Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ranjith Kumavath | + 1100 word(s) | 1100 | 2021-08-31 12:08:48 | | | |
| 2 | Camila Xu | Meta information modification | 1100 | 2021-08-31 13:29:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kumavath, R. Action of Cardiac Glycosides. Encyclopedia. Available online: https://encyclopedia.pub/entry/13747 (accessed on 07 February 2026).
Kumavath R. Action of Cardiac Glycosides. Encyclopedia. Available at: https://encyclopedia.pub/entry/13747. Accessed February 07, 2026.
Kumavath, Ranjith. "Action of Cardiac Glycosides" Encyclopedia, https://encyclopedia.pub/entry/13747 (accessed February 07, 2026).
Kumavath, R. (2021, August 31). Action of Cardiac Glycosides. In Encyclopedia. https://encyclopedia.pub/entry/13747
Kumavath, Ranjith. "Action of Cardiac Glycosides." Encyclopedia. Web. 31 August, 2021.
Copy Citation
Cardiac glycosides are natural sterols and constitute a group of secondary metabolites isolated from plants and animals.
cardiac glycosides
transcription factors
therapeutic target
1. Introduction
Globally, the incidence and mortality of cancer are forecast to rise to 19.3 million cases and 10 million deaths in 2021, making it the second most prevalent cause of death. According to recent reports, female breast cancer (11.7%) has been the most commonly diagnosed cancer, followed by lung (11.4%), colorectal (10%), prostate (7.3%), and stomach (5.6%) cancers. Lung cancer is the highest cause of patient deaths, with an estimated 1.8 million deaths (18%), followed by colorectal (9.4), liver (8.3%), stomach (7.7%), and breast (6.9%) cancers [1]. Some common reasons for cancer include spontaneous or environmentally-induced accumulation of genetic and epigenetic aberrations in the genome, leading to an uncontrolled proliferation of tumor cells [2][3]. It can also result from dysregulation in transcription regulating network proteins such as transcription factors (TFs). TFs are among the most prominent regulatory proteins that bind to specific DNA sequences and regulate gene expression by influencing RNA polymerase activity [4][5]. Thus, researchers worldwide are exploring novel therapeutics that can target these unique mutations in the genome while avoiding the systemic toxicity typical of the traditional chemotherapeutic approach.
In the search for novel therapeutic agents for cancer treatment, natural compounds have always been a focus of scientific attention, and this interest continues to grow. Interest in natural compounds is attributed to several factors such as the minimal side effects compared to chemo or radiation therapy, an extensive array of chemical diversity, and the ease of access and bioprocessing [6][7]. Natural bioactive fractions and pure compounds derived from plants are widely used to treat multiple cancers [8]. In recent years, myriads of plant-derived compounds with anticancer properties were identified that could specifically act on various pathways to inhibit carcinogenesis. Some phytochemically derived anticancer compounds like calebin A, nobiletin, garcinol, CG, and many others target TFs precisely [9][10][11][12]. Cardiac glycosides (CGs) are of particular interest because of their potential as drug repurposing candidates derived from plant and animal sources and are used in various human ailments [13]. CGs are principally involved in the inhibition of the Na+/K+ ATPase pump during muscle contraction in heart failure, and thus, they are clinically approved for the treatment of cardiovascular diseases [14]. Interestingly, the roles of CGs have also been explored for broad application in various cancers. CGs exhibit selective cytotoxic effects on tumor cells; thus, their potential for use as anticancer molecules has increased [15]. Some of the potent inhibitors of cancer cell growth from the group include digitoxin, peruvoside, strophanthidin, bufalin, and ouabain; the principal use of CGs today comes from their ability to inhibit Na+/K+ ATPase pump. However, studies related to the specificity of Na+/K+ ATPase pump in cancer cells have primarily been undertaken to identify its impact on immunogenic cell death that stimulates the cognate immune response.
2. Cardiac Glycosides
CGs are widely distributed naturally-derived cardiotonic steroids obtained predominantly from various plants and amphibian sources and considered highly useful in cardiac and cancer therapeutics [16][17]. Structurally the CG drugs are composed of glycone (sugar) and aglycone (steroid) moieties [18]. The core structure of the CG is characterized by the presence of lactone-containing substituent at the β-17 position (butyrolactone or α-pyrone) and a sugar moiety at the β-3 position of the steroidal core [19]. The CGs aglycone moiety is composed of a steroidal nucleus, and the lactone ring of the aglycone moiety regulates the functional activity of the CG [20]. Besides, the glycone moiety regulates the toxicokinetic and toxicodynamic profiles of the CGs. The most commonly occurring sugars within the glycone moieties are glucose, fructose, galactose, glucuronide, mannose, rhamnose, and digitalose [21][22][23]. The overall potency of the CGs is influenced by the type of sugar attached to the steroid [21]. The addition of the rhamnose was reported to enhance the potency of the CG compound 6 to 35 times. In contrast, the addition of mannose did not exhibit any significant effect on potency [24]. Depending on the R group at position 17, the CGs are structurally classified into two types: (i) cardenolides and (ii) bufadienolides [18]. The cardenolides (lactone 2-furanone) are characterized by the presence of unsaturated five-membered butyrolactone ring at position C-17, and the bufadienolides (lactone 2-pyrone) are identified by the presence of the doubly unsaturated six-member α-pyrone ring.
Previous literature has documented the presence of CGs in the seed, leaf, stem, root, and bark of several plant species, acting as a significant source of arrow poisons and widely distributed in Africa, Asia, and South America. The cardenolides were confined to the angiosperms and abundantly present in Apocynaceae and Asclepiadaceae [18]. Additionally, they were also reported to occur in Brassicaceae, Fabaceae, Malvaceae, Solanaceae, Cruciferae, Sterculiaceae, and Euphorbiaceae [25]. Furthermore, the bufadienolides were found in the plant families, including Crassulaceae, Iridaceae, Hyacinthaceae, Melianthaceae, Ranunculaceae, Santalaceae, and animal sources including toads, fireflies, and snakes [25]. Some of the widely known and characterized CGs include digoxin, digitoxin, ouabain, oleandrin, calotropin, thevetin, convallatoxin, bufalin, marinobufagenin, and telocinobufagin [18][23]. The cardenolides like digoxin and digitoxin were derived from the foxglove species Digitalis lanata and Digitalis purpurea. The African arrow poison ouabain was found in species belonging to the genera Acokanthera and Strophanthus. The toxic oleandrin was obtained from the Apocynaceae plant species called Nerium oleander [26]. The milkweed species belong to Asclepias, and the family Asclepiadaceae, which is reported to produce the toxic cardenolide calotropin. Besides, a poisonous CG like thevetin was found in the South American ornamental shrub Cascabela thevetioides (Kunth) [27], and convallatoxin was reported in the plant Convallaria majalis [28]. The cardiotonic steroid bufalin was found in the dried venom obtained from the parotoid gland of the Chinese bufo toad, and steroids including marinobufagenin and telocinobufagin were isolated from the skin sample secretions of the Brazilian toad, Bufo rubescens, and the giant neotropical marine toad Rhinella marina [29][30].
3. Mode of Action of Cardiac Glycosides
For several decades CG drugs were used in cardiology as folk medicines, diuretics, and emetics to treat cardiac congestion and cardiac arrhythmia [23]. The CG steroids affect cardiac contractility by targeting the cellular Na+/K+ ATPase pump. The inhibition of Na+/K+ ATPase pump (Figure 1) leads to intracellular retention of Na+ and subsequently induces the concentration of intracellular Ca2+ ion mediated by the effect of Na+/Ca2+ membrane exchanges [31]. The elevated level of intracellular Ca2+ concentration causes inotropy and bradycardia. Besides, the accumulation of intracellular Na+ and Ca2+ result in the membrane and ventricular ectopy [31].
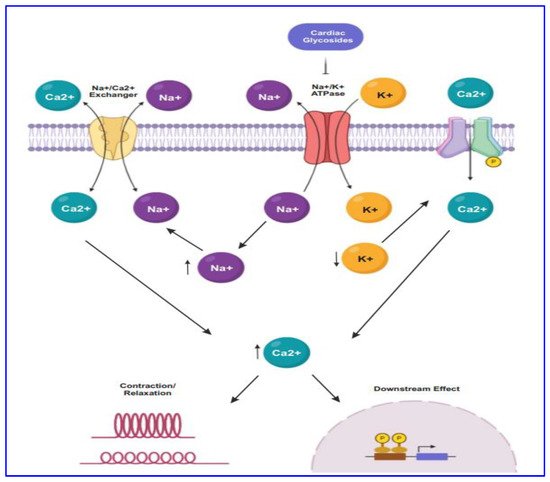
Figure 1. The mode of action of CGs in cancer proceeds through targeting Na+/K+-ATPase by maintaining the concentration of sodium-potassium gradient across the plasma membrane. CG binds to the Na+/K+-ATPase pump, thus inhibiting it, resulting in intracellular retention of Na+ and increasing the concentration of Ca2+. Subsequently, lower expression of Na+/K+-ATPase causes endoplasmic reticulum stress.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Guan, Q.; Yan, H.; Chen, Y.; Zheng, B.; Cai, H.; He, J.; Song, K.; Guo, Y.; Ao, L.; Liu, H. Quantitative or qualitative transcriptional diagnostic signatures? A case study for colorectal cancer. BMC Genom. 2018, 19, 99.
- Takeshima, H.; Ushijima, T. Accumulation of genetic and epigenetic alterations in normal cells and cancer risk. NPJ Precis. Oncol. 2019, 3, 1–8.
- Bhagwat, A.S.; Vakoc, C.R. Targeting transcription factors in cancer. Trends Cancer 2015, 1, 53–65.
- Lambert, M.; Jambon, S.; Depauw, S.; David-Cordonnier, M.-H. Targeting transcription factors for cancer treatment. Molecules 2018, 23, 1479.
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699.
- Thakrar, C.; Patel, K.; D’ancona, G.; Kent, B.D.; Nesbitt, A.; Selsick, H.; Steier, J.; Rosenzweig, I.; Williams, A.J.; Leschziner, G.D.; et al. Effectiveness and side-effect profile of stimulant therapy as monotherapy and in combination in the central hypersomnias in clinical practice. J. Sleep Res. 2017, 27, e12627.
- Zhang, Q.-Y.; Wang, F.-X.; Jia, K.-K.; Kong, L.-D. Natural product interventions for chemotherapy and radiotherapy-induced side effects. Front. Pharmacol. 2018, 9, 1253.
- Aggarwal, V.; Tuli, H.S.; Kaur, J.; Aggarwal, D.; Parashar, G.; Chaturvedi Parashar, N.; Kulkarni, S.; Kaur, G.; Sak, K.; Kumar, M. Garcinol Exhibits Anti-Neoplastic Effects by Targeting Diverse Oncogenic Factors in Tumor Cells. Biomedicines 2020, 8, 103.
- Ashrafizadeh, M.; Zarrabi, A.; Saberifar, S.; Hashemi, F.; Hushmandi, K.; Hashemi, F.; Moghadam, E.R.; Mohammadinejad, R.; Najafi, M.; Garg, M. Nobiletin in Cancer Therapy: How This Plant Derived-Natural Compound Targets Various Oncogene and Onco-Suppressor Pathways. Biomedicines 2020, 8, 110.
- Buhrmann, C.; Shayan, P.; Banik, K.; Kunnumakkara, A.B.; Kubatka, P.; Koklesova, L.; Shakibaei, M. Targeting NF-κB signaling by calebin a, a compound of turmeric, in multicellular tumor microenvironment: Potential role of apoptosis induction in CRC cells. Biomedicines 2020, 8, 236.
- Safe, S.; Kasiappan, R. Natural products as mechanism-based anticancer agents: Sp transcription factors as targets. Phytother. Res. 2016, 30, 1723–1732.
- Karaś, K.; Sałkowska, A.; Dastych, J.; Bachorz, R.A.; Ratajewski, M. Cardiac glycosides with target at direct and indirect interactions with nuclear receptors. Biomed. Pharmacother. 2020, 127, 110106.
- El-Mallakh, R.S.; Brar, K.S.; Yeruva, R.R. Cardiac glycosides in human physiology and disease: Update for entomologists. Insects 2019, 10, 102.
- Reddy, D.; Kumavath, R.; Ghosh, P.; Barh, D. Lanatoside C induces G2/M cell cycle arrest and suppresses cancer cell growth by attenuating MAPK, Wnt, JAK-STAT, and PI3K/AKT/mTOR signaling pathways. Biomolecules 2019, 9, 792.
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Orta, M.L.; Maldonado-Navas, D.; García-Domínguez, I.; López-Lázaro, M. Evaluating the cancer therapeutic potential of cardiac glycosides. BioMed Res. Int. 2014, 2014, 1–9.
- Hou, Y.; Shang, C.; Meng, T.; Lou, W. Anticancer potential of cardiac glycosides and steroid-azole hybrids. Steroids 2021, 171, 108852.
- Morsy, N. Cardiac glycosides in medicinal plants. In Aromatic and Medicinal Plants–Back to Nature; Intechopen: London, UK, 2017; pp. 29–45.
- Cornelius, F.; Kanai, R.; Toyoshima, C. A structural view on the functional importance of the sugar moiety and steroid hydroxyls of cardiotonic steroids in binding to Na, K-ATPase. J. Biol. Chem. 2013, 288, 6602–6616.
- Heasley, B. Chemical synthesis of the cardiotonic steroid glycosides and related natural products. Chem. A Eur. J. 2012, 18, 3092–3120.
- Botelho, A.F.M.; Pierezan, F.; Soto-Blanco, B.; Melo, M.M. A review of cardiac glycosides: Structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon 2019, 158, 63–68.
- Cho, H.-J.; Do, B.-K.; Shim, S.-M.; Kwon, H.; Lee, D.-H.; Nah, A.-H.; Choi, Y.-J.; Lee, S.-Y. Determination of cyanogenic compounds in edible plants by ion chromatography. Toxicol. Res. 2013, 29, 143–147.
- Patel, S. Plant-derived cardiac glycosides: Role in heart ailments and cancer management. Biomed. Pharmacother. 2016, 84, 1036–1041.
- Melero, C.P.; Medarde, M.; San Feliciano, A. A short review on cardiotonic steroids and their aminoguanidine analogues. Molecules 2000, 5, 51–81.
- Hollman, A. Plants and cardiac glycosides. Br. Heart J. 1985, 54, 258.
- Pedroza, H.d.P.; Ferreira, M.G.; Carvalho, J.G.d.; Melo, K.D.A.; Keller, K.M.; Melo, M.M.; Soto-Blanco, B. Concentrações de oleandrina nas folhas de Nerium oleander de diferentes cores da floração. Ciência Rural 2015, 45, 864–866.
- Balderas-López, J.L.; Barbonetti, S.; Pineda-Rosas, E.L.; Tavares-Carvalho, J.C.; Navarrete, A. Cardiac glycosides from Cascabela thevetioides by HPLC-MS analysis. Rev. Bras. Farmacogn. 2019, 29, 441–444.
- Welsh, K.J.; Huang, R.S.P.; Actor, J.K.; Dasgupta, A. Rapid detection of the active cardiac glycoside convallatoxin of lily of the valley using LOCI digoxin assay. Am. J. Clin. Pathol. 2014, 142, 307–312.
- Cunha Filho, G.A.; Schwartz, C.A.; Resck, I.S.; Murta, M.M.; Lemos, S.S.; Castro, M.S.; Kyaw, C.; Pires, O.R., Jr.; Leite, J.R.S.; Bloch, C., Jr. Antimicrobial activity of the bufadienolides marinobufagin and telocinobufagin isolated as major components from skin secretion of the toad Bufo rubescens. Toxicon 2005, 45, 777–782.
- Lenaerts, C.; Wells, M.; Hambÿe, S.; Blankert, B. Marinobufagenin extraction from Rhinella marina toad glands: Alternative approaches for a systematized strategy. J. Sep. Sci. 2019, 42, 1384–1392.
- Roberts, D.M.; Gallapatthy, G.; Dunuwille, A.; Chan, B.S. Pharmacological treatment of cardiac glycoside poisoning. Br. J. Clin. Pharmacol. 2016, 81, 488–495.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
31 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




