Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zohaib Khurshid | + 1963 word(s) | 1963 | 2021-08-31 10:13:10 | | | |
| 2 | Rita Xu | Meta information modification | 1963 | 2021-09-08 03:34:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Khurshid, Z. Hydroxyapatite in Oral Care Products. Encyclopedia. Available online: https://encyclopedia.pub/entry/13976 (accessed on 07 February 2026).
Khurshid Z. Hydroxyapatite in Oral Care Products. Encyclopedia. Available at: https://encyclopedia.pub/entry/13976. Accessed February 07, 2026.
Khurshid, Zohaib. "Hydroxyapatite in Oral Care Products" Encyclopedia, https://encyclopedia.pub/entry/13976 (accessed February 07, 2026).
Khurshid, Z. (2021, September 07). Hydroxyapatite in Oral Care Products. In Encyclopedia. https://encyclopedia.pub/entry/13976
Khurshid, Zohaib. "Hydroxyapatite in Oral Care Products." Encyclopedia. Web. 07 September, 2021.
Copy Citation
Calcium phosphate compounds form the inorganic phases of our mineralised tissues such as bone and teeth, playing an important role in hard tissue engineering and regenerative medicine. In dentistry and oral care products, hydroxyapatite (HA) is a stable and biocompatible calcium phosphate with low solubility being used for various applications such as tooth remineralisation, reduction of tooth sensitivity, oral biofilm control, and tooth whitening.
hydroxyapatite
hydroxyapatite toothpaste
remineralisation
teeth whitening
biofilm
dentine hypersensitivity
1. Introduction
Dental caries is a global disease affecting all ages and sectors of the population. Dental caries remains the most common chronic bacterial driven disease despite advancements in early detection and treatment [1]. According to a study by The Global Burden of Disease in 2017, it is estimated that 2.3 billion people suffer from caries of permanent teeth and more than 530 million children suffer from caries of primary teeth [2]. Untreated caries can progress into the tooth pulp, lead to dental abscesses, cause significant pain and suffering, and ultimately tooth loss [1]. Dental caries is caused by the action of acids on the enamel surface. The acids are produced as a byproduct from bacteria in the dental biofilm (plaque) metabolising the sugars in foods or drinks [1]. The produced acid leads to a loss of calcium and phosphate from the enamel; this process is called demineralization [1]. The gingiva can also become inflamed in response to plaque irritation, diagnosed as gingivitis [3]. The Global Burden of Disease Study 2017 also reported that the prevalence of severe periodontal (gum) diseases affect almost 10% of the global population [2]. Consequently, controlling oral microbial biofilms on the tooth surface is essential to prevent the progression of caries and periodontal disease.
Hydroxyapatite (HA) is a bioactive and non-toxic ceramic that has a close analogy to the inorganic portion of human teeth and bone. It is arranged in a typical lattice structure with the chemical formulation of (Ca10(PO4)6(OH)2) [4]. Depending on the manufacturing technique employed, various synthetic apatites are produced today [5]. HA used for biomedical applications is chemically prepared to achieve tailored properties such as chemical purity, crystal morphology, and crystal size [5]. HA’s bioactive, non-toxic, and osteoconductive properties mean it can form direct chemical bonds with living tissues. Thus, HA is a bioceramic that is widely used as an implantable material in dentistry, maxillofacial and orthopaedic surgery to repair bone defects and as a coating material for metallic implants [6]. Studies of the osteogenesis effectiveness of HA-based coatings have shown favourable results, however, their validity has been critically considered [7]. Most of these favourable results were observed in in vitro studies rather than clinical and in vivo studies, and there is a demand for more standardised comparison criteria of the published research to draw sound clinical conclusions [7]. It has also been noted that the clinical effectiveness is dependent on the specific geometry, pore size, and degradation rate of the HA, whereby a smaller particle size is desirable [8].
The biocompatibility of nano-HA has also made it attractive as a potential novel reinforcing filler in composite restorations [9]. However, so far, nano-HA has been shown to be clinically unsuitable due to high water uptake, high refractive index and hence light scattering [9]. Therefore, no significantly improved properties compared to conventional composites with regard to tooth mechanical properties and biofilm protection [9]. However, there is suggestive more promise with smaller HA particles and increasing total filler amount [9].
The external layer of human teeth, the enamel, is composed of 97% inorganic component, and the dentine is composed of 70% inorganic component; these inorganic phases are mainly made up of HA [10][11]. In the past 50 years, calcium phosphate for daily oral care has been thoroughly researched, especially in preventative dentistry. HA can be extracted from various natural resources such as bovine, ovine, porcine bone and marine structures [12][13][14][15][16]. Trace elements such as zinc, sodium, magnesium and carbonate present in some natural sources of HA have been found to mimic the apatite produced from human bone and influence the physical properties of HA such as crystal structure, morphology and solubility, and also the thermal stability [4]. Naturally sourced HA can also be a more environmentally friendly, sustainable, and economical substitute than synthetic HA [12]. However, using naturally sourced HA in oral care products has not been investigated.
HA dentifrice with synthetic HA for teeth brushing was first produced by NASA (U.S. National Aeronautics and Space Authority) as a repairing material for the astronauts who lost minerals in their teeth and bones due to the absence of gravity in 1970. In 1978, the Japanese company Sangi Co. Ltd. (Tokyo, Japan) developed the world’s first enamel restorative dentifrice. In 1993, nano-HA was approved as an anti-caries agent. Since then, in 2003, nano-HA particle size has been reduced from 100 to 50 nanometers, making it more effective at penetrating below the surface of the enamel [11][17]. Moreover, HA has also been incorporated into oral rinses and gels for oral home care to aid remineralisation and biofilm control [18][19][20].
The ideal properties of a dentifrice include minimal abrasive effect, non-irritating, non-toxic, non-staining, protects against caries and biofilm formation, while being cost-effective and readily available [21]. Many studies have been conducted to test the efficacy of HA, especially in enamel and dentine remineralisation, biofilm control, reducing dentine sensitivity, and tooth whitening [5][10][11][22][23][24][25][26][27][28][29][30]. Currently, only a few reviews report on nanomaterial in oral care products or HA in dentistry which includes restorative, preventative, and regenerative applications [5][10][11][31][32].
2. Hydroxyapatite in Oral Care Products
As HA is naturally found in enamel and dentine, synthetic HA is incorporated into dental products in various examples such as dental cement, fillings, and oral care products, including dentifrice, mouthwash, and gels [8]. HA can be synthesised in different crystallite morphologies such as spherical or needle-like and particle sizes (micro to nano) [5][33]. Currently, there are two types of HA; the nanocrystalline and the microcluster form, both nano and micro-HA are available in oral care products [10][30]. The inorganic component of enamel is made of HA crystallites in the range of ~50 nm in diameter [34]. HA crystallites are tightly packed and strictly organised as enamel prisms [5]. Micro-HA particles are about 5–10 microns in size, and this is significantly larger than enamel HA and dentinal tubules. Therefore these are not as effective in remineralisation and in reducing sensitivity [5].
Nano-HA particle size ranges from 20–100 nm, with a rod-shaped morphology resembling those in natural enamel (Figure 1) [11][22][35]. Nano-HA have a high affinity to bind to substances due to an increased surface area which may improve remineralisation and reduce sensitivity as HA with a 20–50 nm size matches the nano-sized defects due to acidic erosion at the enamel surface [32][36]. Nano-HA are also thought to be more effective than micro-HA in biofilm management. The interaction with microorganisms is only possible if the particles involved are smaller than the microorganisms. Nano-size particles are small enough to directly interact with the bacterial membrane [19][32].
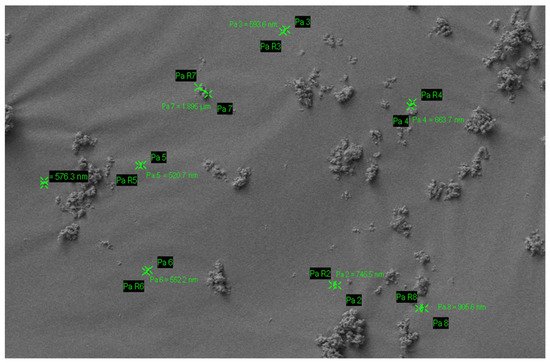
Figure 1. Particle size of nano-hydroxyapatite.
There are currently many toothpastes available in the market claiming to prevent dental caries, gum disease, desensitisation, tooth whitening, and remineralisation of dental hard tissues such as dentine and enamel. The anti-dental caries properties of these kinds of toothpaste are primarily based on potassium nitrates, triclosan, stannous chloride, zinc salt and fluoride compounds such as sodium fluoride, sodium monofluorophosphate, stannous fluoride. For the past few years, HA-based toothpastes have attracted more interest in the market and from manufacturers due to their biocompatibility with hard tissue and substituting capability [37][38][39]. Table 1 summarises the description of commercially available HA-based toothpastes. However, these products are still not as readily available as other products due to higher costs and limited clinical studies.
Table 1. Commercial toothpaste based on hydroxyapatite (nano).
| S. No | Commercial Product | Ingredients | Country |
|---|---|---|---|
| 1 | APAGARD® PREMIO | Aqua, dicalcium phosphate, glycerin, xylitol, hydroxyapatite (nano), silica, Peg-8, sodium lauryl sulfate, cellulose gum, aroma, sodium silicate, trimagnesium phosphate, hydrolyzed conchiolin protein, sodium saccharin, glycyrrhetinic acid, cetylpyridinium chloride, lauryl diethylenediaminoglycine Hcl | Germany |
| 2 | Ela Mint Toothpaste | Water, vegetable glycerin, hydrated silica, sorbitol powder, silica, hydroxyapatite (nano), sodium benzoate, sodium lauroyl sarcosinate, mentha piperita essential (peppermint) oil, Mentha viridis (spearmint) oil, Illicium verum (star anise) oil, Gaultheria procumberis (wintergreen) oil, xylitol, xanthan gum, Stevia rebaudiana extract powder, methylsulfonylmethane, Aloe barbadensis (aloe vera) leaf juice, sodium bicarbonate, Camellia sinensis (green tea) leaf extract, Cucumis sativus (cucumber) fruit extract, Persea gratissima (avocado) fruit extract, Mangifera indica (mango) fruit extract, menthol, Elettaria cardamomum miniscula seed (cardamom), potassium chloride. | USA |
| 3 | Coco Ginger Toothpaste | Glycerin, water, hydrated silica, erythritol, silica, natural flavors (coconut and ginger), hydroxyapatite (nano), xanthan gum, sodium benzoate, Aloe barbadensis (aloe vera) leaf juice, Chamomilla recutita (chamomile) flower extract, methylsulfonylmethane (msm), potassium chloride, sodium bicarbonate, Stevia rebaudiana extract powder, sodium lauroyl sarcosinate. | USA |
| 4 | APAGARD® RIN-SU | Aqua, glycerin, xylitol, hydroxyapatite, xanthan gum, alcohol, polyglyceryl-5 stearate, lauryl diethylenediaminoglycine HCL, aroma, cethylpyridinium chloride | Germany |
| 5 | APADENT® TOTAL CARE | Aqua, dicalcium phosphate, glycerin, hydroxyapatite (nano), silica, peg-8, sodium lauryl sulphate, cellulose gum, aroma, trimagnesium phosphate, pvp, butylene glyium sodium, alcoholic acrylic, sodium sodium, sodium, sodium, sodium, sodylacride, sodylen, sodylacride, sodylacride, sodyl. pyridoxine HCL, lauryl diethylenediaminoglycine HCL, Camellia sinensis leaf extract, Chamomilla recutilla (Matricaria) extract, Salvia officinalis (Sage) leaf extract | Germany |
| 6 | Travel Size Ela Mint Toothpaste | Water, vegetable glycerin, hydrated silica, sorbitol powder, silica, hydroxyapatite (nano), sodium benzoate, sodium lauroyl sarcosinate, Mentha piperita essential (peppermint) oil, Mentha viridis (spearmint) oil, Illicium verum (star anise) oil, Gaultheria procumberis (wintergreen) oil, xylitol, xanthan gum, stevia rebaudiana extract powder, methylsulfonylmethane, Aloe barbadensis (aloe vera) leaf juice, sodium bicarbonate, camellia sinensis (green tea) leaf extract, Cucumis sativus (cucumber) fruit extract, Persea gratissima (avocado) fruit extract, Mangifera indica (mango) fruit extract, menthol, Elettaria cardamomum miniscula seed (cardamom), potassium chloride. | USA |
| 7 | Toothpaste PrevDent® nHAp™ | Aqua, hydrated silica, sorbitol, glycerin, xylitol, potassium nitrate, nano-hydroxyapatite, magnesium aluminum silicate, mentha piperita oil, sodium lauroyl sarcosinate, xanthan gum, phenoxyethanol, potassium chloride, sodium sulfate, sodium saccharin, CI 77891 | The Netherlands |
| 8 | Biorepair® | Sorbitol, Aqua, Silica, PEG32, Glycerin, Aroma, Zinc hydroxyapatite, Na-Myristoyl Sarcosinate, Celulose gum, Citric acid, Na-benzoate, Benzylalcohol, Na-methyl cocoyl taurate, Mentha peprita oil, Na-sacchrine, K-sorbate, Fragaria vesca Juice, Anethole, Phenoxyethanol, Mentho |
Italy |
| 9 | X-PUR Remin® | 10% Nano Medical Hydroxyapatite 10% Xylitol, Water, macrogol 400, zeolite, polyvinylpyrrolidone, glycyrrthetinc acid, cetylpyridinium chloride, glycerin, xylitol silicic anhydride, castor oil, sodium lauroyl glutamate, carragenan, ethanol, carboxymethylcellulose sodium, titanium dioxide, flavour. |
Canada |
| 10 | Biorepair® Advanced Active Shield Anti-Cavities | Aqua, zinc hydroxyapatite, hydrated silica, sorbitol, glycerin, xylitol, silica, aroma, cellulose gum, zinc pca, sodium myristoyl sarcosinate, sodium methyl cocoyl taurate, tetrapotassium pyrophosphate, sodium saccharin, zinc citrate, citric acid, ammonium acryloyldimethyltaurate/VP copolymer, benzyl alcohol, phenoxyethanol, sodium benzoate, limonene. | Italy |
| 11 | GUM SensiVital+ toothpaste | Glycerin, aqua, hydrated silica, isomalt, potassium nitrates, hydroxyapatite, PVM/MA copolymer, lauryl glucoside, PEG-40 hydrogenated castor oil, sodium monofluorophosphate, aroma, cellulose gum, sodium hydroxide, sodium saccharin, cocamidopropyl betaine, hesperidin, sucralose, sodium chloride, cetylpyridinium chloride, sodium benzoate, CI 42090. | Germany |
| 12 | Kinder Karex™ toothpaste | Aqua, hydrogenated starch hydrolysate, hydrated silica, hydroxyapatite, xylitol, silica, cellulose gum, aroma, 1,2-hexanediol, caprylyl glycol, sodium methyl cocoyl taurate, sodium sulfate, sodium cocoyl glycinate, limonene | Germany |
| 13 | NanoXIM •CarePaste |
(Synthetic nano-HA water-based suspension ingredient designed to be easily incorporated in oral care products.) hydroxyapatite (nano), Potassium Chloride, Microbial content, heavy metals. | Portugal |
| 14 | VITIS® whitening toothpaste | Aqua, glycerin, sorbitol, silica, PVP, sodium lauryl sulphate, titanium dioxide, sodium monofluorophosphate, pentasodium triphosphate, perlite, sodium hexametaphosphate, xanthan gum, xylitol, tetrapotassium pyrophosphate, hydroxylapatite (nano), menthone glycerin acetal, sodium saccharin, potassium chloride, sodium methylparaben, potassium acesulfame, aroma. | Spain |
| 15 | INNOVA | Aqua, hydrated silica, hydrogenated starch hydrolysate, glycerin, PEG-8, hydroxyapatite, cellulose gum, aroma, sodium monofluorophosphate, cocamidopropyl betaine, sodium lauroyl sarcosinate, xylitol, propylene glycol, olaflur, Stevia rebaudiana leaf extract, anethole, citric acid, eucalyptol, o-cymen-5-ol, tocopheryl acetate, CI 77891, thymol, calcium lactate, Vitis vinifera seed extract, disodium EDTA, aspergillus/tannic acid ferment extract, glucose, inositol, sodium benzoate, potassium sorbate, limonene. Fluoride content—0,15% (1500 ppm). | Russian |
| 16 | MEGASONEX | Sorbitol, glycerin, hydroxyapatite (nano), water (aqua), silica, xylitol, tetrasodium pyrophosphate, sodium methyl cocoyl taurate, mica, titanium dioxide, sodium carboxymethylcellulose, citric acid, sodium saccharin, aroma | USA |
| 17 | WhiteWashLaboratories | Glycerin, aqua, calcium carbonate, xylitol, hydroxyapatite, potassium nitrate, hydrated silica, tetrasodium pyrophosphate, kaolin, sodium bicarbonate, pentasodium triphosphate, pvp, sodium monofluorophosphate, cocamidopropyl betaine, potassium chloride, xanthan gum, stevioside, Mentha piperita oil, bromelain, l-menthol, papain, urea peroxide, Eucalyptus globulus leaf oil, limonene. | UK |
References
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 1–16.
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858.
- Stamm, J.W. Epidemiology of gingivitis. J. Clin. Periodontol. 1986, 13, 360–366.
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1285–1299.
- Enax, J.; Epple, M. Synthetic hydroxyapatite as a biomimetic oral care agent. Oral Health Prev. Dent 2018, 16, 7–19.
- Adamopoulos, O.; Papadopoulos, T. Nanostructured bioceramics for maxillofacial applications. J. Mater. Sci. Mater. Med. 2007, 18, 1587–1597.
- Surmenev, R.A.; Surmeneva, M.A. A critical review of decades of research on calcium phosphate–based coatings: How far are we from their widespread clinical application? Curr. Opin. Biomed. Eng. 2019, 10, 35–44.
- Habibah, T.U.; Amlani, D.V.; Brizuela, M. Hydroxyapatite Dental Material. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020.
- Domingo, C.; Arcís, R.; López-Macipe, A.; Osorio, R.; Rodríguez-Clemente, R.; Murtra, J.; Fanovich, M.; Toledano, M. Dental composites reinforced with hydroxyapatite: Mechanical behavior and absorption/elution characteristics. J. Biomed. Mater. Res. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2001, 56, 297–305.
- Meyer, F.; Amaechi, B.T.; Fabritius, H.-O.; Enax, J. Overview of calcium phosphates used in biomimetic oral care. Open Dent. J. 2018, 12, 406.
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Annali di Stomatologia 2014, 5, 108.
- Pu’ad, N.A.S.M.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588.
- Ratnayake, J.T.B.; Gould, M.L.; Shavandi, A.; Mucalo, M.; Dias, G.J. Development and characterization of a xenograft material from New Zealand sourced bovine cancellous bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1054–1062.
- Shavandi, A.; Bekhit, A.E.-D.A.; Ali, A.; Sun, Z.; Ratnayake, J.T. Microwave-assisted synthesis of high purity β-tricalcium phosphate crystalline powder from the waste of Green mussel shells (Perna canaliculus). Powder Technol. 2015, 273, 33–39.
- Shavandi, A.; Hou, Y.; Carne, A.; McConnell, M.; Bekhit, A.E.-D.A. Chapter Four—Marine Waste Utilization as a Source of Functional and Health Compounds. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 87, pp. 187–254.
- Shavandi, A.; Wilton, V.; Bekhit, A.E.-D.A. Synthesis of macro and micro porous hydroxyapatite (HA) structure from waste kina (Evechinus chloroticus) shells. J. Taiwan Inst. Chem. Eng. 2016, 65, 437–443.
- Najibfard, K.; Ramalingam, K.; Chedjieu, I.; Amaechi, B. Remineralization of early caries by a nano-hydroxyapatite dentifrice. J. Clin. Dent. 2011, 22, 139.
- Amaechi, B.T.; AbdulAzees, P.A.; Okoye, L.O.; Meyer, F.; Enax, J. Comparison of hydroxyapatite and fluoride oral care gels for remineralization of initial caries: A pH-cycling study. BDJ Open 2020, 6, 1–7.
- Hannig, C.; Basche, S.; Burghardt, T.; Al-Ahmad, A.; Hannig, M. Influence of a mouthwash containing hydroxyapatite microclusters on bacterial adherence in situ. Clin. Oral Investig. 2013, 17, 805–814.
- Hill, R.G.; Gillam, D.G.; Chen, X. The ability of a nano hydroxyapatite toothpaste and oral rinse containing fluoride to protect enamel during an acid challenge using 19F solid state NMR spectroscopy. Mater. Lett. 2015, 156, 69–71.
- Jagtap, A.M.; Kaulage, S.R.; Kanse, S.S.; Shelke, V.D.; Gavade, A.S.; Vambhurkar, G.B.; Todkar, R.R.; Dange, V.N. Preparation and Evaluation of Toothpaste. Asian J. Pharm. Anal. 2018, 8, 191–194.
- Ramis, J.M.; Coelho, C.C.; Córdoba, A.; Quadros, P.A.; Monjo, M. Safety assessment of nano-hydroxyapatite as an oral care ingredient according to the EU cosmetics regulation. Cosmetics 2018, 5, 53.
- Schlagenhauf, U.; Kunzelmann, K.H.; Hannig, C.; May, T.W.; Hösl, H.; Gratza, M.; Viergutz, G.; Nazet, M.; Schamberger, S.; Proff, P. Impact of a non-fluoridated microcrystalline hydroxyapatite dentifrice on enamel caries progression in highly caries-susceptible orthodontic patients: A randomized, controlled 6-month trial. J. Investig. Clin. Dent. 2019, 10, e12399.
- Paszynska, E.; Pawinska, M.; Gawriolek, M.; Kaminska, I.; Otulakowska-Skrzynska, J.; Marczuk-Kolada, G.; Rzatowski, S.; Sokolowska, K.; Olszewska, A.; Schlagenhauf, U. Impact of a toothpaste with microcrystalline hydroxyapatite on the occurrence of early childhood caries: A 1-year randomized clinical trial. Sci. Rep. 2021, 11, 1–15.
- Steinert, S.; Zwanzig, K.; Doenges, H.; Kuchenbecker, J.; Meyer, F.; Enax, J. Daily application of a toothpaste with biomimetic hydroxyapatite and its subjective impact on dentin hypersensitivity, tooth smoothness, tooth whitening, gum bleeding, and feeling of freshness. Biomimetics 2020, 5, 17.
- Steinert, S.; Kuchenbecker, J.; Meyer, F.; Simader, B.; Zwanzig, K.; Enax, J. Whitening Effects of a Novel Oral Care Gel with Biomimetic Hydroxyapatite: A 4-Week Observational Pilot Study. Biomimetics 2020, 5, 65.
- Sarembe, S.; Enax, J.; Morawietz, M.; Kiesow, A.; Meyer, F. In vitro whitening effect of a hydroxyapatite-based oral care gel. Eur. J. Dent. 2020, 14, 335.
- Grocholewicz, K.; Matkowska-Cichocka, G.; Makowiecki, P.; Droździk, A.; Ey-Chmielewska, H.; Dziewulska, A.; Tomasik, M.; Trybek, G.; Janiszewska-Olszowska, J. Effect of nano-hydroxyapatite and ozone on approximal initial caries: A randomized clinical trial. Sci. Rep. 2020, 10, 1–8.
- Demito, C.F.; Costa, J.V.d.; Fracasso, M.d.L.C.; Ramos, A.L. Efficacy of fluoride associated with nano-hydroxyapatite in reducing enamel demineralization adjacent to orthodontic brackets: In situ study. Dent. Press J. Orthod. 2020, 24, 48–55.
- Amaechi, B.T.; AbdulAzees, P.A.; Alshareif, D.O.; Shehata, M.A.; Lima, P.P.d.C.S.; Abdollahi, A.; Kalkhorani, P.S.; Evans, V. Comparative efficacy of a hydroxyapatite and a fluoride toothpaste for prevention and remineralization of dental caries in children. BDJ Open 2019, 5, 1–9.
- Hannig, M.; Hannig, C. Nanomaterials in preventive dentistry. Nat. Nanotechnol. 2010, 5, 565–569.
- Enax, J.; Fabritius, H.-O.; Fabritius-Vilpoux, K.; Amaechi, B.T.; Meyer, F. Modes of action and clinical efficacy of particulate hydroxyapatite in preventive oral health care—State of the art. Open Dent. J. 2019, 13, 274–287.
- Zafar, M.S.; Alnazzawi, A.A.; Alrahabi, M.; Fareed, M.A.; Najeeb, S.; Khurshid, Z. 18—Nanotechnology and nanomaterials in dentistry. In Advanced Dental Biomaterials; Khurshid, Z., Najeeb, S., Zafar, M.S., Sefat, F., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 477–505.
- Kuśnieruk, S.; Wojnarowicz, J.; Chodara, A.; Chudoba, T.; Gierlotka, S.; Lojkowski, W. Influence of hydrothermal synthesis parameters on the properties of hydroxyapatite nanoparticles. Beilstein J. Nanotechnol. 2016, 7, 1586–1601.
- Coelho, C.C.; Grenho, L.; Gomes, P.S.; Quadros, P.A.; Fernandes, M.H. Nano-hydroxyapatite in oral care cosmetics: Characterization and cytotoxicity assessment. Sci. Rep. 2019, 9, 1–10.
- Ramesh, N.; Ratnayake, J.T.B.; Moratti, S.C.; Dias, G.J. Effect of chitosan infiltration on hydroxyapatite scaffolds derived from New Zealand bovine cancellous bones for bone regeneration. Int. J. Biol. Macromol. 2020, 160, 1009–1020.
- Esteves-Oliveira, M.; Santos, N.M.; Meyer-Lückel, H.; Wierichs, R.J.; Rodrigues, J.A. Caries-preventive effect of anti-erosive and nano-hydroxyapatite-containing toothpastes in vitro. Clin. Oral Investig. 2017, 21, 291–300.
- Tschoppe, P.; Zandim, D.L.; Martus, P.; Kielbassa, A.M. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J. Dent. 2011, 39, 430–437.
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic aspects of restorative dentistry biomaterials. Biomimetics 2020, 5, 34.
More
Information
Subjects:
Dentistry, Oral Surgery & Medicine; Biochemistry & Molecular Biology; Engineering, Biomedical
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
08 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




