Heart failure (HF) characterized by cardiac remodeling is a condition in which inflammation and fibrosis play a key role. Dietary supplementation with n-3 polyunsaturated fatty acids (PUFAs) seems to produce good results. In fact, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have anti-inflammatory and antioxidant properties and different cardioprotective mechanisms.
- PUFAs
- heart failure
- HFrEF
- HFpEF
- myocardial infarction (MI)
- cardiac remodeling
- cardiac fibrosis
- inflammation
- cardioprotective mechanism
1. Introduction
Heart failure (HF) arises as a result of various cardiovascular diseases and leads to numerous cases of hospitalization [1][2][3][1,2,3]. It is known that in HF, there is an alteration of the ventricular filling or of the ejection of blood. To this condition are added symptoms such as dyspnea and fatigue as well as pulmonary and peripheral edema [4]. There are many comorbidities associated with heart failure, such as hypertension, diabetes, and obesity or hyperlipidemia, which are responsible for the increased hospitalization and mortality of patients with HF, even of relatively young subjects [5][6][7][8][9][10][5,6,7,8,9,10]. In addition, there has been an increase in HF cases linked to the ageing population [5][11][5,11]. According to Katsi V. et al. 2017, HF patients are categorized on the basis of underlying left ventricular ejection fraction (LVEF) and are mainly distinguished as patients with HF with a reduced ejection fraction (HFrEF) and patients with HF with a preserved ejection fraction (HFpEF) [12]; the latter represents more than 50% of HF cases and has a much less well-understood pathophysiology than HFrEF [2][12][13][14][15][2,12,13,14,15]. HF is characterized by cardiac remodeling that usually occurs following events related to hypertension, myocardial infarction (MI), myocarditis, or heart valve disease and is a condition in which inflammation and fibrosis are determinants [3][16][3,16] ( Figure 1 ).
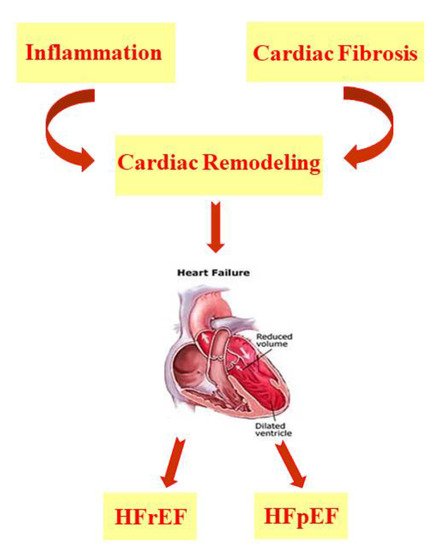
HF is characterized by cardiac remodeling caused by cardiac inflammation and fibrosis; it is distinguished in both HF with a reduced ejection fraction (HFrEF) and HF with a preserved ejection fraction (HFpEF).
In particular, inflammation determines the presence of immune cells in the heart tissue and can be distinguished by sterile myocardial inflammation and non-sterile myocardial inflammation [3]. In order to resolve myocardial inflammation, anti-inflammatory processes are activated, which result in the onset of a pro-fibrotic state. In myocardial fibrosis, extracellular matrix proteins are more abundant and show structural and functional alterations. Both cardiac inflammation and fibrosis are being studied in numerous animal models, as they could be the target of new heart failure therapies [3]. Experimental and clinical studies conducted over the years in patients with acute and chronic heart failure have demonstrated the involvement of both the innate and adaptive immune systems in the disease [17][18][19][17,18,19]. In 2019, the CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) trial, using anti-cytokine therapy with a monoclonal antibody against IL-1β, established that chronic inflammation plays an important role in the pathogenesis of heart failure. Therefore, anti-inflammatory therapies could be administered to heart failure patients with a cardio-inflammatory phenotype [18]. From a pathophysiological point of view, HF has a reduced ATP (adenosine triphosphate) availability, changes in Na + and Ca 2+ handling, and myocytes characterized by oxidative stress. In all of these processes, the mitochondria are involved and therefore play a fundamental role in HF. In fact, the altered mitochondrial structure, dynamics, and function in HF generate an energetic deficit and oxidative stress [2][20][21][22][23][24][25][26][27][28][29][2,20,21,22,23,24,25,26,27,28,29]. In particular, the process known as ROS-induced ROS release is triggered. In this condition, the ROS release from the mitochondria triggers a further release of ROS from neighboring mitochondria; in this way, high levels of ROS are present, which alter the excitation–contraction coupling, generating cardiac remodeling and cell death [2][20][30][31][2,20,30,31]. It has been shown that, in the early stages of HF, oxidative stress and mitochondrial dysfunction determine the onset of a condition known as “cardiomyocyte frailty”, in which myocardial cells undergo apoptosis if there are no protective mechanisms such as autophagy and the overexpression of endogenous antioxidant enzymes [5]. There is also altered function of the endoplasmic reticulum, reduced nitric oxide (NO) release, metalloproteinase (MMP) dysregulation, and a change in cardiac stem cell mobilization. All of this determines an increase in the left ventricle’s stiffness, generating diastolic dysfunction [5]. HF can also arise as a result of a pathological increase in the heart size [32]. Following MI, both the infarcted and non-infarcted areas present ventricular morphological and functional changes, such as greater ventricular preload and afterload and lower systolic volume and cardiac output, conditions that determine HF onset. In order to restore the physiological condition, a neuro-humoral response is activated, which is characterized by a greater sympathetic discharge and hormone synthesis such as angiotensin II and aldosterone, thus increasing peripheral vascular resistance. In the early stages of HF, this represents a compensatory mechanism, but in the long term, it leads to a worsening of symptoms [33]. Regarding the therapy for HFrEF, clinical trials have demonstrated the efficacy of several therapies evaluated at the same time. To date, therapies include treatment with vasodilators, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs) as well as beta-blockers and mineralocorticoid receptor antagonists (MRAs) [34][35][36][34,35,36]. In addition, the US Food and Drug Administration (FDA) has recently approved the sodium–glucose co-transporter 2 (SGLT2) inhibitors as a novel therapeutic approach to treating HFrEF patients, irrespective of the presence or absence of type 2 diabetes (T2D) [37][38][39][40][37,38,39,40]. However, the risk to patients remains high, and further studies are required to define new pharmacological approaches [34][35][36][34,35,36]. With regard to HFpEF, the diagnosis of which is more complex, up until now, clinical studies have not shown the existence of an effective therapy, but treatment is based on the use of diuretics and mineralocorticoid receptor antagonists, and exercise training is recommended [41][42][43][44][41,42,43,44]. At the moment, clinical studies aim at a better pathophysiological characterization in order to identify individual therapies [42][45][42,45]. Furthermore, self-care is very important for the management of HF patients. In particular, the Heart Failure Association (HFA) guidelines focus on maintaining self-care, consisting of regular drug intake and eventual adaptation to changes in the course of the disease. Maintaining self-care also includes a special focus on physical activity and diet. Self-care monitoring and management is also important as well as travel, leisure, smoking, sleep, immunization, and infection prevention [46]. Alongside pharmacological treatment, a non-pharmacological approach could be effective, especially in the early stages of the disease. In particular, it has been shown that many HF patients have a low intake of micronutrients, a condition that could trigger heart failure [5][47][5,47]. Therefore, in the early stages of disease onset, the intake of many plant extracts with antioxidant properties, such as bergamot polyphenolic fraction (BPF) extract, could counteract HF development [5][48][5,48]. In addition, both studies in animal models and in patients with HF indicate that the intake of n-3 polyunsaturated fatty acids (PUFAs) inhibits the onset of interstitial fibrosis and diastolic heart dysfunction [49]. In fact, many researchers are currently studying the use of natural compounds in the prophylaxis and treatment of various diseases, as there are studies that demonstrate their effectiveness in heart diseases, hepatic steatosis, metabolic syndrome, and neurodegenerative diseases [5][50][51][52][53][54][55][5,50,51,52,53,54,55].
2. Heart Failure and PUFAs
PUFAs are long-chain polyunsaturated fatty acids with a carboxyl group (-COOH) at the polar hydrophilic end and a non-polar hydrophobic methyl group (-CH3) at the opposite end [56]. They are divided into two classes, n-3 and n-6 PUFAs, whose precursors are, respectively, α-linolenic acid (ALA, 18:3, Omega-3) and linoleic acid (LA, 18:2, Omega-6), which are defined as essential, as they must be ingested through dietary means [57][58][57,58]. ALA, present in beans, nuts, and flax seeds, is the metabolic precursor of eicosapentaenoic acid (EPA, 20:5 n-3) and docosahexaenoic acid (DHA, 22:6 n-3), while γ-linolenic acid and arachidonic acid (AA) derive from LA, which is mainly present in soy and corn oils [58][59][58,59]. Despite the synthesis pathway in the liver, the highest amount of EPA and DHA is achiever through diet, as they are present in foods of animal origin but, above all, in the oil and flesh derived from marine fish [56][57][58][59][56,57,58,59]. As far as their function is concerned, the n-6 PUFAs and their derivatives have pro-inflammatory action, unlike the n-3 PUFAs and their derivatives, which are powerful anti-inflammatories; therefore, the recommended daily intake of PUFAs is in favor of the latter [59][60][61][62][59,60,61,62]. Meta-analyses of prospective cohort studies indicate that fish consumption is inversely associated with HF risk. On the contrary, the risk of developing HF increases with a higher consumption of meat; therefore, fish can be considered a healthy animal-based dietary source of protein. Obviously, the positive association is also linked to the type of fish consumed and the cooking method. However, fish is considered to be one of the best cardioprotective foods. In interventional studies, it has been reported that the increased consumption of fish or the integration of n-3 PUFAs in diet has a protective action against cardiometabolic risks such as hypertension, inflammation, oxidative stress, and endothelial dysfunction in addition to having lipid-lowering properties [49][63][49,63]. It is interesting to underline that the hypotriglyceridemic, anti-inflammatory, anti-arrhythmic, and anti-thrombotic effects of n-3 PUFAs can be attributed to a modification of the DNA methylation profile in blood leukocytes [64]. Furthermore, both in vivo and clinical studies report the preventive action of n-3 PUFAs on cardiotoxicity and HF, which arise as side effects of anti-cancer therapies [65]. Thanks to their properties, n-3 PUFAs reduce ventricular remodeling and myocardial fibrosis, improving systolic and diastolic ventricular function altered in HF [66]. Furthermore, muscle wasting (understood as loss of muscle mass and strength) and cachexia (understood as weight loss) affect many HF patients, and supplementation with n-3 PUFAs appears to have a beneficial effect on these comorbidities. In fact, both the European Society of Cardiology (ESC) and the American College of Cardiology/American Heart Association (ACC/AHA) guidelines state that the integration of n-3 PUFAs into the diets of HF patients can be considered an additional therapy and is effective in reducing the death and hospitalization risk for these subjects [67]. Additionally, the AHA recommends eating 1–2 seafood meals per week, especially non-fried seafood [68][69][68,69]. In the Australian heart failure guidelines, of all the nutraceuticals considered, only the n-3 PUFAs have a recommendation, even though it is minimal [70]. Some clinical trials did not demonstrate the efficacy of treating HF with n-3 PUFAs, particularly with EPA; this could be caused by the failure to achieve a therapeutically effective concentration in all subjects [71]. Conversely, n-6 PUFAs can be cardiotoxic by acting directly on the heart muscle. In fact, the n-6 PUFAs, while reducing cholesterol levels and showing anti-atherosclerotic properties, can reduce survival after cardiac remodeling; this has been demonstrated in both in vitro and in vivo studies. These studies have shown that LA, but not derivatives such as γ-linolenic acid or arachidonic acid, stimulate the transforming growth factor-β (TGFβ) isoforms by increasing the collagen I/III ratio and lysyl oxidase (LOX) activity, causing an impaired transmitral flow that generates a cardiac “stiffening” with early diastolic dysfunction and normal systolic function [72].
In addition to this, another meta-analysis of randomized controlled trials confirmed lower levels of BNP and serum norepinephrine (sNE) following n-3 PUFA supplementation in HF patients [73][83]. A study in patients with decompensated heart failure reported a significant depletion of EPA and DHA even though this was not correlated with an increased three-year mortality risk [74][84]. Despite the contradictory data on the benefits and optimal doses of n-3 PUFAs, which are very often linked to the design and execution of the study, it seems sufficiently clear that n-3 PUFAs can be used in preventive cardiology. In fact, they show modest but statistically significant positive effects with a progressive trend over time [75][85].
The roles of EPA, DHA, AA, and DGLA have also been studied in acute decompensated HF (ADHF) [76][94]. In particular, the PUFA dosage was evaluated by Nagai T. et al. , 2016, in 685 patients hospitalized for ADHF, with a follow-up period of approximately 560 days, considering all-cause death and aggravation of HF as adverse events [76][94]. Dosages demonstrated that low levels of n-6 PUFAs were present in patients experiencing more severe clinical outcomes. Conversely, there appears to be no correlation between EPA and DHA levels and adverse events. An explanation for the low n-6 PUFA levels in patients hospitalized for ADHF could be linked to the pro-inflammatory role of these fatty acids which therefore could be consumed more often following a more consistent inflammatory response. Decompensated HF also presents a lower synthesis of the potent vasoactive agents, prostaglandin E1 (PGE1), prostacyclin (PG12), and NO, as a consequence of the low AA and DGLA levels. Furthermore, in these patients, the consequences of advanced HF can lead to a condition of malnutrition, which also affects the n-6 PUFA supply. Therefore, the dosing of n-6 PUFAs in ADHF patients could provide information on the risks these patients face after hospitalization [76][94]. In another study on ADHF by Ouchi S. et al., 2017, over the course of about 2 years, 267 hospitalized patients suffering from this disease were monitored [77][95]. In particular, the evaluation of the geriatric nutritional risk index (GNRI), which is representative of the nutritional status of patients, associated with long-term mortality and PUFA levels allowed the authors to draw some conclusions. Indeed, low levels of DHA, DGLA, and AA were independently associated with long-term mortality across various nutritional statuses in this study. The same association was not found for EPA. This difference between EPA and DHA is probably due to the different levels of the two fatty acids in the cardiocyte membranes; in fact, DHA is abundant, as opposed to EPA, thus influencing cell membrane function. Additionally, this study indicated that a the time of admission patients with ADHF present a condition of malnutrition, especially in relation to essential fatty acid intake [77][95]. Furthermore, it has been shown that in ADHF patients, not only low DGLA levels but also a low DGLA/AA ratio are associated with long-term mortality [78][96].
Depression is known to be another one of the comorbidities affecting HF patients, resulting in a worsening of their health conditions [79][103]. Therefore, a preliminary study was conducted on a small number of patients suffering from chronic heart failure (CHF) and major depressive disorder. In the trial called Omega-3 Supplementation for Co-Morbid Depression and Heart Failure Treatment (OCEAN), Jiang W. et al., 2018, evaluated the effect of EPA and DHA supplementation on circulating levels of these PUFAs and on depressive disorders that severely afflict patients with CHF [80][104]. Patients were treated with both a combination of EPA + DHA in a 2:1 ratio and with EPA alone as well as a placebo, for a period of 12 weeks. The results indicate an increase in circulating PUFAs levels as well as an improvement in cognitive depressive symptoms, probably related to more favorable health conditions. These results need to be further investigated based on larger patient groups and for longer treatment times [80][104].
