
Wound dressing
|
Active
|
[145]
|
|
Polylactic acid
|
Fe3O4-COOH
|
Electrospinning
|
Antimicrobial (E. coli and S. aureus)
Drug delivery
|
Active
|
[146]
|
|
Triaxial
|
Nisin
|
Electrospinning
|
Antimicrobial (S. aureus)
|
Active
|
[147]
|
|
Cellulose acetate/polyester urethane
|
Polyhexamethylene biguanide
|
Electrospinning
|
Antimicrobial (E. coli)
Cytotoxicity
Wound healing
|
Active
|
[148]
|
|
Polycaprolactone/gelatine
|
Metronidazole
|
Electrospinning
|
Antimicrobial (F. nucleatum)
Cytotoxicity (L929 Cells)
Drug delivery
|
Active
|
[149]
|
|
Silk fibroin
|
Peptide motif
|
Electrospinning
|
Antimicrobial (S. aureus, E. coli, S. epidermidis and P. aeruginosa)
Wound dressing
|
Active
|
[150]
|
|
Polycaprolactone
|
2-(Methacryloyloxy) ethyl trimethylammonium/polycaprolactone
|
Cross-linking polymerization and electrospinning
|
Antimicrobial (E. coli and S. aureus)
Wound dressing
|
Active
|
[151]
|
|
Nylon 6
|
N-Halamine
|
Electrospinning
|
Antimicrobial (E. coli and S. aureus)
|
Active
|
[152]
|
|
Polycaprolactone
|
Peptide dissolved micro needles
|
Coaxial electrospinning and electrospray deposition
|
Antimicrobial (S. aureus, K. pneumoniae, A. baumannii, and P. aeruginosa)
Chronic wound dressing
|
Active
|
[153]
|