You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wen-Li Hsu | + 1483 word(s) | 1483 | 2021-08-27 11:02:49 | | | |
| 2 | Rita Xu | -7 word(s) | 1476 | 2021-09-03 12:00:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hsu, W. 9,12-Octadecadiynoic Acid. Encyclopedia. Available online: https://encyclopedia.pub/entry/13677 (accessed on 28 December 2025).
Hsu W. 9,12-Octadecadiynoic Acid. Encyclopedia. Available at: https://encyclopedia.pub/entry/13677. Accessed December 28, 2025.
Hsu, Wen-Li. "9,12-Octadecadiynoic Acid" Encyclopedia, https://encyclopedia.pub/entry/13677 (accessed December 28, 2025).
Hsu, W. (2021, August 30). 9,12-Octadecadiynoic Acid. In Encyclopedia. https://encyclopedia.pub/entry/13677
Hsu, Wen-Li. "9,12-Octadecadiynoic Acid." Encyclopedia. Web. 30 August, 2021.
Copy Citation
Human breast milk lipids have major beneficial effects: they promote infant early brain development, growth and health. To identify the relationship between human breast milk lipids and infant neurodevelopment, multivariate analyses that combined lipidomics and psychological Bayley-III scales evaluation were utilized.
human breast milk
lipidomics
9
12-octadecadiynoic acid
neurodevelopment
adaptive behavior
1. Introduction
The World Health Organization (WHO) suggests a global strategy for infant and young child feeding, and that breastfeeding is helpful to improve infant neurodevelopment, growth and health. Human breast milk contains a mixture of substances that contribute to its nutritional value, and human breast milk lipids supply the fundamental source of energy and necessary nutrients for infants [1]. Human breast milk lipids are mainly composed of triglycerides, which are esters derived from three fatty acids and glycerol. In a small population, polyunsaturated fatty acids (PUFAs) constitute only 0.8% to 26% of triglycerides in human breast milk; they are considered some of the most important lipids required for infant short-term and long-term neurodevelopmental outcomes [2]. Although we are continuously learning more about the role of lipids, especially PUFAs in infant neurodevelopment, lipids represent a large group of macronutrients constituting a majority of components that remain largely unstudied and poorly understood.
We identified a correlation between human breast milk lipid composition and infant neurodevelopment. Milk samples were collected from the study subjects within the first month after giving birth in the Kaoping area in Taiwan. Lipids were further isolated from human breast milk and analyzed for their components. Infants’ and children’s neurodevelopmental assessment scores were measured according to Bayley-III scales. The relationship between human breast milk lipid components and neurodevelopment were analyzed by multiple regression. Notably, 9,12-octadecadiynoic acid (C18H28O2) had a significantly positive correlation coefficient (β estimate = 0.28875) with an adaptive behavioral development (Table 1) and was identified from thousands of human breast milk lipids (Figure 1).
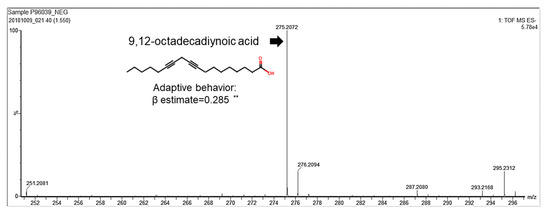
Figure 1. 9,12-octadecadiynoic acid reveals a positive correlation with adaptive behavior. Multivariate analyses between 9,12-octadecadiynoic acid and adaptive behavior of the Bayley-III developmental score adjusted for mother’s age, body mass index and parity number of birth (n = 100). Spearman correlation coefficient of 9,12-octadecadiynoic acid vs. adaptive behavior, correlation coefficient, β estimate = 0.28875; ** p < 0.01.
Table 1. Multivariate analyses between lipid components and the adaptive behavior of the Bayley-III developmental score (n = 100) adjusted for mother’s age, body mass index and parity number of birth.
| Description | Compounds Description (rt_m/z) |
Formula | Category | β estimate (p-Value) |
|---|---|---|---|---|
| Bullatacinone | 1.05_621.4340 | C37H66O7 | Fatty acid | 0.247 (0.013) |
| 9,12-octadecadiynoic acid | 1.55_275.2061 | C18H28O2 | Fatty acid | 0.285 (0.005) |
| 16:1(5Z) | 2.02_253.2221 | C16H30O2 | Fatty acid | −0.203 (0.037) |
9,12-Octadecadiynoic acid can be a derivative from 9,12-octadecadienoic acid (linoleic acid), revealing triple bonds at positions 9 and 12 of linoleic acid. So far, the biological function of 9,12-octadecadiynoic acid is still unclear. This study utilized Caenorhabditis elegans (C. elegans) as a model to investigate how 9,12-octadecadiynoic acid affected neurobehavioral development.
2. Effects of 9,12-Octadecadiynoic Acid Supplementation on Neurobehavioral Indicators in Worms
Our study found that 9,12-octadecadiynoic acid was positively correlated with adaptive behavioral development, which is a complex behavioral development in response to environmental demands. Adaptive behavioral development is defined as the collection of conceptual, social and practical skills learned by people to enable them to function in their everyday lives [3], implicating impact on children’s adaptive capacity to manage risks in unsafe environments. In C. elegans, adaptive behavioral development is an important response to either environmental or internal physiological changes [4]. Neuronal activity has been shown to interact with environmental adaptability that extends further to adaptive behavioral development [5]. Therefore, neurobehavioral indicators are crucial to reflect the condition of the neural system, and the effect of 9,12-octadecadiynoic acid on neurobehavioral development in C. elegans was further investigated.
To avoid the possible metabolic effects on 9,12-octadecadiynoic acid, we firstly examined the worm’s feeding protocol, and confirmed the growth of worms from the L1 to L4 stage with the feeding of UV-killed E. coli OP50. To identify whether 9,12-octadecadiynoic acid affected neuronal activity in C. elegans, locomotive behaviors were evaluated by worm tracking, distance moved, body bends, head thrashes, moving speed and acceleration after larval intake of 9,12-octadecadiynoic acid until young L4 stage. As shown in Figure 2, supplementation with 9,12-octadecadiynoic acid using a dose of 0.1 μM significantly enhanced worms’ locomotive behaviors, but supplementing with 9,12-octadecadiynoic acid doses above 1 μM resulted in the significant inhibition of their locomotive behaviors. Interestingly, the spatial pattern of worm tracking with 0.1 μM 9,12-octadecadiynoic acid supplementation revealed more complications than with other concentrations (Figure 2A).
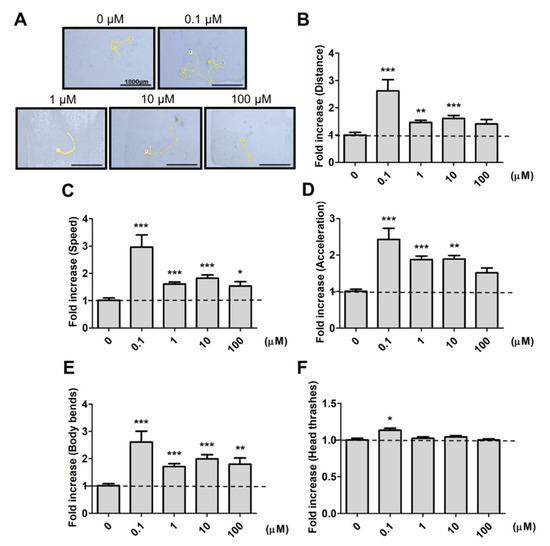
Figure 2. Locomotion evaluation after larval intake of 9,12-octadecadiynoic acid. (A) Worm tracking, (B) moving distance, (C) moving speed, (D) acceleration, (E) body bends and (F)head thrashes. Data (n = 30) were presented as the fold value compared to the control group (mean ± SEM, * p < 0.05; ** p < 0.01; *** p < 0.001).
In addition, an adaptive response to environmental changes can be reflected in foraging activity [6]. We further detected the effect of 9,12-octadecadiynoic acid on foraging ability. A similar result was also observed for foraging behavior; supplementary 9,12-octadecadiynoic acid promoted foraging activity at 0.1 μM, but this activity was delayed at concentrations above 1 μM. Our results imply that a low concentration supplementation with 9,12-octadecadiynoic acid in C. elegans potentially accelerated motor neuronal activity due to the enhancement of neurobehavioral indicators in worms.
3. Effects of 9,12-Octadecadiynoic Acid Supplementation on Aggregative Behavior in Worms
In worms, social interactions, such as aggregative behavior, can be regulated by sensory neurons that respond to environmental stress and maintain survival [7][8]. A family of small molecules, the ascarosides, play important roles as chemical signals regulating aggregative behavior in worms. Therefore, we further investigated whether 9,12-octadecadiynoic acid affected aggregative behaviors that are regulated by ascarosides in C. elegans. After larval intake of 9,12-octadecadiynoic acid from the L1 to L4 stage, aggregative behavior in 20 worms was measured. Although we observed that the aggregation number of worms was increased at high concentrations of supplementary 9,12-octadecadiynoic acid, there was no statistically significant difference with 9,12-octadecadiynoic acid supplementation (Figure 3A,B). It could be that sensory neurons are not importantly involved in 9,12-octadecadiynoic acid-regulated neurobehavioral development. Our results supported that supplementation with 9,12-octadecadiynoic acid under 100 μM in C. elegans did not significantly affect aggregative behavior.
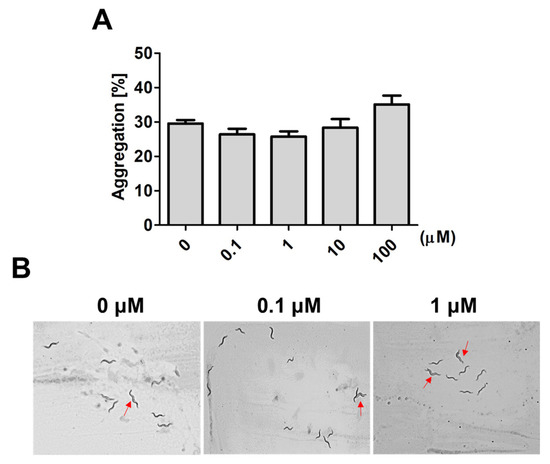
Figure 3. Aggregation behavior analysis after larval intake of 9,12-octadecadiynoic acid. (A) Aggregation behavior of the worms exposed to different concentrations of 9,12-octadecadiynoic acid pretreatment. The data represent the average of three independent experiments (mean ± SEM). (B) Aggregation of the worms (red arrow) on plates.
4. Supplementary 9,12-Octadecadiynoic Acid in Worms Influences Serotonin Synthesis and Serotonin-Related Gene Expression
There are four major neurons in C. elegans larvae, a pair of pharyngeal neurosecretory motor neurons, MSNs, and a pair of chemosensory neurons, ADFs [9]. According to our findings, supplementation with 0.1 μM 9,12-octadecadiynoic acid in worms promoted locomotive behaviors. Motor neuronal activity, especially NSM, could be regulated by supplementation with 9,12-octadecadiynoic acid. Serotonin (5-hydroxytryptamine, 5-HT), which is synthesized in NSMs, belongs to a neurotransmitter group responsible for regulating food sensory activity in a new environment [10]. Therefore, we supposed that serotonin synthesis or serotonin-related gene expression in worms may be affected by supplementing with 9,12-octadecadiynoic acid.
After larval intake of 9,12-octadecadiynoic acid from the L1 to L4 stage, the distribution of serotonin is shown in Figure 4A, and the fluorescence signals of serotonin were especially revealing in the pharyngeal area where MSNs are located. The quantification of the serotonin fluorescence signals revealed the following: Although supplementary 0.1 μM 9,12-octadecadiynoic acid enhanced locomotive behaviors, serotonin synthesis was not increased (Figure 4B). We further detected the expression of tryptophan hydroxylases, cat-4 and tph-1, the key enzymes for serotonin biosynthesis [11], and found that supplementation with 0.1 μM 9,12-octadecadiynoic acid did not accelerate the levels of cat-4 and tph-1 (Figure 4C).
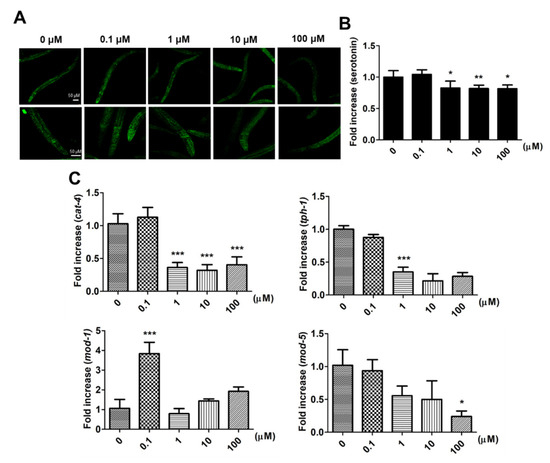
Figure 4. Effect of Effects of 9,12-octadecadiynoic acid on serotonin synthesis and serotonin-related gene expression in C. elegans. (A) Analysis of serotonin (green) in C. elegans by immunohistochemistry staining. The intensity of emitted fluorescence was quantified by using an Olympus confocal microscope as the (B) (mean ± SD, * p < 0.05; ** p < 0.01). (C) Integrated gene serotonin-related expression profiles tested after larval intake of 9,12-octadecadiynoic acid. Values of serotonin-related gene expression were normalized using actin mRNA and represent means relative to the control (mean ± SD, * p < 0.05; *** p < 0.001).
Serotonin transporters, MOD-1 and MOD-5, facilitate the influence of locomotive behaviors in C. elegans via modulating serotonin dynamics [12][13]. To investigate how supplementary 0.1 μM 9,12-octadecadiynoic acid affected locomotive behaviors, the expressions of mod-1 and mod-5 were also examined. Our results indicated that only the level of mod-1 was significantly enhanced by supplementation with 0.1 μM 9,12-octadecadiynoic acid (Figure 4C). However, supplementation with 9,12-octadecadiynoic acid above 1 μM significantly decreased serotonin synthesis and serotonin-related gene expression (Figure 4B,C). These results were similar to the effect of 9,12-octadecadiynoic acid on locomotive behaviors and suggested that the neurosecretory motor neuronal activity in worms is modulated by 9,12-octadecadiynoic acid-regulated serotonin dynamics. This finding implies that 9,12-octadecadiynoic acid significantly regulates locomotive behaviors, especially MSN. Therefore, MOD-1, a serotonin transporters, is involved in 0.1 μM 9,12-octadecadiynoic acid-increased motor neuronal activity and alters neural plasticity in locomotive behaviors.
References
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid Quality in Infant Nutrition: Current Knowledge and Future Opportunities. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 8–17.
- Mortensen, E.L.; Michaelsen, K.F.; Sanders, S.A.; Reinisch, J.M. The association between duration of breastfeeding and adult intelligence. JAMA 2002, 287, 2365–2371.
- Thomas Oakland, P.L.H. Adaptive Behavior Assessment System-II; Elsevier Inc.: Amsterdam, The Netherlands, 2008.
- Lee, I.H.; Procko, C.; Lu, Y.; Shaham, S. Stress-Induced Neural Plasticity Mediated by Glial GPCR REMO-1 Promotes C. elegans Adaptive Behavior. Cell Rep. 2021, 34, 108607.
- Wolf, C.; Linden, D.E. Biological pathways to adaptability--interactions between genome, epigenome, nervous system and environment for adaptive behavior. Genes Brain Behav. 2012, 11, 3–28.
- Pradhan, S.; Quilez, S.; Homer, K.; Hendricks, M. Environmental Programming of Adult Foraging Behavior in C. elegans. Curr. Biol. 2019, 29, 2867–2879.e4.
- Srinivasan, J.; von Reuss, S.H.; Bose, N.; Zaslaver, A.; Mahanti, P.; Ho, M.C.; O’Doherty, O.G.; Edison, A.S.; Sternberg, P.W.; Schroeder, F.C. A modular library of small molecule signals regulates social behaviors in Caenorhabditis elegans. PLoS Biol. 2012, 10, e1001237.
- Artyukhin, A.B.; Yim, J.J.; Cheong Cheong, M.; Avery, L. Starvation-induced collective behavior in C. elegans. Sci. Rep. 2015, 5, 10647.
- Bargmann, C.I.; Chemosensation in C. elegans. In WormBook; WormBook. 2006; pp. 1–29. Available online: http://www.wormbook.org (accessed on 15 July 2021).
- Lee, K.S.; Iwanir, S.; Kopito, R.B.; Scholz, M.; Calarco, J.A.; Biron, D.; Levine, E. Serotonin-dependent kinetics of feeding bursts underlie a graded response to food availability in C. elegans. Nat. Commun. 2017, 8, 14221.
- Cao, X.; Wang, X.; Chen, H.; Li, H.; Tariq, M.; Wang, C.; Zhou, Y.; Liu, Y. Neurotoxicity of nonylphenol exposure on Caenorhabditis elegans induced by reactive oxidative species and disturbance synthesis of serotonin. Environ. Pollut. 2019, 244, 947–957.
- Ranganathan, R.; Sawin, E.R.; Trent, C.; Horvitz, H.R. Mutations in the Caenorhabditis elegans serotonin reuptake transporter MOD-5 reveal serotonin-dependent and -independent activities of fluoxetine. J. Neurosci. 2001, 21, 5871–5884.
- Ranganathan, R.; Cannon, S.C.; Horvitz, H.R. MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature 2000, 408, 470–475.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
891
Revisions:
2 times
(View History)
Update Date:
03 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




