
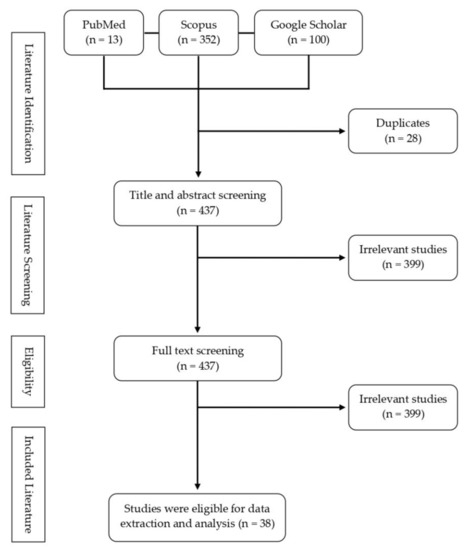
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohammad Basyuni | + 1554 word(s) | 1554 | 2021-08-24 11:41:23 | | | |
| 2 | Beatrix Zheng | + 1494 word(s) | 3048 | 2021-09-03 08:06:28 | | |
Video Upload Options
Cancer is a deadly disease that has become a burden to everyone. There were 9.8 million cancer deaths reported in 2018. Colorectal cancer (CRC) is the third most common type of cancer globally, with 1.80 million cases, and it ranks second with the highest mortality in the world, i.e., 862,000 deaths.
1. Introduction
Cancer is a deadly disease that has become a burden to everyone. There were 9.8 million cancer deaths reported in 2018. Colorectal cancer (CRC) is the third most common type of cancer globally, with 1.80 million cases, and it ranks second with the highest mortality in the world, i.e., 862,000 deaths [1]. The increase in CRC in developing countries is possibly due to an increase in the aging population, modern living habits, dietary habits, and an increase in risk factors for CRC, which include genetic diseases, smoking, alcohol, and lack of exercise. The percentage of CRC deaths in Indonesia in 2014 was 10% of the 103,000 CRC mortality rates in men and 8.5% of the 92,000 in women [2][3]. In Indonesia, CRC is an interesting case. For instance, the median age of colorectal cancer patients is younger than the western population. This means that productive young people are affected more, thus posing a heavy economic burden on their families [4][5][6].
Cancer treatment with chemotherapy agents is still an option, but the multi-drug resistance (MDR) mechanism has resulted in reduced efficacy of chemotherapy drugs [7]. The chemoprevention agents referred to here generally have the role of inhibiting tumor growth through cell cycle arrest mechanisms [8], stimulating apoptosis, or inhibiting the expression of proteins that play a role in MDR [9]. Various efforts are needed to develop new treatment methods for more effective therapy and prevention of degenerative diseases [10]. Alternative options such as the use of medicinal plants in the treatment of degenerative diseases can decrease any side effects [11]. Usually, lower effects may affect low efficacy, so there may be a trade-off. Therefore, there is a need to develop targeted therapy that uses super toxic plant-derived toxins as warheads that are to be conjugated to monoclonal antibodies targeting the CRC-specific antigens [12].
One of the strategies in the discovery and development of drugs for the prevention of degenerative diseases is by exploring natural products, especially plants, that have the potential to be sources of antioxidants. Plants are known to have an important role in drug discovery [13]. Natural products are secondary metabolites produced by plants, animals, and microorganisms in response to external stimulation such as changes in nutrition, infection, and competition [14].
2. Analysis on Results
| Language | Geographical Location | Population Search Terms | Intervention Search Terms |
|---|---|---|---|
| English | Indonesia | Colorectal cancer* OR “colorectal cancer*” | Secondary metabolite* OR natural product* |
| Bahasa Indonesia | Indonesia | “Kanker kolon” atau kanker usus besar* | Produk bahan alam* atau metabolit sekunder* |
| Year | Number of Publication (a) |
|---|---|
| 1990 | 1 |
| 2007 | 1 |
| 2008 | 2 |
| 2012 | 2 |
| 2013 | 1 |
| 2015 | 2 |
| 2016 | 4 |
| 2017 | 5 |
| 2018 | 8 |
| 2019 | 7 |
| 2020 | 5 |
| Types of Publication | Publication Reported (b) |
| Book/Book Chapter | 1 |
| Journal Article | 35 |
| Thesis | 2 |
| Types of Natural Product | Number of Natural Product Reported (a) |
|---|---|
| Phenolic | 8 |
| Phytosterol | 9 |
| Carotenoid | 1 |
| Terpenoid | 17 |
| Alkaloid | 8 |
| Flavonoid | 5 |
| Peptide | 3 |
| Polyketide | 2 |
| Polyisoprenoid | 5 |
| Carbolyc acid | 1 |
| Fatty acid | 5 |
| Glycoside | 2 |
| Aromatic compound | 1 |
| Types of Cell Lines | Number of Used Cell Lines (b) |
| HCT-15 | 3 |
| Colo205 | 1 |
| HT-29 | 5 |
| CaCo-2 | 2 |
| HCT-116 | 6 |
| SW-480 | 1 |
| CRC | 2 |
| Colo320DM | 1 |
| WiDr | 16 |
| ADC | 1 |
| AOM CRC Rat Model | 2 |
| Colorectal Cancer Cytotoxicity Analysis Method | Number of Used Method (c) |
| MTT in vitro assay | 22 |
| In vivo | 3 |
| Others in vitro | 8 |
| Types Method | Colorectal Cancer Cytotoxic Analysis Method | Types of Object/CRC Cell Lines | Types of Natural Products | The Concentration of the Tested Samples | IC50 Value / % Cell Viability / % Inhibition | Cytotoxicity Categorize [18] | Mechanism of Actions |
|---|---|---|---|---|---|---|---|
| In vivo | Colonic lesions induced by azoxymethane (AOM) | Rats | Non-nutritive compounds in fruits, vegetables, and fruits [19] | ― | ― | ― | Control of cell proliferation in ACFs and/or normal-appearing crypts of rats exposed to AOM [19]. |
| Rats | Ethanol extract of Phaleria macrocarpa fruits (mostly flavonoids contains) [20] | ― | ― | ― | The crude ethanolic extract of P. macrocarpa had high antioxidant activity and it modulated the oxidative stress as proved by the up-regulation of glutathione-s-transferase and superoxide dismutase [20]. | ||
| Rats | Water extract of Premna oblongifolia Merr. Leaves (polyphenolic compound) [21] | ― | ― | ― | Natural dietary fiber and antioxidant sources (as found in fruits, vegetables, and plant extracts) may exhibit a protective effect against CRC [21]. | ||
| Xenograft model nude mice (carrying HCT-15 cells) [22] | Mice | Lissoclibadins (polysulfur aromatic alkaloids) from Ascidian lissoclinum [22] | ― | ― | ― | Lissoclibadin 1 suppressed tumor growth in nude mice. Lissoclibadin 1 induced cell death via apoptosis due to the mitochondrial cytochrome dependent activation (intrinsic pathway) of the caspase-9 and caspase-3 cascade pathway [22]. | |
| In vitro | MTT assay [23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43]; MTS assay [44][45][46]; WST assay [22]; SRB assay [47]; apoptosis with double staining method [30][41][43][48]; cell cycle analysis [49] gene expression analysis [32][33]; mitochondrial membrane potential ѱm (MMP), cytochrome c release analysis and NFkB translocation [45]; caspase activity and inhibitor assays [45]; protein extraction, protein array and western blotting analyses [45]; computational molecular docking and statistical analyses [41][46] | HT-29 [20][45][46][50]; HCT-15 [22][23][39]; Colo205 [44]; WiDr [24][26][27][28][30][32][33][34][35][36][37][38][40][41][49]; HCT-116 [25][29][31][42][43][47]; CaCo-2 [45] | Catechin 7-O-apiofuranoside and didesmethyl tocotrienol [20] | 12.5–200 µg/mL | 68% (inhibition) | Not classified | The fraction of P. macrocarpa exhibited the highest activity as anti-proliferative against HT-29 cells. The compounds had antioxidant activity leading to a cytoprotective effect. The mechanisms of this chemoprevention included up-regulation of Bax and proliferation-promoting proteins (PCNA) [20]. |
| Lissoclibadins (polysulfur aromatic alkaloids) from Ascidian lissoclinum [22] | 5 μM | 4.0 μM | Significant / strong | Lissoclibadin 1 exerted the most potent cytotoxic effects and mainly promoted apoptosis through an intrinsic pathway with the activation of a caspase-dependent pathway in HCT-15 cells [22]. | |||
| Leptoclinidamide and (−)-leptoclinidamine from Leptoclinides dubuis [23] | 0 to 27 μM | The compounds not displayed activity against cell line | Not classified | Not active against cancer cell line [20][22]. | |||
| Curacyclin A and B from the latex of Jatropha curcas L. [44] | 1–1000 µg/mL | The compounds did not have any effect on cell line | No cytotoxicity | ||||
| The ethanol extracts from 95 ascidians collected at North Sulawesi, Indonesia; shermilamine B and kuanoniamine D [39] | 0 to 27 μM | 6.7 µM (shermilamine B) and 4.1 μM (kuanoniamine D) | Significant / strong | Shermilamine B and kuanoniamine D was classified in the pyridoacridine alkaloids, which have been known to exhibit various bioactivities such as cytotoxicity, inhibition of topoisomerase II, anti-HIV activity, Ca2+ releasing activity, and intercalation with DNA [39]. | |||
| Crude ethyl acetate extract of endophytic fungi isolated from Annona muricata leaves; alkaloid compounds [24] | 25; 50; 100; 200; 400 µg/mL | 20.80 µg/mL | Moderate | Alkaloid compounds in endophytic fungal extract of isolate Sir-SM2 had a high cytotoxic effect on the colon cancer cell and the lowest toxicity to normal cells compared with other fungal extracts. The compounds have an alkylating activity that can cause breakage and damage of DNA strands, leading to the cancer cells death [24]. | |||
| Fungi derived from the marine sponge Neopetrosia chaliniformis [34], Acanthostrongylophora ingens [40], Aspergillus nomius NC06 [43] | 100 ppm [34] | 70.31% (cell viability) [34] | Not classified | Marine-derived fungus NC06 from sponge N. chaliniformis AR-01 showed the most selective cytotoxicity against the WiDr cell line compared to the Vero cell line [34]. | |||
| 100 µg/mL [40] | 12.88% (cell viability) [40] | Strong cytotoxicity (≤ 50%) | Not presented | ||||
| 100; 10; 1; 0.1 µg/mL [43] | 5.28 µg/mL [44] | Significant / strong | Not presented | ||||
| Polyisoprenoids (polyprenol and dolichol) from Nypa fruticans, Rhizophora mucronata, Ceriops tagal, Avicennia alba, Avicennia marina and Avicennia lanata leaves [27][28][35][36][37][49] | 15.625; 31.25; 62.50; 125; 250; 500 μg/mL [37] | 276 µg/mL (C. tagal) and 278 µg/mL (R. mucronata) [37] | No cytotoxicity | Polyisoprenoids induced apoptosis in the early-apoptosis phase and caused cell cycle arrest in the G0-G1 stage while decreasing the expression of Bcl-2 and cyclin-D1. In addition, the polyisoprenoid had a SI value for classification as highly selective and enables the suppression of COX-2 expression in WiDr cells [35][36][37]. | |||
| 15.625; 31.25; 62.525; 125; 250; 500 µg/mL [27] | 180.2 µg/mL (N. fruticans) [27] | Low | |||||
| 15.625; 31.25; 62.525; 125; 250; 500 μg/mL [28] | 180.186 μg/mL (N. fruticans) [28] | Low | |||||
| 500; 250; 125; 62.5; 31.25 µg/mL [35] | 154.987 µg/mL (A. marina) and 305.928 µg/mL (A. lanata) [35] | Low and no cytotoxicity | |||||
| 500; 250; 125; 62.5; 31.25; 15.625 µg/mL [36] | 173.775 μg/mL (A. alba) [36] | Low | |||||
| Ethyl acetate extract from Trichoderma reesei strain TV221 (EAFTrR) associated with marine sponge: Stylissa flabelliformis [37] | 2000, 1000, 500; 400; 300; 250; 200; 150; 125; 100; 75; 62,5; 50; 25 µg/mL | 88.88 µg/mL | Low | The extract has the potential of having anti-cancer genes through the capability to spur apoptosis. The mechanism of inhibition of cancer cell growth may go by cell cycle arrest, cell cycle delay, or apoptotic mechanism [38]. | |||
| Alpinumisoflavone from Erythrina poeppigiana [41] | 100.0; 50.0; 25.0; 12.5; 6.25; 3.25 μg/mL | 5.63 µg/mL | Significant / strong | Alpinumisoflavone is a flavonoid that has a pyran ring as pyranisoflavonoid. The presence of hydroxyl group in A-ring in position 5 increase the cytotoxic activity of flavonoids. The presence of hydroxyl group in B-ring in positions 4’ is shown to increase the cytotoxicity of flavonoids [41]. | |||
| Dichloromethane extract of Canna indica rhizomes [26] | 2000, 1500, 1000, 750; 500; 250; 125 ppm | 361.83 ppm | No cytotoxicity | The extract contained a compound that could induce apoptotic activity and cell cycle in the WiDr cells [26]. | |||
| Nine lichen species from six different locations in East Java, Indonesia [30] | 1024, 512; 256; 128; 64; 32 μg/mL | 324 μg/mL | No cytotoxicity | Not presented | |||
| Arcangelisia flava L. Merr chloroform extract [31] | 50; 100; 200; 300; 400 µg/mL | 121.637 µg/mL | Low | The chloroform extract of A. Flava was capable to trigger apoptosis in the WiDr cells [32]. | |||
| Piper crocatum Ruiz & Pav ethanol extract [33] | 1; 10; 100; 500; 1000, 2000, 4000 µg/mL | 727 μg/mL | No cytotoxicity | The ethanol extract of P. crocatum had an activity to induce apoptosis and suppress COX-2 protein expression in WiDr cells [33]. | |||
| Peptides from Platycephalus fuscus [46] | 0.005 mg protein/mL | 91.04% (inhibition) | Not classified | The further cell-based study is essential to observe the mechanistic pathways and structure or function relationship of peptides in stimulating apoptosis [46]. | |||
| Cycloart-24-ene-26-ol-3-one from Aglaia exima leaves [45] | 0.39–200 μM | 2.4 µM (HT-29); 5.6 µM (CaCo-2) | Significant / strong | It is bound to tumor necrosis factor-receptor 1 (TNF-R1) leading to the initiation of caspase-8 and, through the activation of Bid, in the activation of caspase-9. This activity causes a reduction in mitochondrial membrane potential (MMP) and the release of cytochrome-C. The activation of caspase-8 and -9 both acts to commit the cancer cells to apoptosis through downstream caspase-3/7 activation, PARP cleavage and the lack of NFkB translocation into the nucleus [45]. | |||
| Seaweeds (extracted in four kind of organic solvents): Gracilaria verrucose [51]; Ulva luctuca and Eucheuma cottonii [29]; Eucheuma Sp. [42] | 200; 100; 50; 25; 12.5; 6.25; 3.125; 1.5625 μg/mL [51] | 43.9 μg/mL (G. verrucose) [51] | Moderate | Not presented | |||
| 51.2; 25.6; 12.8; 6.4; 3.2; 1.6; 0.8; 0.4 µg/mL [29] | 69.3 μg/mL (U. luctuca) and 21.4 μg/mL (E. cottonii) [29] | Low and moderate | |||||
| 51.2; 25.6; 12.8; 6.4; 3.2; 1.6; 0.8; 0.4 μg/mL [42] | 16.82 μg/mL (Eucheuma Sp.) [42] | Significant / strong | |||||
| 2-O-β-glucopyranosil cucurbitacin D, isolated from the ethyl acetate soluble fraction of Benalu batu (Begonia sp.) [25] | 6.25; 12.5; 25; 50 μg/mL | 0.002 μg/mL and 6.88% (cell viability) | Significant / strong | The presence of cucurbitacin type triterpenoid could be a marker compound for Begonia plant species. It exhibited potent cytotoxic activity against HCT-116 via apoptosis induction with a significant percentage of early and late apoptosis [25]. | |||
| Chloroform fraction of Garcinia mangostana fruits hulls [47] | 0.01–100 μM | 15.8 µM | Moderate | The chloroform fraction contained bioactive compounds that induced significant antiproliferative and cytotoxic potentials via induction of apoptosis and cell cycle arrest at G0/G1-phase, necrosis, and apoptosis in HCT-116 cells [47]. | |||
| Polygonumins A from Polygonum minus [31] | 100; 50; 25; 12.5; 6.25; 3.13 µg/mL | 3.24 µg/mL | Significant / strong | The sugar moiety, a sucrose unit, was recognized to be critical to the topoisomerase inhibition activity as antitumor drugs [31]. | |||
| Gyrinops versteegii (Gilg.) Domke leaves extract (chloroform and ethanol solvents). The most abundant compounds detected in both extracts were fatty acids, namely palmitic acid, stearic acid, and pentadecanoic acid [51] | ― | Not determined | ― | Not presented (the first reported study on metabolite profiling of G. versteegii leaves extract, the result supported further study on G. versteegii as the anticancer-resource plant) | |||
| (S)-2-hydroxy-3-(octanoyloxy)propyl tetracosanoate, (S)-3-(((S)-11-acetoxy octadecanoyl)oxy)propane-1,2-diyl diacetate, docosanedioic acid, 2,5-dimethylnonadecane, lupeol, stigmasterol, b-sitosterol, heptadecanoic acid, hexanedioic acid, 1,6-bis[(2R)-ethylhexyl] ester, and 1,3-di-O-[2′,2′-di- (p-phenylene)] were isolated from the leaves of Garcinia daedalanthera Pierre, collected from Indonesia [52] | Not displayed | 19.2 μM (lupeol) | Moderate | Not presented | |||
| Fulvoplumierin; allamcin; allamandin; 2,5-dimethoxy-p-benzoquinone; plumericine; and lignan liriodendrin (from bark or Plumeria rubra) [53] | Not displayed | 0.1 µg/mL (plumericine); 0.3 µg/mL (allamcin and allamandin); 1.3 µg/mL (fulvoplumierin); 1.4 µg/mL (2,5-dimethoxy-p-benzoquinone); 16 µg/mL (liriodendrin) | Significant / strong | Not presented |
3. Discussion and Future Directions
Cancer is the second leading cause of death in the world after cardiovascular disease. Meanwhile, colorectal cancer ranks in the top three in the number of causes of death and is ranked as the second most common cancer type in men and third in women [54][55]. A comprehensive therapy development to treat colorectal cancer is needed to reduce patient mortality. This therapy development is expected to be capable of overcoming the resistance of conventional chemotherapy agents that already exist today. Resistance of WiDr cells to 5-fluorouracil (5-FU)—an antimetabolite chemotherapeutic agent—is mediated by an increased expression of thymidylate synthetase enzyme which is the main inhibitory target of 5-FU [56][57]. WiDr is also one of the cells that have low sensitivity to treatment with 5-FU. WiDr cells are widely applicable in Indonesia due to proper carcinogenesis and tumorigenicity studies and anti-tumor testing on potency disclosure of bioactive compounds from natural products for further development. Besides, WiDr cells are identical toHCT-15 and HT-29 cell lines because they are derived from the same patient and most likely from the same tumor [57]. Overall, WiDr cells are suitable to be used as models in screening the novel compound as a co-chemotherapeutic agent with 5-FU. Combination therapy (co-chemotherapy) of 5-FU with a chemo-preventive agent is an alternative to overcome resistance, increase efficacy, and reduce adverse effects. These facts that lead the research and development of natural products become important for the future directions of medicinal plants in colorectal cancer therapy.
Plants are one of the largest sources of natural products that are used to discover and develop novel chemotherapeutic agents. In particular, several discovered novel compounds from plants have unique mechanisms of action, greater efficacy, or lower adverse effects compared to the conventional chemotherapy drugs currently in use. Bioactive compounds from medicinal plants in Indonesia have also been studied to exhibit anticancer activities in the colorectal, namely terpenoids, phytosterols, alkaloids, phenolics, flavonoids, and polyisoprenoids ( the basic structure for one of several derivatives from those bioactive compounds is presented in Figure 2 ).
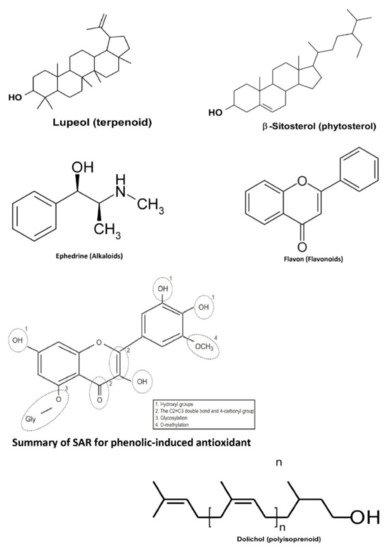
In the current paper, we conducted a systematic review that provided predictions for several secondary metabolites sourced from natural products to be utilized as an alternative treatment against colorectal cancer in Indonesia. They are also prospective candidates for future co-chemotherapy agents in safety, quality, standardization, and efficaciousness. This finding emphasized the potential of several natural products as anticancer agents against colorectal cancer cells.
Leading the discovery is the main component of today’s early pharmaceutical research. The exploration of novel drugs for colorectal cancer therapy that has a fast progression can be a drug repurposing strategy to bypass preclinical steps that usually require laborious and resource-intensive work. In addition, it is also considered that the development of existing agents in the future can be more easily utilized by the community. For those purposes, the exploration of Indonesia’s frequently used natural resources biodiversity is the best choice. In the past, drug development is based on trial and error, so it was costly and time-consuming. Today, molecular modeling, with the aid of computer hardware and software (computational method), has reduced the risk and the process of discovery is more effective in cost and time [58].
4. Conclusions
In summary, this entry revealed bioactive compounds from natural products of Indonesian plants that have been researched and have potential as anticancer agents that are most commonly experienced in men and women including colorectal cancer. The general method used for the analysis of the cytotoxic activity of colorectal cancer cells is the in vitro method using the MTT assay, the most widely used cell line is WiDr. The most studied bioactive compounds that have any activity against colorectal cancer are terpenoids, phytosterols, phenolics, alkaloids, and polyisoprenoids, but other natural products may have the potential to be developed from this study.
References
- WHO. Latest Global Cancer Data: Cancer Burden Rises to 18.1 Million New Cases and 9.6 Million Cancer Deaths in 2018. Available online: https://www.who.int/cancer/PRGlobocanFinal.pdf (accessed on 14 May 2021).
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.; Watanabe, T. Colorectal Cancer. Nat. Rev. Dis. Prim. 2015, 5, 15065.
- Aakif, M.; Balfe, P.; Elfaedy, O.; Awan, F.N.; Pretorius, F.; Silvio, L.; Castinera, C.; Mustafa, H. Study on Colorectal Cancer Presentation, Treatment and Follow-Up. Int. J. Colorectal Dis. 2016, 31, 1361–1362.
- Leung, W.K.; Ho, K.Y.; Kim, W.; Lau, J.Y.W.; Ong, E.; Hilmi, I.; Kullavanijaya, P.; Wang, C.; Li, C.; Fujita, R.; et al. Colorectal Neoplasia in Asia: A Multicenter Colonoscopy Survey in Symptomatic Patients. Gastrointest. Endosc. 2006, 64, 751–759.
- Ng, S.C.; Zeng, Z.; Niewiadomski, O.; Tang, W.; Bell, S.; Kamm, M.A.; Hu, P.; de Silva, J.; Niriella, M.A.; Udara, W.S.A.A.Y.; et al. Early Course of Inflammatory Bowel Disease in a Population-Based Inception Cohort Study from 8 Countries in Asia and Australia. Gastroenterology 2016, 150, 86–95.
- Abdullah, M.; Sudoyo, A.W.; Utoma, A.R.; Fauzi, A.; Rani, A.A. Molecular Profile of Colorectal Cancer in Indonesia: Is There Another Pathway? Gastroenterol. Hepatol. Bed Bench 2012, 5, 71–78.
- Conze, D.; Weiss, L.; Regen, P.S.; Bhushan, A.; Weaver, D.; Johnson, P.; Rincon, M. Autocrine Production of Interleukin 6 Causes Multidrug Resistance in Breast Cancer Cells. Cancer Res. 2001, 61, 8851–8858.
- Shapiro, G.I.; Harper, J.W. Anticancer Drug Targets: Cell Cycle and Checkpoint Control. J. Clin. Investig. 1999, 104, 1645–1653.
- Kitagawa, S. Inhibitory Effects of Polyphenols on P-Glycoprotein-Mediated Transport. Biol. Pharm. Bull. 2006, 29, 1–6.
- Adina, A.B.; Goenadi, F.A.; Handoko, F.F.; Nawangsari, D.A.; Hermawan, A.; Jenie, R.I.; Meiyanto, E. Combination of Ethanolic Extract of Citrus Aurantifolia Peels with Doxorubicin Modulate Cell Cycle and Increase Apoptosis Induction on MCF-7 Cells. Iran. J. Pharm. Res. 2014, 13, 919–926.
- Sudiana, I.K. Patobiologi Molekular Kanker; Salemba Medika: Jakarta, Indonesia, 2008.
- Knödler, M.; Buyel, J.F. Plant-Made Immunotoxin Building Blocks: A Roadmap for Producing Therapeutic Antibody-Toxin Fusions. Biotechnol. Adv. 2021, 47, 107683.
- Cragg, G.M.; Newman, D.J. A Tale of Two Tumor Targets: Topoisomerase I and Tubulin. The Wall and Wani Contribution to Cancer Chemotherapy. J. Nat. Prod. 2004, 67, 232–244.
- Stohrl, W.R. The Role of Natural Products in a Modern Drug Discovery Program. Drug Discov. Today 2000, 5, 39–41.
- Chen, T.R.; Drabkowski, D.; Hay, R.J.; Macy, M.; Peterson, W., Jr. WiDr Is a Derivative of Another Colon Adenocarcinoma Cell Line, HT-29. Cancer Genet. Cytogenet. 1987, 27, 125–134.
- Waly, M.I.; Al-Rawahi, A.S.; Al Riyami, M.; Al-Kindi, M.A.; Al-Issaei, H.K.; Farooq, S.A.; Al-Alawi, A.; Rahman, M.S. Amelioration of Azoxymethane Induced-Carcinogenesis by Reducing Oxidative Stress in Rat Colon by Natural Extracts. BMC Complement. Altern. Med. 2014, 14, 1–10.
- Reddy, B.S. Animal Models for Colon Cancer Chemoprevention. In Encyclopedia of Cancer; Bertino, J.R., 2nd, Ed.; Elsevier Science: New York, NY, USA, 2002; Volume 1, pp. 49–55.
- Kuete, V.; Efferth, T. African Flora Has the Potential to Fight Multidrug Resistance of Cancer. BioMed Res. Int. 2015, 2015, 914813.
- Tanaka, T.; Sugie, S. Inhibition of Colon Carcinogenesis by Dietary Non-Nutritive Compounds. J. Toxicol. Pathol. 2007, 20, 215–235.
- Shwter, A.N.; Abdullah, N.A.; Alshawsh, M.A.; El-Seedi, H.R.; Al-Henhena, N.A.; Khalifa, S.A.M.; Abdulla, M.A. Chemopreventive Effect of Phaleria Macrocarpa on Colorectal Cancer Aberrant Crypt Foci in vivo. J. Ethnopharmacol. 2016, 193, 195–206.
- Nurdin, S.U.; Le Leu, R.K.; Aburto-Medina, A.; Young, G.P.; Stangoulis, J.C.R.; Ball, A.S.; Abbott, C.A. Effects of Dietary Fibre from the Traditional Indonesian Food, Green Cincau (Premna Oblongifolia Merr.) on Preneoplastic Lesions and Short Chain Fatty Acid Production in an Azoxymethane Rat Model of Colon Cancer. Int. J. Mol. Sci. 2018, 19, 2593.
- Tatsuta, T.; Hosono, M.; Rotinsulu, H.; Wewengkang, D.S.; Sumilat, D.A.; Namikoshi, M.; Yamazaki, H. Lissoclibadin 1, a Polysulfur Aromatic Alkaloid from the Indonesian Ascidian Lissoclinum Cf. Badium, Induces Caspase-Dependent Apoptosis in Human Colon Cancer Cells and Suppresses Tumor Growth in Nude Mice. J. Nat. Prod. 2017, 80, 499–502.
- Yamazaki, H.; Wewengkang, D.S.; Nishikawa, T.; Rotinsulu, H.; Mangindaan, R.E.P.; Namikoshi, M. Two New Tryptamine Derivatives, Leptoclinidamide and (−)-Leptoclinidamine B, from an Indonesian Ascidian Leptoclinides Dubius. Mar. Drugs 2012, 10, 349–357.
- Arifni, F.R.; Hasan, A.E.Z.; Julistiono, H.; Bermawie, N.; Riyanti, E.I. Anticancer Activities of Endophytic Fungi Isolated from Soursop Leaves (Annonamuricata L.) against WiDr Cancer Cells. Annu. Res. Rev. Biol. 2017, 18, 1–11.
- Zubair, M.S.; Alarif, W.M.; Ghandourah, M.A.; Anam, S.; Jantan, I. Cytotoxic Activity of 2-o-β-Glucopyranosil Cucurbitacin d from Benalu Batu (Begonia Sp.) Growing in Morowali, Central Sulawesi. Indones. J. Chem. 2020, 20, 766–772.
- Widyarini, S.; Hartanto, N.L.; Pratiwi, R. Phytochemical Analysis and Cytotoxic Activities of Two Distinct Cultivars of Ganyong Rhizomes (Canna Indica) against the Widr Colon Cancer Cell Line. Biodiversitas 2020, 21, 1660–1669.
- Sari, D.P.; Basyuni, M.; Hasibuan, P.A.Z.; Sumardi, S.; Nuryawan, A.; Wati, R. Cytotoxic and Antiproliferative Activity of Polyisoprenoids in Seventeen Mangroves Species against WiDr Colon Cancer Cells. Asian Pac. J. Cancer Prev. 2018, 19, 3393–3400.
- Sari, D.P.; Basyuni, M.; Hasibuan, P.A.Z.; Wati, R. The Inhibition of Polyisoprenoids from Nypa Fruticans Leaves on Cyclooxygenase 2 Expression of WiDr Colon Cancer Cells. Asian J. Pharm. Clin. Res. 2018, 11, 154–157.
- Arsianti, A.; Fadilah, F.; Wibisono, L.K.; Kusmardi, S.; Azizah, N.N.; Putrianingsih, R.; Murniasih, T.; Rasyid, A.; Pangesti, R. Phytochemical Composition and Anticancer Activity of Seaweeds Ulva Lactuca and Eucheuma Cottonii against Breast MCF-7 and Colon HCT-116 Cells. Asian J. Pharm. Clin. Res. 2016, 9, 115–119.
- Nugraha, A.S.; Pratoko, D.K.; Damayanti, Y.D.; Lestari, N.D.; Laksono, T.A.; Addy, H.S.; Untari, L.F.; Kusumawardani, B.; Wangchuk, P. Antibacterial and Anticancer Activities of Nine Lichens of Indonesian Java Island. J. Biol. Act. Prod. Nat. 2019, 9, 39–46.
- Ahmad, R.; Sahidin, I.; Taher, M.; Low, C.; Noor, N.M.; Sillapachaiyaporn, C.; Chuchawankul, S.; Sarachana, T.; Tencomnao, T.; Iskandar, F.; et al. Polygonumins A, a Newly Isolated Compound from the Stem of Polygonum Minus Huds with Potential Medicinal Activities. Sci. Rep. 2018, 8, 1–15.
- Ariati, V. Uji Sitotoksisitas Dan Apoptosis Ekstrak Kloroform Daun Kayu Kuning (Arcangelisia Flava, L. Merr) Terhadap Kultur Sel Kanker Kolon WiDr In Vitro. Undergraduate Thesis, Universitas Jember, Jember, East Java, Indonesia, 2015.
- Utami, M.T. Pengaruh Ekstrak Etanolik Daun Sirih Merah (Piper Crocatum Ruiz and Pav) Terhadap Sel Kanker Kolon WiDr. Sanata Darma University, Yogyakarta, Indonesia, 2015.
- Artasasta, M.A.; Yanwirasati, A.D.; Handayani, D. Cytotoxic Activity Screening of Ethyl Acetate Fungal Extracts Derived from the Marine Sponge Neopetrosia ChaliniformisAR-01. J. Appl. Pharm. Sci. 2017, 7, 174–178.
- Illian, D.N.; Basyuni, M.; Wati, R.; Hasibuan, P.A.Z. Polyisoprenoids from Avicennia Marina and Avicennia Lanata Inhibit WiDr Cells Proliferation. Pharmacogn. Mag. 2018, 14, 513–518.
- Illian, D.N.; Hasibuan, P.A.Z.; Sumardi, N.A.; Wati, R.; Basyuni, M. Anticancer Activity of Polyisoprenoids from Avicennia Alba Blume. in WiDr Cells. Iran. J. Pharm. Res. 2019, 18, 1477–1487.
- Sari, D.P.; Basyuni, M.; Hasibuan, P.A.Z.; Wati, R. Cytotoxic Effect of Polyisoprenoids from Rhizophora Mucronata and Ceriops Tagal Leaves against WiDr Colon Cancer Cell Lines. Sains Malays. 2018, 47, 1953–1959.
- Setyowati, E.P.; Pratiwi, S.; Purwatiningsih, P.I. In-Vitro Cytotoxicity and Apoptosis Mechanism of Ethyl Acetate Extract from Trichoderma Reesei Strain TV221 Associated with Marine Sponge: Stylissa Flabelliformis. J. Appl. Pharm. Sci. 2018, 8, 151–157.
- Sumilat, D.A.; Wewengkang, D.S.; Rotinsulu, H.; Yamazaki, H.; Oda, T.; Ukai, K.; Namikoshi, M. Bioactivity of Extracts from Ascidians Collected in North Sulawesi as Seeds of Marine-Derived Drugs. AACL Bioflux 2018, 11, 516–524.
- Aminah, I.; Putra, A.E.; Arbain, D.; Handayani, D. Screening of Cytotoxic Activities toward WiDr and Vero Cell Lines of Ethyl Acetate Extracts of Fungi Derived from the Marine Sponge Acanthostrongylophora Ingens. J. Appl. Pharm. Sci. 2019, 9, 1–5.
- Herlina, T.; Haraswati, N.; Apriani, R.; Nishinarizki, V.; Gaffar, S.; Supratman, U. Cytotoxic Activity of Alpinumisoflavone from Erythrina Poeppigiana (Leguminosae) Against Colon Cancer (WiDr), Cervical Cancer (Hela), and Hepatoma Cancer (HepG2) Cells. HAYATI J. Biosci. 2019, 26, 96–100.
- Subroto, P.A.M.; Arsianti, A.; Lesmana, E. Phytochemical Analysis and Anticancer Activity of Seaweed Eucheuma Sp. against Colon HCT-116 Cells. 3rd Biomedical Engineering’s Recent Progress in Biomaterials, Drugs Development, and Medical Devices: Proceedings of the International Symposium of Biomediacal Enginering (ISBE) 2018, Jakarta, Indonesia, 6–8 August 2018; Gozan, M., Astutiningsih, S., Wulan, P.P.D.K., Ramahdita, G., Dhelika, R., Kreshanti, P., Eds.; AIP Conference Proceedings: Jakarta, Indonesia, 2019; Volume 2092, p. 030015.
- Artasasta, M.A.; Yanwirasti, T.M.; Djamaan, A.; Handayani, D. Cytotoxic and Antibacterial Activities of Marine Sponge-Derived Fungus Aspergillus Nomius NC06. Rasayan J. Chem. 2019, 12, 1463–1469.
- Insanu, M.; Anggadiredja, J.; Kayser, O. Curcacycline A and B—New Pharmacological Insights to an Old Drug. Int. J. Appl. Res. Nat. Prod. 2012, 5, 26–34.
- Leong, K.H.; Looi, C.Y.; Loong, X.M.; Cheah, F.K.; Supratman, U.; Litaudon, M.; Mustafa, M.R.; Awang, K. Cycloart-24-Ene-26-Ol-3-One, a New Cycloartane Isolated from Leaves of Aglaia Exima Triggers Tumour Necrosis Factor-Receptor 1-Mediated Caspase-Dependent Apoptosis in Colon Cancer Cell Line. PLoS ONE 2016, 11, 1–17.
- Nurdiani, R.; Vasilijevic, T.; Yeager, T.; Singh, T.K.; Donkor, O.N. Bioactive Peptides with Radical Scavenging and Cancer Cell Cytotoxic Activities Derived from Flathead (Platycephalus Fuscus) by-Products. Eur. Food Res. Technol. 2017, 243, 627–637.
- Mohamed, G.A.; Al-Abd, A.M.; El-Halawany, A.M.; Abdalla, H.M.; Ibrahim, S.R.M. New Xanthones and Cytotoxic Constituents from Garcinia Mangostana Fruit Hulls against Human Hepatocellular, Breast, and Colorectal Cancer Cell Lines. J. Ethnopharmacol. 2017, 23, 302–312.
- Istiqomah, M.A.; Hasibuan, P.A.Z.; Sumardi, S.; Nuryawan, A.; Wati, R.; Basyuni, M. Apoptotic with Double-Staining Test, P53, and Cyclooxygenase-2 to Proliferation Colon Cancer Cell (WiDr) of Dolichol in Three Mangrove Leaves. Open Access Maced. J. Med. Sci. 2020, 8, 37–42.
- Istiqomah, M.A.; Hasibuan, P.A.Z.; Sumaiyah, S.; Yusraini, E.; Oku, H.; Basyuni, M. Anticancer Effects of Polyisoprenoid From Nypa Fruticans Leaves by Controlling Expression of P53, EGFR, PI3K, AKT1, and MTOR Genes in Colon Cancer (WiDr) Cells. Nat. Prod. Commun. 2020, 15, 1–8.
- Forestrania, R.C.; Anaya-Eugenio, G.D.; Acuña, U.M.; Ren, Y.; Elya, B.; de Blanco, E.C. Secondary Metabolites from Garcinia Daedalanthera Pierre Leaves (Clusiaceae). Nat. Prod. Res. 2020, 1–7.
- Wardana, T.A.P.; Nuringtyas, T.R.; Wijayanti, N.; Hidayati, L. Phytochemical Analysis of Agarwood (Gyrinops Versteegii (Gilg.) Domke) Leaves Extracts as Anticancer Using GC-MS. In Proceedings of the 2nd International Conference on Science, Mathematics, Environment, and Education, Surakarta, Indonesia, 26–28 July 2019; AIP Conference Proceedings: Surakarta, Indonesia, 2019; Volume 2194, p. 020136.
- Kurniasari, K.D.; Arsianti, A.; Astika, Y.; Mandasari, B.K.D.; Masita, R.; Zulfa, F.R.; Dewi, M.K.; Zagloel, C.R.Z.; Azizah, N.N.; Putrianingsih, R. Phytochemical Analysis and Anticancer Activity of Seaweed Gracilaria Verrucosa against Colorectal HCT-116 Cells. Orient. J. Chem. 2018, 34, 1257–1262.
- Kardono, L.B.S.; Tsauri, S.; Padmawinata, K.; Pezzuto, J.M.; Kinghorn, A.D. Cytotoxic Constituents of the Bark of Plumeria Rubra Collected in Indonesia. J. Nat. Prod. 1990, 53, 1447–1455.
- Kimman, M.; Norman, R.; Jan, S.; Kongston, D.; Woodward, M. The Burden of Cancer in Member Countries of the Association of Southeast Asian Nations (ASEAN). Asian Pac. J. Cancer Prev. 2012, 13, 411–420.
- McDonald, M.; Hertz, R.P.; Lowenthal, S.W.P. The Burden of Cancer in Asia. Available online: https://cdn.pfizer.com/pfizercom/products/cancer_in_asia.pdf (accessed on 14 May 2021).
- Sigmond, J.; Backus, H.H.J.; Wouters, D.; Temmik, O.H.; Jansen, G.; Peters, G.J. Induction of Resistance to the Multitargeted Antifolate Pemetrexed (ALIMTA) in WiDr Human Colon Cancer Cells Is Associated with Thymidylate Synthase Overexpression. Biochem. Pharmacol. 2003, 66, 431–438.
- Ahmed, D.; Eide, P.W.; Eilertsen, I.A.; Danielsen, S.A.; Eknaes, M.; Hektoen, M.; Lind, G.E.; Lothe, R.A. Epigenetic and Genetic Features of 24 Colon Cancer Cell Lines. Oncogenesis 2013, 2, e71.
- Noolvi, M.N.; Patel, H.M.; Bhardwaj, V.; Chauhan, A. Synthesis and in vitro Antitumor Activity of Substituted Quinazoline and Quinoxaline Derivatives: Search for Anticancer Agent. Eur. J. Med. Chem. 2011, 46, 2327–2346.




