
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francesca Oppedisano | + 2411 word(s) | 2411 | 2021-09-02 05:57:03 | | | |
| 2 | Dean Liu | Meta information modification | 2411 | 2021-09-02 11:52:04 | | |
Video Upload Options
Heart failure (HF) characterized by cardiac remodeling is a condition in which inflammation and fibrosis play a key role. Dietary supplementation with n-3 polyunsaturated fatty acids (PUFAs) seems to produce good results. In fact, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have anti-inflammatory and antioxidant properties and different cardioprotective mechanisms.
1. Introduction
Heart failure (HF) arises as a result of various cardiovascular diseases and leads to numerous cases of hospitalization [1][2][3]. It is known that in HF, there is an alteration of the ventricular filling or of the ejection of blood. To this condition are added symptoms such as dyspnea and fatigue as well as pulmonary and peripheral edema [4]. There are many comorbidities associated with heart failure, such as hypertension, diabetes, and obesity or hyperlipidemia, which are responsible for the increased hospitalization and mortality of patients with HF, even of relatively young subjects [5][6][7][8][9][10]. In addition, there has been an increase in HF cases linked to the ageing population [5][11]. According to Katsi V. et al. 2017, HF patients are categorized on the basis of underlying left ventricular ejection fraction (LVEF) and are mainly distinguished as patients with HF with a reduced ejection fraction (HFrEF) and patients with HF with a preserved ejection fraction (HFpEF) [12]; the latter represents more than 50% of HF cases and has a much less well-understood pathophysiology than HFrEF [2][12][13][14][15]. HF is characterized by cardiac remodeling that usually occurs following events related to hypertension, myocardial infarction (MI), myocarditis, or heart valve disease and is a condition in which inflammation and fibrosis are determinants [3][16] ( Figure 1 ).
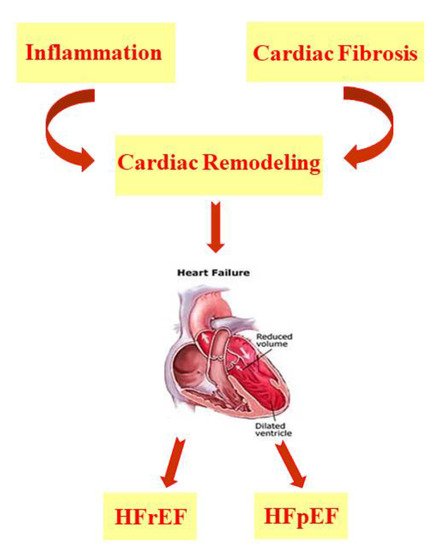
HF is characterized by cardiac remodeling caused by cardiac inflammation and fibrosis; it is distinguished in both HF with a reduced ejection fraction (HFrEF) and HF with a preserved ejection fraction (HFpEF).
In particular, inflammation determines the presence of immune cells in the heart tissue and can be distinguished by sterile myocardial inflammation and non-sterile myocardial inflammation [3]. In order to resolve myocardial inflammation, anti-inflammatory processes are activated, which result in the onset of a pro-fibrotic state. In myocardial fibrosis, extracellular matrix proteins are more abundant and show structural and functional alterations. Both cardiac inflammation and fibrosis are being studied in numerous animal models, as they could be the target of new heart failure therapies [3]. Experimental and clinical studies conducted over the years in patients with acute and chronic heart failure have demonstrated the involvement of both the innate and adaptive immune systems in the disease [17][18][19]. In 2019, the CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) trial, using anti-cytokine therapy with a monoclonal antibody against IL-1β, established that chronic inflammation plays an important role in the pathogenesis of heart failure. Therefore, anti-inflammatory therapies could be administered to heart failure patients with a cardio-inflammatory phenotype [18]. From a pathophysiological point of view, HF has a reduced ATP (adenosine triphosphate) availability, changes in Na + and Ca 2+ handling, and myocytes characterized by oxidative stress. In all of these processes, the mitochondria are involved and therefore play a fundamental role in HF. In fact, the altered mitochondrial structure, dynamics, and function in HF generate an energetic deficit and oxidative stress [2][20][21][22][23][24][25][26][27][28][29]. In particular, the process known as ROS-induced ROS release is triggered. In this condition, the ROS release from the mitochondria triggers a further release of ROS from neighboring mitochondria; in this way, high levels of ROS are present, which alter the excitation–contraction coupling, generating cardiac remodeling and cell death [2][20][30][31]. It has been shown that, in the early stages of HF, oxidative stress and mitochondrial dysfunction determine the onset of a condition known as “cardiomyocyte frailty”, in which myocardial cells undergo apoptosis if there are no protective mechanisms such as autophagy and the overexpression of endogenous antioxidant enzymes [5]. There is also altered function of the endoplasmic reticulum, reduced nitric oxide (NO) release, metalloproteinase (MMP) dysregulation, and a change in cardiac stem cell mobilization. All of this determines an increase in the left ventricle’s stiffness, generating diastolic dysfunction [5]. HF can also arise as a result of a pathological increase in the heart size [32]. Following MI, both the infarcted and non-infarcted areas present ventricular morphological and functional changes, such as greater ventricular preload and afterload and lower systolic volume and cardiac output, conditions that determine HF onset. In order to restore the physiological condition, a neuro-humoral response is activated, which is characterized by a greater sympathetic discharge and hormone synthesis such as angiotensin II and aldosterone, thus increasing peripheral vascular resistance. In the early stages of HF, this represents a compensatory mechanism, but in the long term, it leads to a worsening of symptoms [33]. Regarding the therapy for HFrEF, clinical trials have demonstrated the efficacy of several therapies evaluated at the same time. To date, therapies include treatment with vasodilators, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs) as well as beta-blockers and mineralocorticoid receptor antagonists (MRAs) [34][35][36]. In addition, the US Food and Drug Administration (FDA) has recently approved the sodium–glucose co-transporter 2 (SGLT2) inhibitors as a novel therapeutic approach to treating HFrEF patients, irrespective of the presence or absence of type 2 diabetes (T2D) [37][38][39][40]. However, the risk to patients remains high, and further studies are required to define new pharmacological approaches [34][35][36]. With regard to HFpEF, the diagnosis of which is more complex, up until now, clinical studies have not shown the existence of an effective therapy, but treatment is based on the use of diuretics and mineralocorticoid receptor antagonists, and exercise training is recommended [41][42][43][44]. At the moment, clinical studies aim at a better pathophysiological characterization in order to identify individual therapies [42][45]. Furthermore, self-care is very important for the management of HF patients. In particular, the Heart Failure Association (HFA) guidelines focus on maintaining self-care, consisting of regular drug intake and eventual adaptation to changes in the course of the disease. Maintaining self-care also includes a special focus on physical activity and diet. Self-care monitoring and management is also important as well as travel, leisure, smoking, sleep, immunization, and infection prevention [46]. Alongside pharmacological treatment, a non-pharmacological approach could be effective, especially in the early stages of the disease. In particular, it has been shown that many HF patients have a low intake of micronutrients, a condition that could trigger heart failure [5][47]. Therefore, in the early stages of disease onset, the intake of many plant extracts with antioxidant properties, such as bergamot polyphenolic fraction (BPF) extract, could counteract HF development [5][48]. In addition, both studies in animal models and in patients with HF indicate that the intake of n-3 polyunsaturated fatty acids (PUFAs) inhibits the onset of interstitial fibrosis and diastolic heart dysfunction [49]. In fact, many researchers are currently studying the use of natural compounds in the prophylaxis and treatment of various diseases, as there are studies that demonstrate their effectiveness in heart diseases, hepatic steatosis, metabolic syndrome, and neurodegenerative diseases [5][50][51][52][53][54][55].
2. Heart Failure and PUFAs
PUFAs are long-chain polyunsaturated fatty acids with a carboxyl group (-COOH) at the polar hydrophilic end and a non-polar hydrophobic methyl group (-CH3) at the opposite end [56]. They are divided into two classes, n-3 and n-6 PUFAs, whose precursors are, respectively, α-linolenic acid (ALA, 18:3, Omega-3) and linoleic acid (LA, 18:2, Omega-6), which are defined as essential, as they must be ingested through dietary means [57][58]. ALA, present in beans, nuts, and flax seeds, is the metabolic precursor of eicosapentaenoic acid (EPA, 20:5 n-3) and docosahexaenoic acid (DHA, 22:6 n-3), while γ-linolenic acid and arachidonic acid (AA) derive from LA, which is mainly present in soy and corn oils [58][59]. Despite the synthesis pathway in the liver, the highest amount of EPA and DHA is achiever through diet, as they are present in foods of animal origin but, above all, in the oil and flesh derived from marine fish [56][57][58][59]. As far as their function is concerned, the n-6 PUFAs and their derivatives have pro-inflammatory action, unlike the n-3 PUFAs and their derivatives, which are powerful anti-inflammatories; therefore, the recommended daily intake of PUFAs is in favor of the latter [59][60][61][62]. Meta-analyses of prospective cohort studies indicate that fish consumption is inversely associated with HF risk. On the contrary, the risk of developing HF increases with a higher consumption of meat; therefore, fish can be considered a healthy animal-based dietary source of protein. Obviously, the positive association is also linked to the type of fish consumed and the cooking method. However, fish is considered to be one of the best cardioprotective foods. In interventional studies, it has been reported that the increased consumption of fish or the integration of n-3 PUFAs in diet has a protective action against cardiometabolic risks such as hypertension, inflammation, oxidative stress, and endothelial dysfunction in addition to having lipid-lowering properties [49][63]. It is interesting to underline that the hypotriglyceridemic, anti-inflammatory, anti-arrhythmic, and anti-thrombotic effects of n-3 PUFAs can be attributed to a modification of the DNA methylation profile in blood leukocytes [64]. Furthermore, both in vivo and clinical studies report the preventive action of n-3 PUFAs on cardiotoxicity and HF, which arise as side effects of anti-cancer therapies [65]. Thanks to their properties, n-3 PUFAs reduce ventricular remodeling and myocardial fibrosis, improving systolic and diastolic ventricular function altered in HF [66]. Furthermore, muscle wasting (understood as loss of muscle mass and strength) and cachexia (understood as weight loss) affect many HF patients, and supplementation with n-3 PUFAs appears to have a beneficial effect on these comorbidities. In fact, both the European Society of Cardiology (ESC) and the American College of Cardiology/American Heart Association (ACC/AHA) guidelines state that the integration of n-3 PUFAs into the diets of HF patients can be considered an additional therapy and is effective in reducing the death and hospitalization risk for these subjects [67]. Additionally, the AHA recommends eating 1–2 seafood meals per week, especially non-fried seafood [68][69]. In the Australian heart failure guidelines, of all the nutraceuticals considered, only the n-3 PUFAs have a recommendation, even though it is minimal [70]. Some clinical trials did not demonstrate the efficacy of treating HF with n-3 PUFAs, particularly with EPA; this could be caused by the failure to achieve a therapeutically effective concentration in all subjects [71]. Conversely, n-6 PUFAs can be cardiotoxic by acting directly on the heart muscle. In fact, the n-6 PUFAs, while reducing cholesterol levels and showing anti-atherosclerotic properties, can reduce survival after cardiac remodeling; this has been demonstrated in both in vitro and in vivo studies. These studies have shown that LA, but not derivatives such as γ-linolenic acid or arachidonic acid, stimulate the transforming growth factor-β (TGFβ) isoforms by increasing the collagen I/III ratio and lysyl oxidase (LOX) activity, causing an impaired transmitral flow that generates a cardiac “stiffening” with early diastolic dysfunction and normal systolic function [72].
In addition to this, another meta-analysis of randomized controlled trials confirmed lower levels of BNP and serum norepinephrine (sNE) following n-3 PUFA supplementation in HF patients [73]. A study in patients with decompensated heart failure reported a significant depletion of EPA and DHA even though this was not correlated with an increased three-year mortality risk [74]. Despite the contradictory data on the benefits and optimal doses of n-3 PUFAs, which are very often linked to the design and execution of the study, it seems sufficiently clear that n-3 PUFAs can be used in preventive cardiology. In fact, they show modest but statistically significant positive effects with a progressive trend over time [75].
The roles of EPA, DHA, AA, and DGLA have also been studied in acute decompensated HF (ADHF) [76]. In particular, the PUFA dosage was evaluated by Nagai T. et al. , 2016, in 685 patients hospitalized for ADHF, with a follow-up period of approximately 560 days, considering all-cause death and aggravation of HF as adverse events [76]. Dosages demonstrated that low levels of n-6 PUFAs were present in patients experiencing more severe clinical outcomes. Conversely, there appears to be no correlation between EPA and DHA levels and adverse events. An explanation for the low n-6 PUFA levels in patients hospitalized for ADHF could be linked to the pro-inflammatory role of these fatty acids which therefore could be consumed more often following a more consistent inflammatory response. Decompensated HF also presents a lower synthesis of the potent vasoactive agents, prostaglandin E1 (PGE1), prostacyclin (PG12), and NO, as a consequence of the low AA and DGLA levels. Furthermore, in these patients, the consequences of advanced HF can lead to a condition of malnutrition, which also affects the n-6 PUFA supply. Therefore, the dosing of n-6 PUFAs in ADHF patients could provide information on the risks these patients face after hospitalization [76]. In another study on ADHF by Ouchi S. et al., 2017, over the course of about 2 years, 267 hospitalized patients suffering from this disease were monitored [77]. In particular, the evaluation of the geriatric nutritional risk index (GNRI), which is representative of the nutritional status of patients, associated with long-term mortality and PUFA levels allowed the authors to draw some conclusions. Indeed, low levels of DHA, DGLA, and AA were independently associated with long-term mortality across various nutritional statuses in this study. The same association was not found for EPA. This difference between EPA and DHA is probably due to the different levels of the two fatty acids in the cardiocyte membranes; in fact, DHA is abundant, as opposed to EPA, thus influencing cell membrane function. Additionally, this study indicated that a the time of admission patients with ADHF present a condition of malnutrition, especially in relation to essential fatty acid intake [77]. Furthermore, it has been shown that in ADHF patients, not only low DGLA levels but also a low DGLA/AA ratio are associated with long-term mortality [78].
Depression is known to be another one of the comorbidities affecting HF patients, resulting in a worsening of their health conditions [79]. Therefore, a preliminary study was conducted on a small number of patients suffering from chronic heart failure (CHF) and major depressive disorder. In the trial called Omega-3 Supplementation for Co-Morbid Depression and Heart Failure Treatment (OCEAN), Jiang W. et al., 2018, evaluated the effect of EPA and DHA supplementation on circulating levels of these PUFAs and on depressive disorders that severely afflict patients with CHF [80]. Patients were treated with both a combination of EPA + DHA in a 2:1 ratio and with EPA alone as well as a placebo, for a period of 12 weeks. The results indicate an increase in circulating PUFAs levels as well as an improvement in cognitive depressive symptoms, probably related to more favorable health conditions. These results need to be further investigated based on larger patient groups and for longer treatment times [80].
References
- Chia, N.; Fulcher, J.; Keech, A. Beta-blocker, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, nitrate-hydralazine, diuretics, aldosterone antagonist, ivabradine, devices and digoxin (BANDAID2): An evidence-based mnemonic for the treatment of systolic heart failure. Intern. Med. J. 2016, 46, 653–662.
- Bertero, E.; Maack, C. Calcium Signaling and Reactive Oxygen Species in Mitochondria. Circ. Res. 2018, 122, 1460–1478.
- Bacmeister, L.; Schwarzl, M.; Warnke, S.; Stoffers, B.; Blankenberg, S.; Westermann, D.; Lindner, D. Inflammation and fibrosis in murine models of heart failure. Basic Res. Cardiol. 2019, 114, 1–35.
- Chaudhry, S.P.; Stewart, G.C. Advanced Heart Failure: Prevalence, Natural History, and Prognosis. Heart Fail. Clin. 2016, 12, 323–333.
- Mollace, V.; Rosano, G.M.C.; Anker, S.D.; Coats, A.J.S.; Seferovic, P.; Mollace, R.; Tavernese, A.; Gliozzi, M.; Musolino, V.; Carresi, C.; et al. Pathophysiological Basis for Nutraceutical Supplementation in Heart Failure: A Comprehensive Review. Nutrients 2021, 13, 257.
- Chamberlain, A.M.; Boyd, C.M.; Manemann, S.M.; Dunlay, S.M.; Gerber, Y.; Killian, J.M.; Weston, S.A.; Roger, V.L. Risk Factors for Heart Failure in the Community: Differences by Age and Ejection Fraction. Am. J. Med. 2020, 133, e237–e248.
- Evangelista, I.; Nuti, R.; Picchioni, T.; Dotta, F.; Palazzuoli, A. Molecular Dysfunction and Phenotypic Derangement in Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2019, 20, 3264.
- Mehta, A.; Bhattacharya, S.; Estep, J.; Faiman, C. Diabetes and Heart Failure: A Marriage of Inconvenience. Clin. Geriatr. Med. 2020, 36, 447–455.
- Rao, V.N.; Fudim, M.; Mentz, R.J.; Michos, E.D.; Felker, G.M. Regional adiposity and heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2020, 22, 1540–1550.
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356.
- Florio, M.C.; Magenta, A.; Beji, S.; Lakatta, E.G.; Capogrossi, M.C. Aging, MicroRNAs, and Heart Failure. Curr. Probl. Cardiol. 2020, 45, 1–23.
- Katsi, V.; Georgiopoulos, G.; Laina, A.; Koutli, E.; Parissis, J.; Tsioufis, C.; Nihoyannopoulos, P.; Tousoulis, D. Left ventricular ejection fraction as therapeutic target: Is it the ideal marker? Heart Fail. Rev. 2017, 22, 641–655.
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V.; et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019, 568, 351–356.
- Deng, Y.; Xie, M.; Li, Q.; Xu, X.; Ou, W.; Zhang, Y.; Xiao, H.; Yu, H.; Zheng, Y.; Liang, Y.; et al. Targeting Mitochondria-Inflammation Circuit by β-Hydroxybutyrate Mitigates HFpEF. Circ. Res. 2021, 128, 232–245.
- Juni, R.P.; Kuster, D.W.D.; Goebel, M.; Helmes, M.; Musters, R.J.P.; van der Velden, J.; Koolwijk, P.; Paulus, W.J.; van Hinsbergh, V.W.M. Cardiac Microvascular Endothelial Enhancement of Cardiomyocyte Function Is Impaired by Inflammation and Restored by Empagliflozin. JACC Basic Transl. Sci. 2019, 4, 575–591.
- Hanna, A.; Frangogiannis, N.G. Inflammatory Cytokines and Chemokines as Therapeutic Targets in Heart Failure. Cardiovasc. Drugs Ther. 2020, 34, 849–863.
- Chiurchiù, V.; Leuti, A.; Saracini, S.; Fontana, D.; Finamore, P.; Giua, R.; Padovini, L.; Incalzi, R.A.; Maccarrone, M. Resolution of inflammation is altered in chronic heart failure and entails a dysfunctional responsiveness of T lymphocytes. FASEB J. 2019, 33, 909–916.
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 2020, 17, 269–285.
- Duncan, S.E.; Gao, S.; Sarhene, M.; Coffie, J.W.; Linhua, D.; Bao, X.; Jing, Z.; Li, S.; Guo, R.; Su, J.; et al. Macrophage Activities in Myocardial Infarction and Heart Failure. Cardiol. Res. Pract. 2020, 2020, 4375127.
- Keshavarz-Bahaghighat, H.; Darwesh, A.M.; Sosnowski, D.K.; Seubert, J.M. Mitochondrial Dysfunction and Inflammaging in Heart Failure: Novel Roles of CYP-Derived Epoxylipids. Cells 2020, 9, 1565.
- Gortan Cappellari, G.; Aleksova, A.; Dal Ferro, M.; Cannatà, A.; Semolic, A.; Zanetti, M.; Springer, J.; Anker, S.D.; Giacca, M.; Sinagra, G.; et al. Preserved Skeletal Muscle Mitochondrial Function, Redox State, Inflammation and Mass in Obese Mice with Chronic Heart Failure. Nutrients 2020, 12, 3393.
- Cortassa, S.; Juhaszova, M.; Aon, M.A.; Zorov, D.B.; Sollott, S.J. Mitochondrial Ca2+, redox environment and ROS emission in heart failure: Two sides of the same coin? J. Mol. Cell. Cardiol. 2021, 151, 113–125.
- Dey, S.; DeMazumder, D.; Sidor, A.; Foster, D.B.; O’Rourke, B. Mitochondrial ROS Drive Sudden Cardiac Death and Chronic Proteome Remodeling in Heart Failure. Circ. Res. 2018, 123, 356–371.
- Dietl, A.; Maack, C. Targeting Mitochondrial Calcium Handling and Reactive Oxygen Species in Heart Failure. Curr. Heart Fail. Rep. 2017, 14, 338–349.
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864.
- Kumar, V.; Santhosh Kumar, T.R.; Kartha, C.C. Mitochondrial membrane transporters and metabolic switch in heart failure. Heart Fail. Rev. 2019, 24, 255–267.
- Sabbah, H.N. Targeting the Mitochondria in Heart Failure: A Translational Perspective. JACC Basic Transl. Sci. 2020, 5, 88–106.
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435.
- Xu, H.X.; Cui, S.M.; Zhang, Y.M.; Ren, J. Mitochondrial Ca2+ regulation in the etiology of heart failure: Physiological and pathophysiological implications. Acta Pharmacol. Sin. 2020, 41, 1301–1309.
- Kiyuna, L.A.; Albuquerque, R.P.E.; Chen, C.H.; Mochly Rosen, D.; Ferreira, J.C.B. Targeting mitochondrial dysfunction and oxidative stress in heart failure: Challenges and opportunities. Free Radic. Biol. Med. 2018, 129, 155–168.
- Münzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of Oxidative Stress on the Heart and Vasculature: Part 2 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 212–229.
- Tham, Y.K.; Bernardo, B.C.; Huynh, K.; Ooi, J.Y.Y.; Gao, X.M.; Kiriazis, H.; Giles, C.; Meikle, P.J.; McMullen, J.R. Lipidomic Profiles of the Heart and Circulation in Response to Exercise versus Cardiac Pathology: A Resource of Potential Biomarkers and Drug Targets. Cell Rep. 2018, 24, 2757–2772.
- Blanco-Rivero, J.; Couto, G.K.; Paula, S.M.; Fontes, M.T.; Rossoni, L.V. Enhanced sympathetic neurotransduction in the superior mesenteric artery in a rat model of heart failure: Role of noradrenaline and ATP. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H563–H574.
- Bhatt, A.S.; Abraham, W.T.; Lindenfeld, J.; Bristow, M.; Carson, P.E.; Felker, G.M.; Fonarow, G.C.; Greene, S.J.; Psotka, M.A.; Solomon, S.D.; et al. Treatment of HF in an Era of Multiple Therapies: Statement From the HF Collaboratory. JACC Heart Fail. 2021, 9, 1–12.
- Al-Gobari, M.; Al-Aqeel, S.; Gueyffier, F.; Burnand, B. Effectiveness of drug interventions to prevent sudden cardiac death in patients with heart failure and reduced ejection fraction: An overview of systematic reviews. BMJ Open. 2018, 8, 1–14.
- Rossignol, P.; Hernandez, A.F.; Solomon, S.D.; Zannad, F. Heart failure drug treatment. Lancet. 2019, 393, 1034–1044.
- Rosano, G.; Quek, D.; Martínez, F. Sodium-Glucose Co-transporter 2 Inhibitors in Heart Failure: Recent Data and Implications for Practice. Card. Fail. Rev. 2020, 6, 1–9.
- Nakagawa, Y.; Kuwahara, K. Sodium-Glucose Cotransporter-2 inhibitors are potential therapeutic agents for treatment of non-diabetic heart failure patients. J. Cardiol. 2020, 76, 123–131.
- Grubić Rotkvić, P.; Cigrovski Berković, M.; Bulj, N.; Rotkvić, L.; Ćelap, I. Sodium-glucose cotransporter 2 inhibitors’ mechanisms of action in heart failure. World J. Diabetes 2020, 11, 269–279.
- Shaw, J.A.; Cooper, M.E. Contemporary Management of Heart Failure in Patients with Diabetes. Diabetes Care. 2020, 43, 2895–2903.
- Dick, S.A.; Epelman, S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circ. Res. 2016, 119, 159–176.
- Borlaug, B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2020, 17, 559–573.
- Nair, N. Epidemiology and pathogenesis of heart failure with preserved ejection fraction. Rev. Cardiovasc. Med. 2020, 21, 531–540.
- Von Haehling, S.; Arzt, M.; Doehner, W.; Edelmann, F.; Evertz, R.; Ebner, N.; Herrmann-Lingen, C.; Garfias Macedo, T.; Koziolek, M.; Noutsias, M.; et al. Improving exercise capacity and quality of life using non-invasive heart failure treatments: Evidence from clinical trials. Eur. J. Heart Fail. 2021, 23, 92–113.
- Wintrich, J.; Kindermann, I.; Ukena, C.; Selejan, S.; Werner, C.; Maack, C.; Laufs, U.; Tschöpe, C.; Anker, S.D.; Lam, C.S.P.; et al. Therapeutic approaches in heart failure with preserved ejection fraction: Past, present, and future. Clin. Res. Cardiol. 2020, 109, 1079–1098.
- Jaarsma, T.; Hill, L.; Bayes-Genis, A.; La Rocca, H.B.; Castiello, T.; Čelutkienė, J.; Marques-Sule, E.; Plymen, C.M.; Piper, S.E.; Riegel, B.; et al. Self-care of heart failure patients: Practical management recommendations from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2021, 23, 157–174.
- Rahman, A.; Jafry, S.; Jeejeebhoy, K.; Nagpal, A.D.; Pisani, B.; Agarwala, R. Malnutrition and Cachexia in Heart Failure. JPEN J. Parenter. Enteral. Nutr. 2016, 40, 475–486.
- Carresi, C.; Musolino, V.; Gliozzi, M.; Maiuolo, J.; Mollace, R.; Nucera, S.; Maretta, A.; Sergi, D.; Muscoli, S.; Gratteri, S.; et al. Anti-oxidant effect of bergamot polyphenolic fraction counteracts doxorubicin-induced cardiomyopathy: Role of autophagy and c-kitposCD45negCD31neg cardiac stem cell activation. J. Mol. Cell. Cardiol. 2018, 119, 10–18.
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, M.C.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306.
- Ganguly, R.; Hasanally, D.; Stamenkovic, A.; Maddaford, T.G.; Chaudhary, R.; Pierce, G.N.; Ravandi, A. Alpha linolenic acid decreases apoptosis and oxidized phospholipids in cardiomyocytes during ischemia/reperfusion. Mol. Cell. Biochem. 2018, 437, 163–175.
- Oppedisano, F.; Muscoli, C.; Musolino, V.; Carresi, C.; Macrì, R.; Giancotta, C.; Bosco, F.; Maiuolo, J.; Scarano, F.; Paone, S.; et al. The Protective effect of Cynara Cardunculus extract in diet-induced NAFLD: Involvement of OCTN1 and OCTN2 Transporter Subfamily. Nutrients 2020, 12, 1435.
- Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Scarano, F.; Bosco, F.; Nucera, S.; Maiuolo, J.; Macrì, R.; Ruga, S.; et al. The Effect of natural antioxidants in the development of Metabolic Syndrome: Focus on Bergamot Polyphenolic Fraction. Nutrients 2020, 12, 1504.
- Oppedisano, F.; Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Scicchitano, M.; Scarano, F.; Bosco, F.; Macrì, R.; et al. The Potential for Natural Antioxidant Supplementation in the Early Stages of Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 2618.
- Lauro, F.; Giancotti, L.A.; Ilari, S.; Dagostino, C.; Gliozzi, M.; Morabito, C.; Malafoglia, V.; Raffaeli, W.; Muraca, M.; Goffredo, B.M.; et al. Inhibition of Spinal Oxidative Stress by Bergamot Polyphenolic Fraction Attenuates the Development of Morphine Induced Tolerance and Hyperalgesia in Mice. PLoS ONE 2016, 11, e0156039.
- Gliozzi, M.; Maiuolo, J.; Oppedisano, F.; Mollace, V. The effect of bergamot polyphenolic fraction in patients with non alcoholic liver steato-hepatitis and metabolic syndrome. PharmaNutrition 2016, 4S, S27–S31.
- Aarsetoey, H.; Grundt, H.; Nygaard, O.; Nilsen, D.W. The role of long-chained marine N-3 polyunsaturated Fatty acids in cardiovascular disease. Cardiol. Res. Pract. 2012, 2012, 1–15.
- Adkins, Y.; Kelley, D.S. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J. Nutr. Biochem. 2010, 21, 781–792.
- Landa-Juárez, A.Y.; Ortiz, M.I.; Castañeda-Hernández, G.; Chávez-Piña, A.E. Participation of potassium channels in the antinociceptive effect of docosahexaenoic acid in the rat formalin test. Eur. J. Pharmacol. 2016, 793, 95–100.
- Behl, T.; Kotwani, A. Omega-3 fatty acids in prevention of diabetic retinopathy. J. Pharm. Pharmacol. 2017, 69, 946–954.
- Akbar, U.; Yang, M.; Kurian, D.; Mohan, C. Omega-3 Fatty Acids in Rheumatic Diseases: A Critical Review. J. Clin. Rheumatol. 2017, 23, 330–339.
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56.
- Messamore, E.; Almeida, D.M.; Jandacek, R.J.; McNamara, R.K. Polyunsaturated fatty acids and recurrent mood disorders: Phenomenology, mechanisms, and clinical application. Prog. Lipid Res. 2017, 66, 1–13.
- Jayedi, A.; Shab-Bidar, S. Fish Consumption and the Risk of Chronic Disease: An Umbrella Review of Meta-Analyses of Prospective Cohort Studies. Adv. Nutr. 2020, 11, 1123–1133.
- Napoli, C.; Benincasa, G.; Donatelli, F.; Ambrosio, G. Precision medicine in distinct heart failure phenotypes: Focus on clinical epigenetics. Am. Heart J. 2020, 224, 113–128.
- Serini, S.; Ottes Vasconcelos, R.; Nascimento Gomes, R.; Calviello, G. Protective Effects of ω-3 PUFA in Anthracycline-Induced Cardiotoxicity: A Critical Review. Int. J. Mol. Sci. 2017, 18, 2689.
- Sakamoto, A.; Saotome, M.; Iguchi, K.; Maekawa, Y. Marine-Derived Omega-3 Polyunsaturated Fatty Acids and Heart Failure: Current Understanding for Basic to Clinical Relevance. Int. J. Mol. Sci. 2019, 20, 4025.
- von Haehling, S.; Ebner, N.; Dos Santos, M.R.; Springer, J.; Anker, S.D. Muscle wasting and cachexia in heart failure: Mechanisms and therapies. Nat. Rev. Cardiol. 2017, 14, 323–341.
- O’Keefe, E.L.; Harris, W.S.; DiNicolantonio, J.J.; Elagizi, A.; Milani, R.V.; Lavie, C.J.; O’Keefe, J.H. Sea Change for Marine Omega-3s: Randomized Trials Show Fish Oil Reduces Cardiovascular Events. Mayo Clin. Proc. 2019, 94, 2524–2533.
- Rimm, E.B.; Appel, L.J.; Chiuve, S.E.; Djoussé, L.; Engler, M.B.; Kris-Etherton, P.M.; Mozaffarian, D.; Siscovick, D.S.; Lichtenstein, A.H. American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory from the American Heart Association. Circulation 2018, 138, e35–e47.
- Hopper, I.; Connell, C.; Briffa, T.; De Pasquale, C.G.; Driscoll, A.; Kistler, P.M.; Macdonald, P.S.; Sindone, A.; Thomas, L.; Atherton, J.J. Nutraceuticals in Patients With Heart Failure: A Systematic Review. J. Card. Fail. 2020, 26, 166–179.
- O’Connell, T.D.; Block, R.C.; Huang, S.P.; Shearer, G.C. ω3-Polyunsaturated fatty acids for heart failure: Effects of dose on efficacy and novel signaling through free fatty acid receptor 4. J. Mol. Cell Cardiol. 2017, 103, 74–92.
- Beam, J.; Botta, A.; Ye, J.; Soliman, H.; Matier, B.J.; Forrest, M.; MacLeod, K.M.; Ghosh, S. Excess Linoleic Acid Increases Collagen I/III Ratio and “Stiffens” the Heart Muscle Following High Fat Diets. J. Biol. Chem. 2015, 290, 23371–23384.
- Wang, C.; Xiong, B.; Huang, J. The Role of Omega-3 Polyunsaturated Fatty Acids in Heart Failure: A Meta-Analysis of Randomised Controlled Trials. Nutrients. 2016, 9, 18.
- Berliner, D.; Mattern, S.; Wellige, M.; Malsch, C.; Güder, G.; Brenner, S.; Morbach, C.; Deubner, N.; Breunig, M.; Kiefl, R.; et al. The omega-3 index in patients with heart failure: A prospective cohort study. Prostaglandins Leukot. Essent. Fatty Acids. 2019, 140, 34–41.
- Kones, R.; Howell, S.; Rumana, U. n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: Principles, Practices, Pitfalls, and Promises - A Contemporary Review. Med. Princ. Pract. 2017, 26, 497–508.
- Nagai, T.; Honda, Y.; Sugano, Y.; Nishimura, K.; Nakai, M.; Honda, S.; Iwakami, N.; Okada, A.; Asaumi, Y.; Aiba, T.; et al. Circulating Omega-6, But Not Omega-3 Polyunsaturated Fatty Acids, Are Associated with Clinical Outcomes in Patients with Acute Decompensated Heart Failure. PLoS ONE 2016, 11, e0165841.
- Ouchi, S.; Miyazaki, T.; Shimada, K.; Sugita, Y.; Shimizu, M.; Murata, A.; Kato, T.; Aikawa, T.; Suda, S.; Shiozawa, T.; et al. Low Docosahexaenoic Acid, Dihomo-Gamma-Linolenic Acid, and Arachidonic Acid Levels Associated with Long-Term Mortality in Patients with Acute Decompensated Heart Failure in Different Nutritional Statuses. Nutrients. 2017, 9, 956.
- Ouchi, S.; Miyazaki, T.; Shimada, K.; Sugita, Y.; Shimizu, M.; Murata, A.; Kato, T.; Aikawa, T.; Suda, S.; Shiozawa, T.; et al. Decreased circulating dihomo-gamma-linolenic acid levels are associated with total mortality in patients with acute cardiovascular disease and acute decompensated heart failure. Lipids Health Dis. 2017, 16, 1–8.
- Sbolli, M.; Fiuzat, M.; Cani, D.; O’Connor, C.M. Depression and heart failure: The lonely comorbidity. Eur. J. Heart Fail. 2020, 22, 2007–2017.
- Jiang, W.; Whellan, D.J.; Adams, K.F.; Babyak, M.A.; Boyle, S.H.; Wilson, J.L.; Patel, C.B.; Rogers, J.G.; Harris, W.S.; O’Connor, C.M. Long-Chain Omega-3 Fatty Acid Supplements in Depressed Heart Failure Patients: Results of the OCEAN Trial. JACC Heart Fail. 2018, 6, 833–843.




