| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hammad Ullah | + 2461 word(s) | 2461 | 2020-07-14 08:54:45 | | | |
| 2 | Nora Tang | Meta information modification | 2461 | 2020-07-22 08:48:12 | | | | |
| 3 | Nora Tang | Meta information modification | 2461 | 2020-07-22 08:49:39 | | |
Video Upload Options
Abstract
Gastrointestinal (GI) diseases, which include gastrointestinal reflux disease, gastric ulceration, inflammatory bowel disease, and other functional GI disorders, have become prevalent in a large part of the world population. Metabolic syndrome (MS) is cluster of disorders including obesity, hyperglycemia, hyperlipidemia, and hypertension, and is associated with high rate of morbidity and mortality. Gut dysbiosis is one of the contributing factors to the pathogenesis of both GI disorder and MS, and restoration of normal flora can provide a potential protective approach in both these conditions. Bioactive dietary components are known to play a significant role in the maintenance of health and wellness, as they have the potential to modify risk factors for a large number of serious disorders. Different classes of functional dietary components, such as dietary fibers, probiotics, prebiotics, polyunsaturated fatty acids, polyphenols, and spices, possess positive impacts on human health and can be useful as alternative treatments for GI disorders and metabolic dysregulation, as they can modify the risk factors associated with these pathologies. Their regular intake in sufficient amounts also aids in the restoration of normal intestinal flora, resulting in positive regulation of insulin signaling, metabolic pathways and immune responses, and reduction of low-grade chronic inflammation. This review is designed to focus on the health benefits of bioactive dietary components, with the aim of preventing the development or halting the progression of GI disorders and MS through an improvement of the most important risk factors including gut dysbiosis.
1. Definition
Gastrointestinal (GI) disorders, whose prevalence has increased over the last few decades, are characterized by physiological and morphological abnormalities of the GI system that often occur in combination and include motility disorders, visceral hypersensitivity, altered mucosal and immune function, and altered intestinal microbiota [1][2]. Metabolic syndrome (MS) is a condition of low-grade chronic inflammation due to both genetic and environmental factors, including a number of risk factors for serious diseases such as hyperglycemia, abdominal obesity, hyperlipidemia, and hypertension [3][4].
2. Introduction
Among GI disorders, inflammatory bowel diseases (IBD), including Crohn’s disease (CD), a chronic bowel disease that causes patches of inflammation in the GI tract [5], and ulcerative colitis (UC), which affects only the inner wall of the colon, are the most serious diseases [6]. Other common, idiopathic and chronic inflammatory disorders of the GI tract include diverticular disease, a chronic condition of small pockets of bowel, and irritable bowel syndrome (IBS) defined as an “abdominal discomfort associated with altered bowel habits” [7]. The main causes of GI disorders are genetic predisposition to the disease, pharmacological therapies (i.e., antibiotics, not limited to those administered for human use but also potentially including those used in farm animals and crops and ingested with the resultant foods), non-steroidal anti-inflammatory drugs (NSAIDs) (i.e., aspirin, ibuprofen, diclofenac), and unhealthy lifestyles, including irregular eating, physical inactivity, smoking, and low fiber diets [8][9][10][11]. Moreover, about 50% global population is affected by Helicobacter pylori, a Gram-negative bacterial pathogen, which might cause IBD and functional GI disorders [12]. All GI disorders commonly manifest abdominal pain, constipation, diarrhea, abdominal distention, gastric acidity, gastrointestinal reflux disease (GERD), GI tract (GIT) bleeding, malabsorption or malnutrition, and intestinal obstruction [13][14]. Some of the drugs currently in practice for the treatment of GI disorders include laxatives, anti-diarrheals, opioids, anti-emetics, motility enhancers, and anti-acidity, anti-ulcer, and anti-inflammatory agents [15]. Conventional therapies for IBD include corticosteroids, immunosuppressants and anti-tumor necrosis factor (TNF)-α antibodies, often correlated with a risk of opportunistic infections and dysplasias, with expensive consequences on health system management [16][17]. Evidence suggests that the Western pattern diet (WPD) has led to the wide spread of GERD. Studies have shown the prevalence of GERD to be 18.1–27.8% (North Americans), 8.8–25.9% (Europe), 11.6% (Australia), 23% (South America), 2.5–7.8% (East Asia), and 8.7–33.1% (Middle East) [18]. The prevalence of dyspepsia may vary from country to country, however, the pooled prevalence is reported by Ford et al. to be 21% [19]. In the adult population of the United States, diarrhea results in over 128,000 hospitalizations and 3000 deaths [20][21]. Similarly, available literature suggests that 12% of the worldwide population has had constipation [22]. Nyrop et al. (2007) have estimated the average annual direct health care cost of some GI disorders, per patient population visiting GI clinic, as $5049 (IBS), $6140 (diarrhea), $7522 (constipation), and $7646 (abdominal pain) [23]. Despite recent progression in knowledge of pathophysiological mechanisms involved in GI disorders, their etiopathogenesis has not yet been completely clarified and there is no marker that can lead to their definitive diagnosis. Some etiological factors have been identified, such as visceral hypersensitivity, infections, genetic and epigenetic factors, stress, and changes in the intestinal microbiota leading to dysbiosis [24][25].
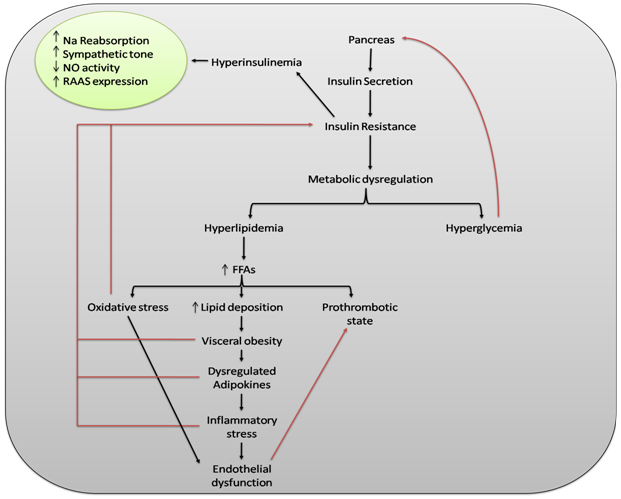
The clinical significance of MS was highlighted by Reaven in 1988, while describing the role of insulin resistance in human disease [26]. An untreated and persistent state of MS may lead to a number of pathologies, the most common of which are cardiovascular disorders (coronary heart disease, cardiac failure, and stroke), type 2 diabetes mellitus, and hepatic abnormalities such as hepatic steatosis [27][28][29]. As reported by Sadeghi, a variety of metabolic disturbances, including hypertension, hyperglycemia, and gout, were already described in the 1920s [30]. Later, in 1947, Vague described the obesity phenotype as being affected by metabolic abnormalities, type 2 diabetes mellitus (T2DM), and cardiovascular disease [31]. The WHO definition and the European Group for the Study of Insulin Resistance agree over the inclusion of glucose intolerance and insulin resistance as essential components of MS [32][33]. Several definitions of said syndrome are in use, but since 2004, the International Diabetes Federation (IDF) has established a unified definition of MS, highlighting the key role of obesity as a risk factor for the diseases reported above [34]. The main risk factors for the development of MS are positive family history [35], smoking [36], excessive alcohol intake [37], aging, low social-economic status, postmenopausal status [38], sedentary life style [39], unhealthy dietary patterns [40], and intake of some medications (atypical antipsychotics) [41]. Figure 1 shows the impact of a dysregulated metabolism on the human body by illustrating the pathogenic pathway of MS. Metabolic disorders share common pathophysiological mechanisms and thus different pharmacologic entities are used in combination for therapeutic purposes [42]. Effective interventions may include physical exercise, dietary modifications, and pharmacological agents such as insulin sensitizers, renin-angiotensin-aldosterone system (RAAS) inhibitors, statins, and fibrates [43][44]. Over the past two decades, a large worldwide increase in people with MS has taken place, associated with a global epidemic of obesity and T2DM. About 20–25% of the world adult population is living with MS [45]. WHO estimates that about 650 million people have been living with obesity, 422 million with diabetes, and 1.13 billion with hypertension [46][47][48].
Figure 1. The impact of a dysregulated metabolism on the human body. The central element of metabolic syndrome (MS) is insulin resistance, which leads to metabolic dysregulation and eventually results in hyperglycemia and hyperlipidemia. Hyperglycemia stimulates β-cells of the pancreas and thus produces more insulin, which causes hyperinsulinemia. Hyperinsulinemia increases sympathetic tone, RAAS expression, and sodium reabsorption through nephrons while decreasing NO activity. Dysregulated fat metabolism results in increased production of FFAs and thus lipid deposition increases, resulting in visceral obesity. Visceral obesity causes dysregulation of adipocytokines and pro-inflammatory processes and leads to inflammatory stress. Increased production of FFAs also causes oxidative stress and prothrombotic states via other mechanisms. Oxidative and inflammatory stresses may lead to endothelial dysfunction, which may further contribute to a prothrombotic state. Oxidative stress, visceral obesity, dysregulated adipocytokines, and pro-inflammatory cytokines further contribute to insulin resistance. Increase (↑); decrease (↓); sodium (Na); nitric oxide (NO); renin-angiotensin-aldosterone system (RAAS); free fatty acids (FFAs).
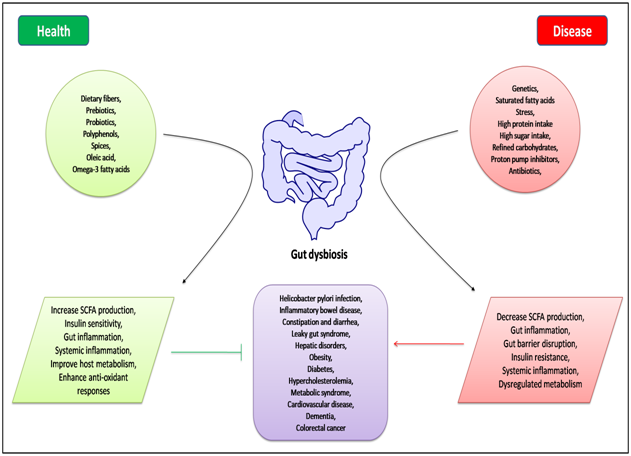
Growing evidence suggests that dysbiosis of the gut microbiota, due to multiple intrinsic and extrinsic factors such as genetic variations, diet, stress, and pharmacological therapy [49], is associated with the pathogenesis of both intestinal and extra-intestinal diseases (Figure 2). In fact, the interaction between microbiota and the host immune system is considered to be a key element for a new understanding of the pathogenesis of a large spectrum of diseases including GI disorders and MS [50]. Current studies highlight the role of a healthy gut microbiota in modulating the onset of various GI diseases such as IBD, colon cancer, celiac disease, and IBS. In addition, dysbiosis is one of the driving factors of a dysregulated metabolism. Hur et al. report the important role of gut microbiota in the pathogenesis of T2DM by influencing body weight, pro-inflammatory activity, and insulin resistance [51]. A pivotal role of gut microbiota is the fermentation of dietary polysaccharides that the human body cannot otherwise digest. Dietary fibers consist of the indigestible portion of plant food carbohydrates, containing insoluble and soluble fibers. Soluble fibers are digested by enzymes derived from the gut microbiota into short-chain fatty acids (SCFAs). SCFAs (butyrate, acetate, and propionate) are absorbed in the intestines and used as energy by the host. In addition to their role as energy substrates, SCFAs act as regulators of food or energy intake and inflammation [52][53]. In particular, butyrate promotes regeneration and protection of intestinal cells, the production of mucin, the reduction of hypercholesterolemia levels, as well as the release of hormones and/or neurotransmitters important for the regulation of intestinal motility and of insulin resistance [54]. An improvement in abdominal pain following the intake of butyrate has been observed in IBS patients due to an alteration in neurotransmitter release and a reduction in the hypersensitivity of intestinal mechanoreceptors, which may result in decreasing luminal pressure and/or peristalsis [55][56].
Figure 2. Schematic representation of intrinsic and extrinsic factors responsible for gut dysbiosis, and their role in health and disease.
Today, lifestyle and diet are recognized as the cornerstones of prevention of pathologies such as cardiovascular diseases, T2DM, MS, and GI disorders. Moreover, the modulation of intestinal dysbiosis through dietary supplements, according to the latest evidence, is used to restore the equilibrium of gut microbiota. Considering that dysbiosis can be a common link between GI disorders and MS, and a correct diet can be used to restore the altered microbiota and is considered to be the first approach to treat both GI disorders and MS, the aim of this review is to summarize the current knowledge of the protective roles of functional dietary components in GI and MS, and to assist in the derivation of a general perspective of these broad areas.
3. Methodology
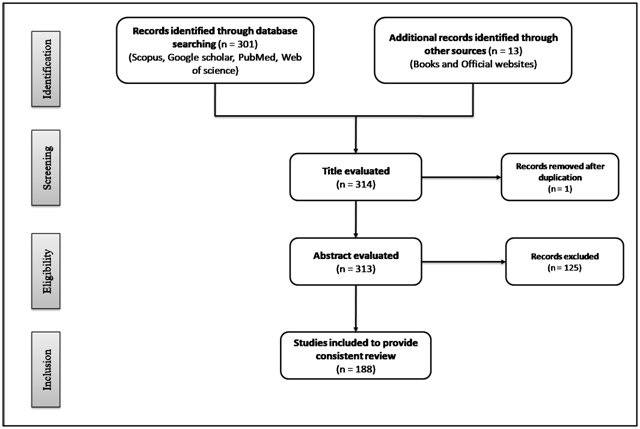
The present study consists of an up-to-date review of the literature covering the health benefits of functional dietary components with special reference to gut microbial modulation in GI disorders and MS. Various electronic databases were used for the literature search, including Scopus, Google scholar, PubMed and Web of Science, using the keywords “metabolic syndrome”, “gastrointestinal disorders”, “dietary food components”, “nutraceuticals”, “functional foods”, “dietary fibers”, “probiotics”, “prebiotics”, “saturated fatty acids”, “short chain fatty acids”, “monounsaturated fatty acids”, “polyunsaturated fatty acids” “polyphenols”, and “spices”. The criteria for selecting articles were “studies reported in English because of language barriers” and, “pre-clinical and clinical studies related to dietary food components”. The results returned 301 papers published up to the 2020 date. Of these articles, 175 were selected, summarized, and critically discussed so as to provide a consistent review. Some books and official websites (World Health Organization and, Food and Drug Organization) were also used for citing specific data within the scope of present study. Figure 3 illustrates the PRISMA flow diagram for study selection. In the following sections, functional dietary components are discussed, with their positive role in GI disorders and MS through the modulation of their most important risk factors, including gut microbes.
Figure 3. PRISMA flow diagram, showing the process of study selection.
4. Bioactive Dietary Components and Gastrointestinal Disorders
The health of the digestive system is important for its appropriate physiological functions, where said physiological parameters largely depend on the type of food ingested and the presence of bioactive components therein [57]. The major factors associated with the development of GI disorders are the use of medications for the treatment of chronic disorders [58], cultural attitudes [59], and socioeconomic factors [60]. The undesirable effects of drugs used for the treatment of GI disorders have led scientists and clinicians to focus on the use of alternative options. The use of dietary approaches, functional foods, and food supplement-based approaches are core parts of alternative treatments [61].
5. Bioactive Dietary Components and Metabolic Syndrome
Changes in nutritional composition of the diet and in energy expenditure can lead to an unhealthy state characterized by a low intake of dietary fibers and polyunsaturated fatty acids and a high consumption of sugars, total fats, cholesterol, and refined carbohydrates, creating an alarming situation of increasing incidence of MS risk factors. Traditional dietary approaches are known to play a substantial role in improving the overall health of an individual and in the prevention of metabolic dysregulation, but these may lack efficacy in achieving long term goals, possibly due to poor compliance. Thus, science has turned its attention towards nutrients with the ability to modulate gut microbiota or other biochemical pathways, with the ultimate goal of preventing MS. These include dietary fibers, prebiotics, probiotics, polyunsaturated fatty acids, and polyphenols [62][63][64].
6. Conclusions
Gut dysbiosis is one of the considerable factors associated with the pathogenesis of GI disorders and MS, by alteration of host’s immune responses and energy homeostasis, which may result in the upstream regulation of inflammatory cascades, insulin resistance, and impairment of the body’s metabolism [45]. Besides gut dysbiosis, researchers also explained the direct link between GI and metabolic disorders. Low-grade chronic inflammatory states in obesity usually facilitate the development and progression of other disorders including IBD [65]. This leads to the statement that prevention of metabolic disorders may also prevent or decrease the frequency of GI disorders like IBD. Despite the availability of a number of therapeutic options, none can provide an ultimate cure with a favorable safety profile. The undesirable effects of drugs have led scientists to consider the use of alternative treatments, including food supplements and functional foods. A review of available scientific literature reveals the health benefits of functional dietary components and their capacity for disease prevention. These have received considerable interest due to their potential nutritional, safety, and protective beneficial effects. In addition to the alteration of other mechanistic pathways, certain dietary components modify gut microbiota, which could provide an alternative approach to reduce a wide range of chronic disorders. In light of the literature available from pre-clinical and clinical studies, the regular consumption of bioactive dietary components in adequate amounts can be said to promote the growth of beneficial bacteria, decrease the inflammatory cascade, regulate intestinal immunity, improve lactose intolerance, enhance the digestive capability of the GI tract, upregulate digestive enzymes, and can improve insulin sensitivity and metabolic pathways. However, the limited available scientific evidence coming from human studies suggests that more in depth clinical trials on these agents in human populations are essential to make these treatments more competitive in the global market of functional foods.
References
- Oshima, T.; Miwa, H. Epidemiology of functional gastrointestinal disorders in Japan and in the world. J. Neurogastroenterol. Motil. 2015, 21, 320.
- Drossman, D.A. Functional gastrointestinal disorders: History, pathophysiology, clinical features, and Rome IV. Gastroenterology 2016, 150, 1262–1279.
- Toro-Martín, D.; Arsenault, B.J.; Després, J.-P.; Vohl, M.-C. Precision nutrition: A review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients 2017, 9, 913.
- Engin, A. The definition and prevalence of obesity and metabolic syndrome. In Obesity and Lipotoxicity; Springer: Cham, Switzerland, 2017; pp. 1–17.
- Lichtenstein, G.R.; Loftus, E.V.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; Sands, B.E. ACG clinical guideline: Management of Crohn’s disease in adults. Am. J. Gastroenterol. 2018, 113, 481–517.
- Danese, S.; Fiorino, G.; Peyrin-Biroulet, L. Positioning therapies in ulcerative colitis. Clin. Gastroenterol. Hepatol. 2020, 18, 1280–1290.
- Barbara, G.; Scaioli, E.; Barbaro, M.R.; Biagi, E.; Laghi, L.; Cremon, C.; Marasco, G.; Colecchia, A.; Picone, G.; Salfi, N. Gut microbiota, metabolome and immune signatures in patients with uncomplicated diverticular disease. Gut 2017, 66, 1252–1261.
- Holtmann, G.; Shah, A.; Morrison, M. Pathophysiology of functional gastrointestinal disorders: A holistic overview. Dig. Dis. 2017, 35, 5–13.
- Philpott, H.; Nandurkar, S.; Lubel, J.; Gibson, P.R. Republished: Drug-induced gastrointestinal disorders. Postgrad. Med. J. 2014, 90, 411–419.
- Francino, M. Antibiotics and the human gut microbiome: Dysbioses and accumulation of resistances. Front. Microbiol. 2016, 6, 1543.
- Scarborough, P.; Bhatnagar, P.; Wickramasinghe, K.K.; Allender, S.; Foster, C.; Rayner, M. The economic burden of ill health due to diet, physical inactivity, smoking, alcohol and obesity in the UK: An update to 2006–07 NHS costs. J. Public Health 2011, 33, 527–535.
- Sachs, G.; Scott, D.R. Helicobacter pylori: Eradication or preservation. F1000 Med. Rep. 2012, 4, 4.
- Ananthakrishnan, A.N.; Xavier, R.J. Gastrointestinal diseases. In Hunter’s Tropical Medicine and Emerging Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 16–26.
- McQuaid, K.R. Drugs used in the treatment of gastrointestinal diseases. In Basic and Clinical Pharmacology, 12th ed.; Katzung, B.G., Ed.; McGraw-Hill Education/Medical: New York, NY, USA, 2012; pp. 1081–1114.
- Gangarosa, L.M.; Seibert, D.G. Drugs used in gastrointestinal disorders. In Modern Pharmacology with Clinical Applications, 5th ed.; Craig, C.R., Stitzel, R.E., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004; pp. 470–483.
- Hindryckx, P.; Novak, G.; Bonovas, S.; Peyrin-Biroulet, L.; Danese, S. Infection risk with biologic therapy in patients with inflammatory bowel disease. Clin. Pharmacol. Ther. 2017, 102, 633–641.
- Parfitt, J.R.; Driman, D.K. Pathological effects of drugs on the gastrointestinal tract: A review. Hum. Pathol. 2007, 38, 527–536.
- El-Serag, H.B.; Sweet, S.; Winchester, C.C.; Dent, J. Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut 2014, 63, 871–880.
- Ford, A.C.; Marwaha, A.; Sood, R.; Moayyedi, P. Global prevalence of, and risk factors for, uninvestigated dyspepsia: A meta-analysis. Gut 2015, 64, 1049–1057.
- Scallan, E.; Griffin, P.M.; Angulo, F.J.; Tauxe, R.V.; Hoekstra, R.M. Foodborne illness acquired in the United States—Unspecified agents. Emerg. Infect. Dis. 2011, 17, 16–22.
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15.
- Wald, A.; Scarpignato, C.; Mueller-Lissner, S.; Kamm, M.; Hinkel, U.; Helfrich, I.; Schuijt, C.; Mandel, K. A multinational survey of prevalence and patterns of laxative use among adults with self-defined constipation. Aliment. Pharmacol. Ther. 2008, 28, 917–930.
- Nyrop, K.; Palsson, O.; Levy, R.; Korff, M.V.; Feld, A.; Turner, M.; Whitehead, W. Costs of health care for irritable bowel syndrome, chronic constipation, functional diarrhoea and functional abdominal pain. Aliment. Pharmacol. Ther. 2007, 26, 237–248.
- Oświęcimska, J.; Szymlak, A.; Roczniak, W.; Girczys-Połedniok, K.; Kwiecień, J. New insights into the pathogenesis and treatment of irritable bowel syndrome. Adv. Med. Sci. 2017, 62, 17–30.
- Aguirre, J.E.; Winston, J.; Sarna, S.K. Neonatal immune challenge followed by adult immune challenge induces epigenetic-susceptibility to aggravated visceral hypersensitivity. Neurogastroenterol. Motil. 2017, 29, e13081.
- Sadeghi, M. The metabolic syndrome. Arya Atheroscler. 2010, 2, 1–2.
- Vague, J. Sexual differentiation, a factor affecting the forms of obesity. La Presse Médicale 1947, 30, 339–340.
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553.
- Balkau, B. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet. Med. 1999, 16, 442–443.
- Zimmet, P.; Alberti, K.; Shaw, J. International Diabetes Federation: The IDF consensus worldwide definition of the metabolic syndrome. Diabetes Voice 2005, 50, 31–33.
- Lipińska, A.; Koczaj-Bremer, M.; Jankowski, K.; Kaźmierczak, A.; Ciurzyński, M.; Ou-Pokrzewińska, A.; Mikocka, E.; Lewandowski, Z.; Demkow, U.; Pruszczyk, P. Does family history of metabolic syndrome affect the metabolic profile phenotype in young healthy individuals? Diabetol. Metab. Syndr. 2014, 6, 75.
- Sun, K.; Liu, J.; Ning, G. Active smoking and risk of metabolic syndrome: A meta-analysis of prospective studies. PLoS ONE 2012, 7, e47791.
- Fan, A.Z.; Russell, M.; Naimi, T.; Li, Y.; Liao, Y.; Jiles, R.; Mokdad, A.H. Patterns of alcohol consumption and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 3833–3838.
- Park, Y.-W.; Zhu, S.; Palaniappan, L.; Heshka, S.; Carnethon, M.R.; Heymsfield, S.B. The metabolic syndrome: Prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch. Intern. Med. 2003, 163, 427–436.
- Gennuso, K.P.; Gangnon, R.E.; Thraen-Borowski, K.M.; Colbert, L.H. Dose–response relationships between sedentary behaviour and the metabolic syndrome and its components. Diabetologia 2015, 58, 485–492.
- Lutsey, P.L.; Steffen, L.M.; Stevens, J. Dietary intake and the development of the metabolic syndrome. Circulation 2008, 117, 754–761.
- Lamberti, J.S.; Olson, D.; Crilly, J.F.; Olivares, T.; Williams, G.C.; Tu, X.; Tang, W.; Wiener, K.; Dvorin, S.; Dietz, M.B. Prevalence of the metabolic syndrome among patients receiving clozapine. Am. J. Psychiatry 2006, 163, 1273–1276.
- Lim, S.; Eckel, R.H. Pharmacological treatment and therapeutic perspectives of metabolic syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 329–341.
- Rask Larsen, J.; Dima, L.; Correll, C.U.; Manu, P. The pharmacological management of metabolic syndrome. Expert Rev. Clin. Pharmacol. 2018, 11, 397–410.
- Prasad, H.; Ryan, D.A.; Celzo, M.F.; Stapleton, D. Metabolic syndrome: Definition and therapeutic implications. Postgrad. Med. 2012, 124, 21–30.
- Stern, M.P.; Williams, K.; González-Villalpando, C.; Hunt, K.J.; Haffner, S.M. Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care 2004, 27, 2676–2681.
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 2 March 2020).
- WHO. Hypertension. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 2 March 2020).
- WHO. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 2 March 2020).
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191.
- Tang, T.W.; Chen, H.-C.; Chen, C.-Y.; Yen, C.Y.; Lin, C.-J.; Prajnamitra, R.P.; Chen, L.-L.; Ruan, S.-C.; Lin, J.-H.; Lin, P.-J. Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation 2019, 139, 647–659.
- Hur, K.Y.; Lee, M.-S. Gut microbiota and metabolic disorders. Diabetes Metab. J. 2015, 39, 198–203.
- Terrapon, N.; Henrissat, B. How do gut microbes break down dietary fiber? Trends Biochem. Sci. 2014, 39, 156–158.
- Prasad, K.N.; Bondy, S.C. Dietary fibers and their fermented short-chain fatty acids in prevention of human diseases. Bioact. Carbohydr. Diet. Fibre 2019, 17, 100170.
- Barbara, G.; Feinle-Bisset, C.; Ghoshal, U.C.; Santos, J.; Vanner, S.J.; Vergnolle, N.; Zoetendal, E.G.; Quigley, E.M. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology 2016, 150, 1305–1318.
- Kannampalli, P.; Shaker, R.; Sengupta, J.N. Colonic butyrate-algesic or analgesic? Neurogastroenterol. Motil. 2011, 23, 975–979.
- Tana, C.; Umesaki, Y.; Imaoka, A.; Handa, T.; Kanazawa, M.; Fukudo, S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol. Motil. 2010, 22, 512.
- Talukder, J. Nutraceuticals in gastrointestinal conditions. In Nutraceuticals in Veterinary Medicine; Gupta, R.C., Srivastava, A., Lall, R., Eds.; Springer: Cham, Switzerland, 2019; pp. 467–479.
- Myasoedova, E.; Talley, N.J.; Manek, N.J.; Crowson, C.S. Prevalence and risk factors of gastrointestinal disorders in patients with rheumatoid arthritis: Results from a population-based survey in Olmsted County, Minnesota. Gastroenterol. Res. Pract. 2011, 2011, 1–7.
- Chuah, K.-H.; Mahadeva, S. Cultural factors influencing functional gastrointestinal disorders in the east. J. Neurogastroenterol. Motil. 2018, 24, 536.
- Ribaldone, D.G.; Pellicano, R.; Actis, G.C. Inflammation in gastrointestinal disorders: Prevalent socioeconomic factors. Clin. Exp. Gastroenterol. 2019, 12, 321.
- Gul, K.; Singh, A.; Jabeen, R. Nutraceuticals and functional foods: The foods for the future world. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627.
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607.
- Mongraw-Chaffin, M.; Foster, M.C.; Anderson, C.A.; Burke, G.L.; Haq, N.; Kalyani, R.R.; Ouyang, P.; Sibley, C.T.; Tracy, R.; Woodward, M. Metabolically healthy obesity, transition to metabolic syndrome, and cardiovascular risk. J. Am. Coll. Cardiol. 2018, 71, 1857–1865.
- Åberg, F.; Helenius-Hietala, J.; Puukka, P.; Färkkilä, M.; Jula, A. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology 2018, 67, 2141–2149.
- Alberti, K.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645.
- Ríos-Hoyo, A.; Cortés, M.J.; Rios-Ontiveros, H.; Meaney, E.; Ceballos, G.; Gutierrez-Salmean, G. Obesity, metabolic syndrome, and dietary therapeutical approaches with a special focus on nutraceuticals (polyphenols): A mini-review. Int. J. Vitam. Nutr. Res. 2014, 84, 113–123.
- Davì, G.; Santilli, F.; Patrono, C. Nutraceuticals in diabetes and metabolic syndrome. Cardiovasc. Ther. 2010, 28, 216–226.
- Chakraborty, R.; Das, L. Nutraceuticals and their role in human health: A review. In Nutraceuticals and Functional Foods in Human Health and Disease Prevention; Bagchi, D., Preuss, H.G., Swaroop, A., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 61–72.
- Szilagyi, A. Relationship (s) between obesity and inflammatory bowel diseases: Possible intertwined pathogenic mechanisms. Clin. J. Gastroenterol. 2019, 13, 139–152.