
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kamel Mhalhel | + 1810 word(s) | 1810 | 2021-08-30 09:59:50 | | | |
| 2 | Kamel Mhalhel | -1 word(s) | 1809 | 2021-08-31 13:26:19 | | | | |
| 3 | Nora Tang | + 131 word(s) | 1940 | 2021-09-01 04:18:05 | | |
Video Upload Options
Acid-sensing ion channels (ASICs) are Na+channels gated by extracellular H+and are widely expressed in the mammalian central and peripheral nervous systems. ASICs are part of the degenerin/epithelial sodium (Na) channel (DEG/ENaC) superfamily whose feature is high permeability to Na that could be blocked by amiloride. Structurally, ASICs consist of two hydrophobic transmembrane domains (TMD) of 20 amino acids approximately, TMD1 and TMD2, a large domain of around 370 amino acids forming an extracellular loop of 14 conserved cysteines, and a kind of short cytoplasmic amino and carboxyl termini of 35–90 amino acids.
1. Introduction
During the last decade, different types of ion channels, including acid-sensing ionic channels (ASICs), calcium-activated potassium (SK1), voltage-gated potassium channels (KV2), and transient receptor potential (TRP) family members, have been identified and investigated in many vertebrate and invertebrate species. Encoded by several genes in all cells type, ion channels show a wide diversity in molecular structure, selectivity to ions, and mechanisms of operation. However, they share the general structural feature of a pore that provides a pathway for charged ions to cross the plasma membrane down their electrochemical gradient. The present chapter covers the intriguing acid-sensing ionic channels in different zebrafish organs, highlighting their structures and function.
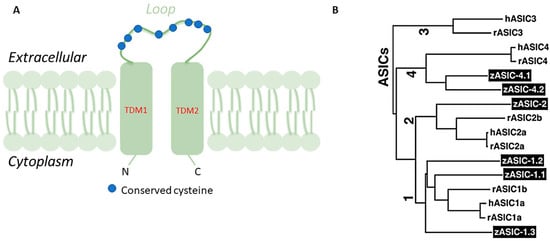
Acid-sensing ion channels (ASICs) are Na+ channels gated by extracellular H+ and are widely expressed in the mammalian central and peripheral nerve systems [1]. ASICs are part of the degenerin/epithelial sodium (Na) channel (DEG/ENaC) superfamily whose feature is high permeability to Na that could be blocked by amiloride. Structurally, ASICs consist of two hydrophobic transmembrane domains (TMD) of 20 amino acids approximately, TMD1 and TMD2, a large domain of around 370 amino acids forming an extracellular loop of 14 conserved cysteines, and a kind of short cytoplasmic amino and carboxyl termini of 35–90 amino acids (Figure 1 A) [2][3][4]. Six ASIC proteins, encoded by four genes, have been identified as ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4 in mammals [5]. Functionally, ASICs monitor moderate deviations from the physiological values of extracellular pH and also participate in mechanoreception and nociception [2][6][7][8][9] or taste receptors [10][11]. ASICs are homo- or hetero-oligomeric assemblies of individual subunits [12][13][14]. The asic1a subunit is widely expressed in the central and peripheral nervous systems and contributes to synaptic transmission [6][15][16]. asic1b and asic3 are expressed in the peripheral nervous system where they would seem to be involved in pain perception [8][17][18][19]. The analgesic effects associated with the inhibition of those ASICs in animals suggest a role in pain. Particularly, in mice with targeted disruptions of the ASIC3 demonstrated the role of ASIC3 in modulating high-intensity stimuli perception, of great importance for the production of pain after tissue inflammation [8][17][19]. asic2a is extensively expressed in the brain and contributes to synaptic transmission [14][18]. asic4 transcripts are abundantly distributed in the adult wild-type mouse brain [20], but in humans, asic4 mRNA is expressed mainly in the pituitary gland while its expression in other parts of the brain is weak [21]. Moreover, ASICs are expressed in dorsal root ganglia (DRG), afferent gastrointestinal neurons [16] and in neurons of the myenteric and submucous plexuses [22].
Fish are, phylogenetically, the closest vertebrate group to amphibians and gave rise to mammals. Living in aquatic ecosystems, fish have different life histories and ecological pressures casting their evolution compared to mammals. An examination of the research findings from fish and mammals pointed up the extent of evolutionary conservation of ASICs across these animal taxa. Indeed, the proton-mediated activation of ASICs was highlighted in teleosts, while in a jawless fish and cartilaginous fish, ASICs were not activated by protons [23]. In addition, while in mammals the major routes for sodium chloride absorption are electroneutral absorption via the combined activity of electroneutral Na+/H+ exchangers and Cl−/HCO3−exchanger beside the electrogenic absorption via epithelial Na+ channels (ENaC) at the mucosal side of the intestinal epithelium, the Na+ uptake in the teleost was channelized by the ASIC family members [24]. ASIC, localized on the apical membranes of the ionocytes in FW trout gills, takes up Na+ from environmental FW in exchange for H+ through vacuolar-type H+ -ATPase (VHA) [25]. In zebrafish, the involvement of the ASICs channels in Na+ uptake was proved [26].
The zebrafish is a model organism for studies in genetics and developmental biology that offers very advantageous features over other vertebrate models, including high fecundity, clear embryos, rapid development, straightforward analysis of gene expression, and detection of developmental abnormalities in mutant fish [27][28][29]. Similar to mammals, the zebrafish has six ASIC subunits denominated zASIC1.1, zASIC1.2, zASIC1.3, zASIC2, zASIC4.1, and zASIC4.2, encoded by six different genes. zASICs share the basic functional properties of mammalian ASICs: receptors of extracellular H+, Na+ selectivity, and the inhibiting effects of amiloride [30]. zasic1.1 , zasic2 , and zasic4.1 are orthologs of mammalian asic1, asic2, and asic4, respectively, while zasic1.2 and zasic1.3 are paralogs of zasic1.1 and, finally, zasic4.2, is a paralog of zasic4.1 (Figure 1 B) [30]. The proteins coded by zasics have molecular masses of around 60 kDa and share 60–75% of amino acid identity with rat and human ASICs [31]. Single ASIC subunits assemble to create functional homomeric or heteromeric ASICs with different surface expression levels on the plasma membrane and consequently different properties. A small region post-TMD has been found to be important in the gating mechanisms of channels in the asic4 gene family [31]. ASICs have been demonstrated in zebrafish sensory organs, gut, gills, and brain using immunohistochemistry, molecular biology techniques, in situ hybridization, Western blot, and RT-PCR, where they ensure nerve transduction (Table 1). Additionally, as in rainbow trout, the ASICs involvement in sodium uptake has been demonstrated in adult zebrafish [31]. However, in larval zebrafish, the ASICs role in Na+ uptake has not been confirmed employing pharmacological and loss of function genetic approaches [32].
2. ASICs in Zebrafish Sensory Organs
Fish are able to transmit chemical or mechanical stimuli from the aquatic environment through a well-organized sensory system. The sensory organs in fish are represented by the olfactory rosette, lateral line, inner ear, taste buds, and chemosensory cells scattered throughout the epidermis and gills. ASICs have been observed in all the sensory organs of zebrafish, although with differences in the distribution between adult and larvae (Table 1) [33,34,35].
| ASIC1 | ASIC2 | ASIC3 | ASIC4 | ZASIC1 | ZASIC2 | ZASIC3 | ZASIC4 | |
|---|---|---|---|---|---|---|---|---|
| Neuromast | + | + | + | + | - | - | - | - |
| Inner ear | + | + | + | - | - | - | - | - |
| Taste buds | + | + | - | + | - | - | - | - |
| Olfactory epithelium | - | + | - | - | - | - | - | - |
| Retina | + | + | - | + | + | - | - | - |
| Gills | - | - | - | + | - | - | - | - |
| Intestine | - | + | - | - | - | - | - | - |
| Brain | - | - | - | - | + | + | - | + |
3. ASICs in Zebrafish Gills
The gills of teleosts are composed of gill arches that provide support for the primary lamellae considered the functional unit of the gill, and are interspersed with five gill slits called chambers [33]. Their epithelium contains ion regulatory cells and, in addition, provides support for the secondary lamellae. The secondary lamellae represent the respiratory unit of the gill and extend from both sides and perpendicular to the longitudinal axis of the main filament [33]. The respiratory lamellae are formed by two epithelial layers separated by spaces due to the presence of pillar cells, through which blood circulates [33]. Freshwater fish, including teleosts, compensate for the loss of ions in a hypotonic environment through the uptake of Na+, Cl−, and Ca2+ ions, which occurs through the activity of particular gill cells, specialized ionocytes defined as mitochondria-rich cells. Four types of ionocytes called mitochondria-rich cells (MRCs) involved in Na+ uptake have been demonstrated in zebrafish gills: VHA-rich cells (HR cells), NKA-rich cells (NaR cells), cells expressing Na+ /Cl− cotransporter (NCC cells), and K+-secreting cells (KS cells) [26]. Furthermore, a previous study has shown the involvement of skin keratinocytes in hypotonicity perception and the contribution of the aforenamed in the activation of innate immunity at an early developmental stage of zebrafish embryos through a transient potential receptor vanilloid 4 (TRPV4)/Ca2+ /TGF-b–activated kinase 1 (TAK1)/NF-kB, by means of pharmacological and genetic inhibition experiments [34]. The expression pattern of different ASIC subunit mRNAs in zebrafish gill tissue was demonstrated by RT-PCR technique. All six ASIC mRNAs were observed in zebrafish gills. Expression of ASIC4.2, by immunoprecipitation, also identified a single band corresponding to ~65 kDa [26]. Furthermore, by immunohistochemistry, cells positive for anti-ASIC4.2 were found in the gills and interlamellar region of the gills. To clarify whether ASIC4.2 colocalizes with NaR cells or HR cells, the gills were double labelled with ASIC4.2 and VHA or NKA, a marker for HR and NaR-type MRC, respectively. Cells immunoreactive for anti-ASIC4.2 were also immunoreactive for anti-VHA. Finally, pharmacological studies in which specific ASIC inhibitors blocked Na+ uptake demonstrated the role of ASIC4.2 in regulating Na+ uptake in zebrafish exposed to low and ultra-low-sodium media at gill level [26].
4. ASICs in Zebrafish Gut
As in mammals, the enteric nervous system of adult zebrafish is organized into two plexuses, the myenteric and submucosal. Structurally, the myenteric plexus consists mainly of enteric neurons, while the submucous plexus has few neurons and numerous nerve fibers [35]. Specific ASIC2 immunoreactivity was found in the enteric nervous system of adult zebrafish as well as in scattered populations of enteroendocrine cells. Particularly, a subpopulation of neurons and nerve fibers were positive to ASIC2, mainly in the myenteric plexus and occasionally in the submucous one. Immunoreactivity for ASIC2 was also found in enteroendocrine epithelial cells in the gut wall. Most of these ASIC2 immunoreactive cells showed a central soma and two processes directed to the organ lumen and the submucous layer, where sometimes they were found close to ASIC2-positive nerve-fiber profiles [36].
5. ASICS in Zebrafish Brain
ASICs are widely expressed in the nervous system of zebrafish embryos and larvae. The expression of six zasic genes in zebrafish neurons was demonstrated through the in situ hybridization technique [30]. In general, the distribution patterns of zasic genes were different. Particularly, zasic1.1 was demonstrated within 30 h postfertilization (hpf) at anterior and posterior lateral line ganglia and optic sensory neurons and at 48 hpf, also in the trigeminal ganglia [30]. At 72 and 96 hpf, expression was observable throughout the central nervous system except for the eyes [30]. zasic1.2 was evident, although with weak expression at 48 hpf, in the ventral thalamus, ventral midbrain, ventral cerebellum and in the dorsal thalamus, hypothalamus and telencephalon along the anterior commissure. zasic1. 2 at 48 hpf was present in the dorsal midbrain (dMb) and olfactory bulb, whereas from 96 hpf it was also expressed in the tectum. zasic1.3 was expressed at 30 hpf in the ganglia of the lateral line while between 30 and 72 hpf in the telencephalon it was expressed along the anterior commissure tract, in the ventral thalamus, ventral midbrain, and ventral cerebellum [30]. By 48 hpf, expression was also evident in the dorsal thalamus and hypothalamus. At 96 hpf, expression was also strong in the habenula. zasic2 was observed at 30 hpf along the anterior commissure tract and at 48 hpf, also in the preoptic area, ventral thalamus, and ventral midbrain. At 72 and 96 hpf, it was present in the whole brain except for the dorsal forebrain and was also expressed in retinal ganglion cells. zasic4.1 at 48 hpf showed a similar pattern to that of zasic1.2 . zasic4.1 was expressed in the dorsal midbrain and retinal ganglion cells. zasic4.2 as early as 24 and 30 hpf was expressed along the anterior commissure tract and in cells along the commissure tract. At 48 hpf, it was observable in the preoptic zone, posterior hypothalamus, ventral midbrain, cerebellum, and retinal ganglion cells [30].
References
- Kress, M.; Waldmann, R. Chapter 8 Acid Sensing Ionic Channels. In Current Topics in Membranes; Academic Press: Cambridge, MA, USA, 2006; Volume 57, pp. 241–276. ISBN 978-0-12-815456-4.
- Sherwood, T.W.; Frey, E.N.; Askwith, C.C. Structure and Activity of the Acid-Sensing Ion Channels. Am. J. Physiol. Cell Physiol. 2012, 303, C699–C710.
- Hanukoglu, I. ASIC and ENaC Type Sodium Channels: Conformational States and the Structures of the Ion Selectivity Filters. FEBS J. 2017, 284, 525–545.
- Gründer, S.; Chen, X. Structure, Function, and Pharmacology of Acid-Sensing Ion Channels (ASICs): Focus on ASIC1a. Int. J. Physiol. Pathophysiol. Pharm. 2010, 2, 73–94.
- Krishtal, O. Receptor for Protons: First Observations on Acid Sensing Ion Channels. Neuropharmacology 2015, 94, 4–8.
- Holzer, P. Acid-Sensitive Ion Channels and Receptors. In Sensory Nerves; Canning, B.J., Spina, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 283–332. ISBN 978-3-540-79090-7.
- Delmas, P.; Coste, B. Mechano-Gated Ion Channels in Sensory Systems. Cell 2013, 155, 278–284.
- Chen, C.-C.; Zimmer, A.; Sun, W.-H.; Hall, J.; Brownstein, M.J.; Zimmer, A. A Role for ASIC3 in the Modulation of High-Intensity Pain Stimuli. Proc. Natl. Acad. Sci. USA 2002, 99, 8992.
- Zha, X. Acid-Sensing Ion Channels: Trafficking and Synaptic Function. Mol. Brain 2013, 6, 1.
- Lin, W.; Ogura, T.; Kinnamon, S.C. Acid-Activated Cation Currents in Rat Vallate Taste Receptor Cells. J. Neurophysiol. 2002, 88, 133–141.
- Ugawa, S. Identification of Sour-Taste Receptor Genes. Anat. Sci. Int. 2003, 78, 205–210.
- Krishtal, O. The ASICs: Signaling Molecules? Modulators? Trends Neurosci. 2003, 26, 477–483.
- Holzer, P. Acid-Sensitive Ion Channels in Gastrointestinal Function. Curr. Opin. Pharmacol. 2003, 3, 618–625.
- Wemmie, J.A.; Price, M.P.; Welsh, M.J. Acid-Sensing Ion Channels: Advances, Questions and Therapeutic Opportunities. Trends Neurosci. 2006, 29, 578–586.
- Lingueglia, E. Acid-Sensing Ion Channels in Sensory Perception. J. Biol. Chem. 2007, 282, 17325–17329.
- Holzer, P. Acid Sensing by Visceral Afferent Neurones. Acta Physiol. 2011, 201, 63–75.
- Lingueglia, E. Les canaux ioniques ASIC dans la douleur. Biol. Aujourd’hui 2014, 208, 13–20.
- Hill, A.S.; Ben-Shahar, Y. The Synaptic Action of Degenerin/Epithelial Sodium Channels. Channels 2018, 12, 262–275.
- Li, W.-G.; Xu, T.-L. ASIC3 Channels in Multimodal Sensory Perception. ACS Chem. Neurosci. 2011, 2, 26–37.
- Hoshikawa, M.; Kato, A.; Hojo, H.; Shibata, Y.; Kumamoto, N.; Watanabe, M.; Ugawa, S. Distribution of ASIC4 Transcripts in the Adult Wild-Type Mouse Brain. Neurosci. Lett. 2017, 651, 57–64.
- Gründer, S.; Geissler, H.S.; Bässler, E.L.; Ruppersberg, J.P. A New Member of Acid-Sensing Ion Channels from Pituitary Gland. Neuroreport 2000, 11, 1607–1611.
- Yiangou, Y.; Facer, P.; Smith, J.; Sangameswaran, L.; Eglen, R.; Birch, R.; Knowles, C.; Williams, N.; Anand, P. Increased Acid-Sensing Ion Channel ASIC-3 in Inflamed Human Intestine. Eur. J. Gastroenterol. Hepatol. 2001, 13, 891–896.
- Sneddon, L.U. Evolution of Nociception and Pain: Evidence from Fish Models. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20190290.
- Takei, Y. The Digestive Tract as an Essential Organ for Water Acquisition in Marine Teleosts: Lessons from Euryhaline Eels. Zool. Lett. 2021, 7, 10.
- Dymowska, A.K.; Schultz, A.G.; Blair, S.D.; Chamot, D.; Goss, G.G. Acid-Sensing Ion Channels Are Involved in Epithelial Na+ Uptake in the Rainbow Trout Oncorhynchus Mykiss. Am. J. Physiol. Cell Physiol. 2014, 307, C255–C265.
- Dymowska, A.K.; Boyle, D.; Schultz, A.G.; Goss, G.G. The Role of Acid-Sensing Ion Channels in Epithelial Na+ Uptake in Adult Zebrafish (Danio Rerio). J. Exp. Biol. 2015, 218, 1244–1251.
- He, Y.; Bao, B.; Li, H. Using Zebrafish as a Model to Study the Role of Epigenetics in Hearing Loss. Expert Opin. Drug Discov. 2017, 12, 967–975.
- Nicolson, T. The Genetics of Hearing and Balance in Zebrafish. Annu. Rev. Genet. 2005, 39, 9–22.
- Orlando, L. Odor Detection in Zebrafish. Trends Neurosci. 2001, 24, 257–258.
- Paukert, M.; Sidi, S.; Russell, C.; Siba, M.; Wilson, S.W.; Nicolson, T.; Gründer, S. A Family of Acid-Sensing Ion Channels from the Zebrafish: Widespread Expression in the Central Nervous System Suggests a Conserved Role in Neuronal Communication. J. Biol. Chem. 2004, 279, 18783–18791.
- Chen, X.; Polleichtner, G.; Kadurin, I.; Gründer, S. Zebrafish Acid-Sensing Ion Channel (ASIC) 4, Characterization of Homo- and Heteromeric Channels, and Identification of Regions Important for Activation by H+. J. Biol. Chem. 2007, 282, 30406–30413.
- Zimmer, A.M.; Dymowska, A.K.; Kumai, Y.; Goss, G.G.; Perry, S.F.; Kwong, R.W.M. Assessing the Role of the Acid-Sensing Ion Channel ASIC4b in Sodium Uptake by Larval Zebrafish. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2018, 226, 1–10.
- Wilson, J.M.; Laurent, P. Fish Gill Morphology: Inside Out. J. Exp. Zool. 2002, 293, 192–213.
- Galindo-Villegas, J.; Montalban-Arques, A.; Liarte, S.; de Oliveira, S.; Pardo-Pastor, C.; Rubio-Moscardo, F.; Meseguer, J.; Valverde, M.A.; Mulero, V. TRPV4-Mediated Detection of Hyposmotic Stress by Skin Keratinocytes Activates Developmental Immunity. J. Immunol. 2016, 196, 738–749.
- Olsson, C. Autonomic Innervation of the Fish Gut. Acta Histochem. 2009, 111, 185–195.
- Levanti, M.B.; Guerrera, M.C.; Calavia, M.G.; Ciriaco, E.; Montalbano, G.; Cobo, J.; Germanà, A.; Vega, J.A. Acid-Sensing Ion Channel 2 (ASIC2) in the Intestine of Adult Zebrafish. Neurosci. Lett. 2011, 494, 24–28.




