
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Suresh Thangudu | + 2069 word(s) | 2069 | 2021-08-11 05:41:09 | | | |
| 2 | Catherine Yang | Meta information modification | 2069 | 2021-08-31 08:40:07 | | |
Video Upload Options
Peroxidase (POD) mimetic nanozymes converts endogenous H2O2 to water (H2O) and reactive oxygen species (ROS) in a hypoxic tumor microenvironment is a fascinating approach.
1. Introduction
Cancer is one of the leading causes of human mortality [1]. Major problems associated with cancer treatment include the reoccurrence of tumors, tumor metastasis and resistance to chemo drugs [2][3]. Chemotherapy, radiotherapy (RT), and surgery are currently the most efficient treatment modalities, but have significant drawbacks including damage to normal cells, tumor reoccurrence, poor visualization and tumor hypoxia [4][5][6]. Therapeutic and diagnostic strategies for treating the cancer efficiently are needed. Recently, significant attention has been focused on nanomaterial-mediated phototherapies such as photothermal therapy (NmPTT) and photodynamic therapy (NmPDT) for the treatment of many diseases including cancers, bacterial infections, etc. [7][8][9][10][11][12]. NmPTT relies on photothermal heat and NmPDT mainly relies on reactive oxygen species (ROS). Compared to traditional therapeutic modalities, NmPDT is highly selectivity, can be remote controlled, has low systemic toxicity and is noninvasive [13][14]. In terms of its mechanism, PDT is strongly dependent on oxygen, and insufficient oxygen in the tumor microenvironment (tumor hypoxia) makes PDT less effective in in vivo systems [6]. Several factors contribute to tumor hypoxia, such as excess extracellular matrix, low pH values, and immunosuppressive factors resulting from altered metabolic pathways and abnormal tumor vasculature [15]. Several studies have found that tumor hypoxia promotes tumor growth [16][17]. Promising results have been achieved in addressing tumor hypoxia by supplying tumors with oxygen using oxygen carriers such as Hb oxygen carriers, non- Hb oxygen carriers and hybrid proteins, etc. [18][19]. However, limited loading efficiency and release of O 2 on oxygen nanocarriers is still a limiting factor [20]. To this end, increased attention has been focused on generating O 2 on nanomaterials, specifically on enzyme mimetic nanomaterials, known as “nanozymes”, to overcome tumor hypoxia and mediate cancer therapeutics [21]. Two different therapeutic approaches, direct killing (increasing ROS) and indirect killing (depletion of ROS) have been used to investigate cancer therapeutics using various nanozymes, mainly peroxidase (POD), oxidase (OXD), superoxide dismutase (SOD) and catalase (CAT) mimetic nanomaterials [22]. The approach of increasing ROS promotes therapeutic efficiency particularly with the use of POD mimetics by overcoming tumor hypoxia in oxygen-dependent PDT. POD mimetic nanozymes catalyze the endogenous H 2O 2 and produce H 2O and ROS in the tumor microenvironment. However, a detailed review of POD mimetic nanozymes reports for cancer therapeutics is still lacking.
2. Nanozymes
Enzymes can serve as biological catalysts (e.g., POD, OXD SOD and CAT, etc.) for various in vivo biological reactions [23]. Like all catalysts, enzymes have two fundamental properties: firstly, they increase the catalytic reaction without being consumed themselves, and secondly, they increase the reaction rates without altering the chemical equilibrium. In general, natural enzymes consist mainly of two parts: a protein and a metallic cofactor. The protein part contains various functional groups and facilitates absorption of the substrate and provides an active site for substrate binding, whereas the metallic part (generally metal ion or metallic complex) facilitates electron transmission. The simultaneous action of the two parts enhances the enzyme’s catalytic activity [24]. Due to superior catalytic activity and excellent substrate specificity, several enzymes have been used in various applications such as agrochemical production, pharmaceutical processes, food industry applications and biomedical applications [25][26][27]. However, the practical applications of enzymes are restricted due to some serious limitations. As shown in Figure 1 , enzymes have disadvantages such as low operational stability, low sensitivity and high cost, etc. Thus, an alternative strategy to mimic natural enzymes and enhance catalytic reactions is urgently required.
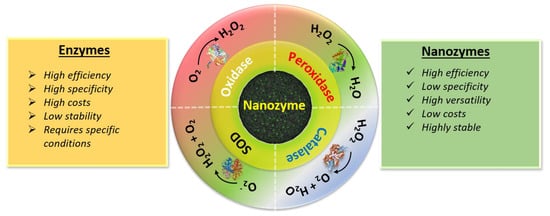
Thus, considerable effort has been devoted to developing nanozymes which are similar to natural enzymes and can effectively catalyze the conversion of enzyme substrates under mild conditions, and exhibit similar catalytic efficiency and enzymatic reaction kinetics [28]. As shown in Figure 1 , nanozymes exhibit several advantages over natural enzymes, including multifunctionality, tunability of catalytic activities, low cost, production scalability, recyclability and high stability. Moreover, these nanozymes can function in ambient conditions. Nanozyme activity can be tuned by simply varying their shape, structure, and composition, and considerable effort has focused on investigating the theoretical mechanisms and kinetics of nanozymes [29].
The advantages of nanozymes have led to their use in energy, environmental and biomedical applications. For instance, nanozymes can be used to qualitatively and quantitatively detect environmental toxins such as ions, molecules and organic compounds [30]. Nanozymes have also been used to treat bacterial infections, offering exciting broad-spectrum antimicrobial properties with negligible cytotoxicities [31]. They have also been used in biosensors for the rapid, reliable, and highly sensitive detection of various diseases [32][33]. Recently, nanomaterial-based nanozymes have been extensively investigated for use in the treatment of cancer through overcoming tumor hypoxia, since oxygen plays a pivotal role in cancer development and treatment [34]. Various enzyme mimetic nanomaterials, such POD OXD, SOD and CAT mimetic nanozymes, have been extensively studied for use in cancer therapeutics [34]. The present review mainly focuses on POD mimetic nanomaterials in cancer theranostic applications, and offers a detailed discussion of current advances and future perspectives.
Peroxidase is a natural enzyme found in wide variety of organisms, from plants to humans to bacteria [35]. The main function of the peroxidase enzyme is the decomposition of H 2O 2 to nontoxic components. H 2O 2 is a toxic byproduct formed by respiration of O 2 [36]. Peroxide enzymes act as detoxifying agents for free radicals (e.g., glutathione peroxidase) and also aid defense against invading pathogens (e.g., myeloperoxidase). Peroxidases are also widely used in bioanalytical and clinical chemistry applications for the detection of analytes via colorimetric assays. As mentioned earlier, to overcome the drawbacks of natural enzymes, significant effort has gone into developing effective alternative POD mimetic enzymatic strategies.
3. Current Trends and Future Perspectives
As discussed earlier, several POD mimetic nanozymes have been successfully used to overcome tumor hypoxia. However, the mechanism by which current POD mimetic NMs generate cytotoxic ROS mainly relies on the amount of intracellular H 2O 2 and pH. Intracellular H 2O 2 concentrations are very low, estimated at around (50 − 100 × 10 −6 M) [37][38]. As a result, most nanozymes have limited therapeutic efficiency in the tumor microenvironment, and catalytic nanozyme therapy alone is not comparable to combination therapy. To this end, some studies have shown that ROS generation on nanozymes can be improved by photothermal therapy [37][39][40]. Zhang et al. fabricated a viruslike Fe 3O 4@Bi 2S 3 nanocatalyst (F-BS NCs) by simple ultrasound. Synergistic coupling of POD mimetic Fe 3O 4 NPs with a narrow band gap semiconductor Bi2S3 (BS) significantly increased POD mimetic activity [41]. As shown in Figure 2 , MNP particles exhibit better enzyme POD-like catalytic activity under mild temperatures compared to at room temperature/no temperature applied. As a result, POD-like activity promotes the conversion frequency of Fe 3+ /Fe 2 + under mild hyperthermia effect on BS under 808 nm laser irradiation [42][43]. Furthermore, POD enzymatic reaction in the tumor microenvironment improves the yield of ROS and resists the cancer under this mild photothermal effect.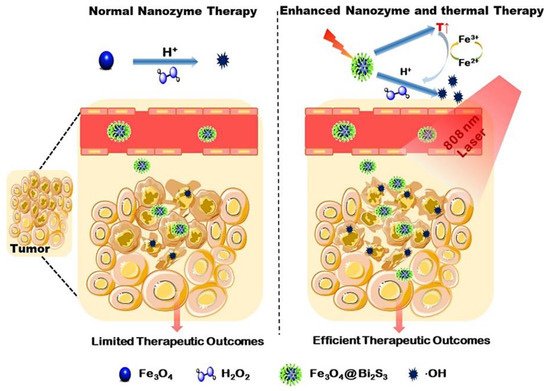
The synergistic effects of dual enzyme mimetic nanostructures in tumor therapy were later investigated. Gao et al. reported a dual inorganic nanozyme-based nanoplatform of Gold (Au) NPs and Fe 3O 4 NPs co-loaded mesoporous silica materials for nanocatalytic tumor therapy [44]. Since Au NPs as a GOx mimic, so it will also catalyze β-D-glucose oxidation into gluconic acid and H 2O 2, which is subsequently catalyzed by the peroxidase-mimic Fe 3O 4 NPs to liberate high-toxic hydroxyl radicals to induce tumor-cell death by the typical Fenton-based catalytic reaction. To further enhance the efficacy of dual nanozyme catalytic therapy, Yi et al. fabricated Wonton-like Bismuth@poly vinyl pyrrolidine@gold platinum (Bi@PVP@AuPt) NPs which exhibited both POD and oxidaselike activity. The stable dual enzymatic behavior of NPs will produce oxygen in hypoxic tumors. Applying the hyperthermia effect to the dual nanozyme significantly promotes ROS generation, resulting in good therapeutic outcomes under combined photothermal and nanocatalytic treatment. Xu et al. designed glucose–oxidase (GOx)-loaded biomimetic Au–Ag hollow nanotriangles (Au–Ag–GOx HTNs) for NIR light-triggered tumor therapy by regulating the tumor environment, where GOx in HTNs triggers the generation of gluconic acid and H 2O 2 [45]. Subsequently, H 2O 2 will be converted to O 2 on the POD mimetic HTNs, eventually boosting the formation of •OH radicals under NIR II light for efficient tumor therapy. Dong et al. reported ceria nanozymes decorating uniform Bismuth sulfide nanorods (Bi 2S 3 NRs) with dendritic mesoporous silica (Bi 2S 3@DMSN) material for tumor catalytic therapy [46]. Synthesized nanozymes exhibited a dual enzyme mimic such as POD and CAT properties under acidic conditions, significantly overcoming tumor hypoxia and elevating oxidative stress under hyperthermia. Recently, Alizadeh et al. reported pH-switchable POD and CAT mimic activities of hierarchical Co(OH) 2/ FeOOH/WO 3 ternary nanoflowers [47]. The POD activities of the as-synthesized nanoflowers were dominant at acidic pH whereas the CAT activities were dominant at basic pH. Indeed, these catalytic materials produced ROS by decomposition of H 2O 2 in both acidic and basic condition, resulting in good anticancer behavior as well as cancer cell detection. Building on these advancements in dual nanozymes and their efficacy in tumor treatment, Ai et al. fabricated a manganese dioxide encapsulated selenium-melanin (Se@Me@MnO 2) multishell nanozyme for intracellular antioxidation [48]. Se@Me@MnO 2 nanozyme exhibits multiple enzyme activities such as CAT, SOD and glutathione peroxidase (GPx). This multishell platform can effectively scavenge the ROS species via synergistic and fast electron transfer between Se, Me and MnO 2. These multienzyme mimetic nanoplatforms are good candidates for future tumor therapy applications.
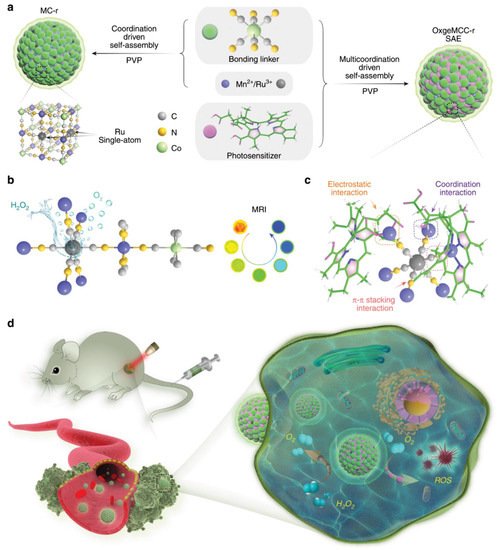
Single-atom-based nanozymes are being developed for various applications. Cost effective and atomically dispersed single atom metal centers can significantly enhance enzyme mimetic properties by maximizing the atomic utilization efficiency and density of active sites. As a result, single atom-based platforms have been developed for various kinds of enzymelike properties such as POD, SOD, CAT, OXD, etc. [49]. By using the enzyme mimetic properties of single atoms, Xu et al. fabricated a zinc-based single atom supported by a metal organic framework and observed excellent POD-like properties [50]. These POD-like activities further help to efficiently deactivate in vivo bacterial infections. Huo et al. fabricated PEGylated single-atom Fe-containing nanocatalysts (PSAF NCs) atoms and observed that the present Fe-based single atom could effectively trigger intracellular H 2O 2 and selectively generate hydroxyl radicals (•OH) in acidic tumor environments [51]. More recently, Wang et al. fabricated single-atom ruthenium as the active catalytic site anchored in a metal-organic framework Mn 3[Co(CN) 6]2 with encapsulated chlorin e6 (Ce 6), which serves as a catalaselike nanozyme for oxygen generation [52]. Figure 3 presents a schematic representation of the detailed fabrication and in vivo applications. Single-atom Ru loading content can degrade intracellular H 2O 2 to O 2 to relieve hypoxia in solid tumors, leading to enhanced ROS generation, and finally causing apoptotic cell death both in vitro and in vivo.
Despite nanozymes offering significant advantages such as high stability, low cost, long-term stability and large scale production, several considerations/improvements are needed for future practical applications. Some key considerations for future nanozymes in cancer applications are as follows.
4. Conclusions
In summary, this review presents past and current advancements in the development of nanozymes, especially POD mimetic nanomaterials for oxygen-dependent phototherapy, with a detailed explanation of the mechanisms and roles of POD mimetic nanozymes in cancer therapeutics. The main obstacle of effective tumor phototherapy is tumor hypoxia. Most phototherapy modalities are oxygen-dependent, and tumor microenvironments contain insufficient oxygen for effective therapeutic application. To overcome this limitation, we review the advantages of POD mimetic nanomaterials which can catalyze endogenous H 2O 2 to H 2O and ROS, thus overcoming tumor hypoxia. Further therapeutic improvement can be achieved through combining therapeutic platforms such as mild PTT-induced enhancement of nanozyme activities and dual, multienzyme strategies. Future perspectives and challenges facing the continued development of nanozyme applications are discussed to elucidate directions for future nanozyme-based therapeutics and to transform clinical settings. We believe the present review helps to provide a more systematic understanding of the advantages, mechanisms and challenges of nanozymes and will facilitate the development of novel POD mimetic nanozymes for efficient cancer phototherapy applications.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The lingering mysteries of metastatic recurrence in breast cancer. Br. J. Cancer 2021, 124, 13–26.
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233.
- Azim, H.A., Jr.; de Azambuja, E.; Colozza, M.; Bines, J.; Piccart, M.J. Long-term toxic effects of adjuvant chemotherapy in breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 1939–1947.
- Majeed, H.; Gupta, V. Adverse Effects Of Radiation Therapy. In StatPearls; StatPearls Publishing Copyright © 2021; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021.
- Brown, J.M. Tumor hypoxia in cancer therapy. Methods Enzymol. 2007, 435, 297–321.
- Vankayala, R.; Hwang, K.C. Near-Infrared-Light-Activatable Nanomaterial-Mediated Phototheranostic Nanomedicines: An Emerging Paradigm for Cancer Treatment. Adv. Mater. 2018, 30, 1706320.
- Thangudu, S.; Cheng, F.-Y.; Su, C.-H. Advancements in the Blood–Brain Barrier Penetrating Nanoplatforms for Brain Related Disease Diagnostics and Therapeutic Applications. Polymers 2020, 12, 3055.
- Thangudu, S. Next Generation Nanomaterials: Smart Nanomaterials, Significance, and Biomedical Applications. In Applications of Nanomaterials in Human Health; Khan, F.A., Ed.; Springer: Singapore, 2020; pp. 287–312.
- Thangudu, S.; Kulkarni, S.S.; Vankayala, R.; Chiang, C.-S.; Hwang, K.C. Photosensitized reactive chlorine species-mediated therapeutic destruction of drug-resistant bacteria using plasmonic core–shell [email protected] nanocubes as an external nanomedicine. Nanoscale 2020, 12, 12970–12984.
- Korupalli, C.; Kalluru, P.; Nuthalapati, K.; Kuthala, N.; Thangudu, S.; Vankayala, R. Recent Advances of Polyaniline-Based Biomaterials for Phototherapeutic Treatments of Tumors and Bacterial Infections. Bioengineering 2020, 7, 94.
- Thangudu, S.; Kaur, N.; Korupalli, C.; Sharma, V.; Kalluru, P.; Vankayala, R. Recent Advances on Near Infrared Light Responsive Multifunctional Nanostructures for Phototheranostic Applications. Biomater. Sci. 2021.
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387.
- Maloth, K.N.; Velpula, N.; Kodangal, S.; Sangmesh, M.; Vallamchetla, K.; Ugrappa, S.; Meka, N. Photodynamic Therapy—A Non-invasive Treatment Modality for Precancerous Lesions. J. Lasers Med. Sci. 2016, 7, 30–36.
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92.
- Brahimi-Horn, M.C.; Chiche, J.; Pouysségur, J. Hypoxia and cancer. J. Mol. Med. 2007, 85, 1301–1307.
- Thomas, S.; Harding, M.A.; Smith, S.C.; Overdevest, J.B.; Nitz, M.D.; Frierson, H.F.; Tomlins, S.A.; Kristiansen, G.; Theodorescu, D. CD24 Is an Effector of HIF-1–Driven Primary Tumor Growth and Metastasis. Cancer Res. 2012, 72, 5600–5612.
- Wang, H.; Li, J.; Wang, Y.; Gong, X.; Xu, X.; Wang, J.; Li, Y.; Sha, X.; Zhang, Z. Nanoparticles-mediated reoxygenation strategy relieves tumor hypoxia for enhanced cancer therapy. J. Control. Release 2020, 319, 25–45.
- Luo, Z.; Tian, H.; Liu, L.; Chen, Z.; Liang, R.; Chen, Z.; Wu, Z.; Ma, A.; Zheng, M.; Cai, L. Tumor-targeted hybrid protein oxygen carrier to simultaneously enhance hypoxia-dampened chemotherapy and photodynamic therapy at a single dose. Theranostics 2018, 8, 3584–3596.
- Sun, Y.; Zhao, D.; Wang, G.; Wang, Y.; Cao, L.; Sun, J.; Jiang, Q.; He, Z. Recent progress of hypoxia-modulated multifunctional nanomedicines to enhance photodynamic therapy: Opportunities, challenges, and future development. Acta Pharm. Sin. B 2020, 10, 1382–1396.
- Sutrisno, L.; Hu, Y.; Hou, Y.; Cai, K.; Li, M.; Luo, Z. Progress of Iron-Based Nanozymes for Antitumor Therapy. Front. Chem. 2020, 8.
- Zhang, Y.; Jin, Y.; Cui, H.; Yan, X.; Fan, K. Nanozyme-based catalytic theranostics. RSC Adv. 2020, 10, 10–20.
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41.
- Hong, S.; Zhang, Q.-L.; Zheng, D.-W.; Zhang, C.; Zhang, Y.; Ye, J.-J.; Cheng, H.; Zhang, X.-Z. Enzyme Mimicking Based on the Natural Melanin Particles from Human Hair. iScience 2020, 23.
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238.
- Cormode, D.P.; Gao, L.; Koo, H. Emerging Biomedical Applications of Enzyme-Like Catalytic Nanomaterials. Trends Biotechnol. 2018, 36, 15–29.
- Alcalde, M.; Ferrer, M.; Plou, F.J.; Ballesteros, A. Environmental biocatalysis: From remediation with enzymes to novel green processes. Trends Biotechnol. 2006, 24, 281–287.
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200.
- Lou-Franco, J.; Das, B.; Elliott, C.; Cao, C. Gold Nanozymes: From Concept to Biomedical Applications. Nano-Micro Lett. 2020, 13, 10.
- Meng, Y.; Li, W.; Pan, X.; Gadd, G.M. Applications of nanozymes in the environment. Environ. Sci. Nano 2020, 7, 1305–1318.
- Yang, D.; Chen, Z.; Gao, Z.; Tammina, S.K.; Yang, Y. Nanozymes used for antimicrobials and their applications. Colloids Surf. B Biointerfaces 2020, 195, 111252.
- Tao, X.; Wang, X.; Liu, B.; Liu, J. Conjugation of antibodies and aptamers on nanozymes for developing biosensors. Biosens. Bioelectron. 2020, 168, 112537.
- Mahmudunnabi, R.G.; Farhana, F.Z.; Kashaninejad, N.; Firoz, S.H.; Shim, Y.-B.; Shiddiky, M.J.A. Nanozyme-based electrochemical biosensors for disease biomarker detection. Analyst 2020, 145, 4398–4420.
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704.
- Khan, A.A.; Rahmani, A.H.; Aldebasi, Y.H.; Aly, S.M. Biochemical and pathological studies on peroxidases—An updated review. Glob. J. Health Sci. 2014, 6, 87–98.
- Mahaseth, T.; Kuzminov, A. Potentiation of hydrogen peroxide toxicity: From catalase inhibition to stable DNA-iron complexes. Mutat. Res./Rev. Mutat. Res. 2017, 773, 274–281.
- Li, S.; Shang, L.; Xu, B.; Wang, S.; Gu, K.; Wu, Q.; Sun, Y.; Zhang, Q.; Yang, H.; Zhang, F.; et al. A Nanozyme with Photo-Enhanced Dual Enzyme-Like Activities for Deep Pancreatic Cancer Therapy. Angew. Chem. Int. Ed. 2019, 58, 12624–12631.
- Ma, Z.; Jia, X.; Bai, J.; Ruan, Y.; Wang, C.; Li, J.; Zhang, M.; Jiang, X. MnO2 Gatekeeper: An Intelligent and O2-Evolving Shell for Preventing Premature Release of High Cargo Payload Core, Overcoming Tumor Hypoxia, and Acidic H2O2-Sensitive MRI. Adv. Funct. Mater. 2017, 27, 1604258.
- Fan, L.; Xu, X.; Zhu, C.; Han, J.; Gao, L.; Xi, J.; Guo, R. Tumor Catalytic-Photothermal Therapy with Yolk-Shell [email protected] Nanozymes. ACS Appl. Mater. Interfaces 2018, 10, 4502–4511.
- Feng, W.; Han, X.; Wang, R.; Gao, X.; Hu, P.; Yue, W.; Chen, Y.; Shi, J. Nanocatalysts-Augmented and Photothermal-Enhanced Tumor-Specific Sequential Nanocatalytic Therapy in Both NIR-I and NIR-II Biowindows. Adv. Mater. 2019, 31, 1805919.
- Zhao, Y.; Ding, B.; Xiao, X.; Jiang, F.; Wang, M.; Hou, Z.; Xing, B.; Teng, B.; Cheng, Z.; Ma, P.a.; et al. Virus-Like [email protected] Nanozymes with Resistance-Free Apoptotic Hyperthermia-Augmented Nanozymitic Activity for Enhanced Synergetic Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 11320–11328.
- Liu, Y.; Zhen, W.; Wang, Y.; Liu, J.; Jin, L.; Zhang, T.; Zhang, S.; Zhao, Y.; Song, S.; Li, C.; et al. One-Dimensional Fe2P Acts as a Fenton Agent in Response to NIR II Light and Ultrasound for Deep Tumor Synergetic Theranostics. Angew. Chem. Int. Ed. 2019, 58, 2407–2412.
- Tang, Z.; Zhang, H.; Liu, Y.; Ni, D.; Zhang, H.; Zhang, J.; Yao, Z.; He, M.; Shi, J.; Bu, W. Antiferromagnetic Pyrite as the Tumor Microenvironment-Mediated Nanoplatform for Self-Enhanced Tumor Imaging and Therapy. Adv. Mater. 2017, 29, 1701683.
- Gao, S.; Lin, H.; Zhang, H.; Yao, H.; Chen, Y.; Shi, J. Nanocatalytic Tumor Therapy by Biomimetic Dual Inorganic Nanozyme-Catalyzed Cascade Reaction. Adv. Sci. 2019, 6, 1801733.
- Xu, M.; Lu, Q.; Song, Y.; Yang, L.; Ren, C.; Li, W.; Liu, P.; Wang, Y.; Zhu, Y.; Li, N. NIR-II driven plasmon-enhanced cascade reaction for tumor microenvironment-regulated catalytic therapy based on bio-breakable Au–Ag nanozyme. Nano Res. 2020, 13, 2118–2129.
- Dong, S.; Dong, Y.; Jia, T.; Liu, S.; Liu, J.; Yang, D.; He, F.; Gai, S.; Yang, P.; Lin, J. GSH-Depleted Nanozymes with Hyperthermia-Enhanced Dual Enzyme-Mimic Activities for Tumor Nanocatalytic Therapy. Adv. Mater. 2020, 32, 2002439.
- Alizadeh, N.; Salimi, A.; Sham, T.-K.; Bazylewski, P.; Fanchini, G.; Fathi, F.; Soleimani, F. Hierarchical Co(OH)2/FeOOH/WO3 ternary nanoflowers as a dual-function enzyme with pH-switchable peroxidase and catalase mimic activities for cancer cell detection and enhanced photodynamic therapy. Chem. Eng. J. 2021, 417, 129134.
- Ai, Y.; You, J.; Gao, J.; Wang, J.; Sun, H.-B.; Ding, M.; Liang, Q. Multi-shell nanocomposites based multienzyme mimetics for efficient intracellular antioxidation. Nano Res. 2021.
- Pei, J.; Zhao, R.; Mu, X.; Wang, J.; Liu, C.; Zhang, X.-D. Single-atom nanozymes for biological applications. Biomater. Sci. 2020, 8, 6428–6441.
- Xu, B.; Wang, H.; Wang, W.; Gao, L.; Li, S.; Pan, X.; Wang, H.; Yang, H.; Meng, X.; Wu, Q.; et al. A Single-Atom Nanozyme for Wound Disinfection Applications. Angew. Chem. (Int. Ed. Engl.) 2019, 58, 4911–4916.
- Huo, M.; Wang, L.; Wang, Y.; Chen, Y.; Shi, J. Nanocatalytic Tumor Therapy by Single-Atom Catalysts. ACS Nano 2019, 13, 2643–2653.
- Wang, D.; Wu, H.; Phua, S.Z.F.; Yang, G.; Qi Lim, W.; Gu, L.; Qian, C.; Wang, H.; Guo, Z.; Chen, H.; et al. Self-assembled single-atom nanozyme for enhanced photodynamic therapy treatment of tumor. Nat. Commun. 2020, 11, 357.




