Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hassan Yahaya | + 3466 word(s) | 3466 | 2021-08-25 04:16:08 | | | |
| 2 | Peter Tang | Meta information modification | 3466 | 2021-08-30 03:39:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yahaya, H. Drug-Resistance of Candida glabrata. Encyclopedia. Available online: https://encyclopedia.pub/entry/13655 (accessed on 08 February 2026).
Yahaya H. Drug-Resistance of Candida glabrata. Encyclopedia. Available at: https://encyclopedia.pub/entry/13655. Accessed February 08, 2026.
Yahaya, Hassan. "Drug-Resistance of Candida glabrata" Encyclopedia, https://encyclopedia.pub/entry/13655 (accessed February 08, 2026).
Yahaya, H. (2021, August 27). Drug-Resistance of Candida glabrata. In Encyclopedia. https://encyclopedia.pub/entry/13655
Yahaya, Hassan. "Drug-Resistance of Candida glabrata." Encyclopedia. Web. 27 August, 2021.
Copy Citation
Candida glabrata is a yeast of increasing medical relevance, particularly in critically ill patients. It is the second most isolated Candida species associated with invasive candidiasis (IC) behind C. albicans. The attributed higher incidence is primarily due to an increase in the acquired immunodeficiency syndrome (AIDS) population, cancer, and diabetic patients. The elderly population and the frequent use of indwelling medical devices are also predisposing factors.
Candida glabrata
candidiasis
virulence factors
biofilm
antifungal drug resistance
1. Introduction
Invasive candidiasis (IC) is a clinical condition that is not associated with a single Candida species. Each Candida species holds unique characteristics comparative to invasive potential, virulence, and antifungal susceptibility pattern [1]. It is an infection with many clinical manifestations that potentially affect any organs. Invasive candidiasis is associated with nosocomial bloodstream infections (BSIs) in tertiary health facilities worldwide [2]. Candida species also pose a significant threat to patients in the intensive care unit (ICU) with consequential mortality outcomes. They are the most commonly associated health care reported cases [3]. Major risk factors for Candida infections include prolonged usage of broad-spectrum antibiotics, immunocompromised state of the host, and the use of medical devices in surgery including catheters [3][4]. Candida species commonly cause invasive nosocomial infections in immunocompromised patients [5]. It accounts for 70–90% of all aggressive mycoses [6]. The increasing isolation of non-albicans species suggests increasing pathogenicity of these species with varying degrees of clinical symptoms [7].
Candida glabrata is an asexual, haploid yeast of the clade Nakaseomyces. It was initially named Cryptococcus glabrata. It then changed to Torulopsis glabrata in 1894, but the Candida genus was described in 1913 [8][9]. Candida glabrata is a successful pathogen colonising epithelial surfaces (mouth, gastrointestinal tract, vagina, skin, and present in stool) as healthy microbial flora with no age specificity [10]. Candida glabrata is commonly found in the environment, particularly on flowers, leaves, surfaces, water, and soil. It is the second most frequently isolated cause of candidiasis after Candida albicans. It accounts for approximately 15–25% of invasive clinical cases [8][11][12]. In fact, C. glabrata is the second most common species found in the United States and North-western Europe [1][11]. Increasing incidence of C. glabrata among Candida species as a cause of BSI in U.S. ICUs between 1989 and 1999 in a survey showed that C. glabrata ranked second to C. albicans accounting for 20% to 24% of all Candida BSIs [12]. Invasive candidiasis due to C. glabrata causes substantial morbidity and mortality of approximately 40–60%, perhaps due to the inherent low susceptibility of C. glabrata to the most commonly used azoles [3].
The usual route of C. glabrata to reach the bloodstream is through the breach of natural barriers, such as the use of catheters, trauma, or surgery [13]. However, disease susceptibility increases due to certain conditions such as AIDS and tuberculosis (TB), immunosuppressive use and cancer drugs, prolonged antibiotic therapy, and prolonged hospitalisation [14]. Increasing isolation frequency of C. glabrata is associated with old age, as reported by Zhang et al. [15]. Accordingly, C. glabrata was isolated more from patients in the age group >70 years than the other age groups (58.2% vs. 41.8%) out of 193 samples collected. A switch from normal flora to the pathogenic state may occur, leading to disease setting in, ranging from superficial (mucosal and skin) to systemic with an alarming mortality rate [16].
Virulence refers to the traits required for establishing a disease. However, strictly speaking, virulence factors have direct interaction and causing damage to the host cells [17]. Changes in the state of either the host or the microbe can affect the degree of virulence [18]. Many available factors facilitate the pathogenicity of Candida species. These include enzyme secretion, cellular adhesion, host defence evasion, and biofilm formation [7]. The infection thrives best in the presence of Candida species-specific virulence factors such as the presence of hyphae for invasion into host tissues [19]. Candida albicans filament exists in two distinct morphologies: hyphae and pseudohyphae. The expression of a specific gene set determines each morphology. The morphologies are critical as virulence factors occurring in most Candida species [20][21]. However, Galocha et al. [13] viewed that the pathogenicity of C. glabrata appears to be independent of the morphology of the yeast as this species is incapable of hyphae formation. Despite that, C. glabrata lacks several pathogenic attributes, critical in other Candida species, including polymorphic switching [22][23]; pathogenic relevance is alarming.
Candida albicans and C. glabrata show a significant difference in their mechanisms of virulence. Candida glabrata pathogenicity is associated with many virulence factors [24]. One of the most crucial factors is that it does not provoke a strong reaction by the host’s immune system. The treatment approach for C. glabrata infections is challenging due to the limited knowledge of its pathogenicity. The reduced antifungal drug susceptibility and the limited choices of effective antifungal agents are also challenging in treatment, as described by Yu et al. [25]. Other virulent factors include biofilm formation associated with adherence to host epithelial surfaces and hospital medical devices [7]. Despite the less destructive nature of C. glabrata in comparison to C. albicans, a high mortality rate associated with C. glabrata and rapidity of disease spread would argue otherwise [26]. Candida glabrata seems to have evolved a strategy based on secrecy, evasion, and persistence without causing severe damage in murine models [27]. Skrzypek et al. [28] also believed that C. glabrata exhibits a unique escape mechanism from the immune system and subsequently survives cellular engulfment and can resist antifungal treatment (Figure 1).
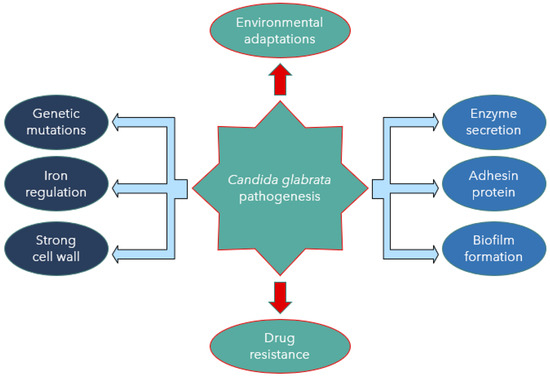
Figure 1. Candida glabrata pathogenesis mediated by virulence factors.
2. Candida glabrata Virulence Factors
2.1. Enzyme Secretion
Secretion of hydrolytic enzymes is a significant determinant of pathogenicity in C. albicans and other non-albicans species. The enzymes protect against host defence reactions [29]. Phospholipases, proteinases, and haemolysins are powerful enzymes used by fungi to invade and infect susceptible hosts [30]. Candida glabrata secretes hydrolytic enzymes (e.g., phospholipases, proteases, and haemolysins) to destroy host tissues [19]. In addition to enzyme secretion, it is thought that host cell penetration occurs via endocytosis induction [13]. The study conducted by Nahas et al. [31] reported three gene families of phosphatases (CgPMU1-3) encoding phosphatase enzymes of different specificity. Accordingly, CgPMU2 was identified as analogous to the PHO5 gene found in S. cerevisiae. It serves as the phosphate-starvation inducible acid phosphatase gene. Almost all known candidal extracellular endopeptidases belong to the aspartic proteinase (Sap) class observed based on sequence analysis, proteolytic activity assay, and secretion of signal detection. Candida glabrata does not possess normal Sap genes in its genome [32]. In this context, C. glabrata is exceptional from this rule because the cell wall is associated with serine protease, Cwp1 (ORF: CBS138)—a gelatinolytic enzyme [24].
2.2. Adhesin Cell-Like Protein
Candida species initiate infection through adherence to host epithelial tissue and colonisation within the host [25]. Candida cell surface proteins involved in specific adherence to surfaces are described as adhesins, and they are critical in mediating biofilms’ formation [7]. Candida glabrata lacks yeast-to-hyphae switching, it grows only in the yeast form, contrary to the virulent switch of C. albicans. A significant virulence factor of C. glabrata is its ability to adhere firmly to many different substrates [3].
Cell surface adhesins in Candida species, particularly C. glabrata or C. albicans, have developed in large gene families [33]. The agglutinin-like sequence (Als) protein family and hyphae wall protein (Hwp1) in C. albicans are critical for the fungal adherence to host epithelial cells [34]. Unlike C. albicans, the main adhesins useful in C. glabrata originated from the epithelial adhesin (EPA) family. These adhesins facilitate C. glabrata attachment to host epithelial cells and assist in macrophage entry [25]. One such cluster includes a lectin-like EPA family. According to the mass spectrometric analysis obtained by De Groot et al. [35], 23 cell wall proteins were identified, including four novel adhesin-like proteins, Awp1/2/3/4 and Epa6. De Groot et al. [35] also reported that C. glabrata contains a unique, high number of genes encoding glycosylphosphatidylinositol (GPI) proteins from different clusters. Both (EPA and GPI) proteins are essential in adherence to human epithelial surfaces and biofilm formation. Cell wall components mediate interactions between C. glabrata and susceptible host, facilitating tissue adhesion and invasion. In addition, they are involved in biofilm formation, triggering the host immune response, and may confer resistance to antifungal drugs [36][37]. Notably, adhesin-like proteins in the cell wall depend on the stage of growth and the genetic background of the invading C. glabrata. Thus, the cells reflected alterations of adhesion capacity and cell surface hydrophobicity.
2.3. Biofilm Formation
Biofilms are considered biological communities formed by microorganisms with a high degree of organisation, structure, coordination, and functionality encased in a self-created extracellular matrix [36]. According to Kumar et al. [9], biofilm is a complex extracellular network of multi-layered microbial structures on biotic or abiotic surfaces shaped by microbe-microbe and organism–surface cooperation. The extracellular matrix defines the biofilm formed by all Candida species. In addition, the matrix contributes to pathogenicity by increasing drug tolerance and promoting immune evasion [38]. Biofilms formed by Candida species, including C. parapsilosis, C. tropicalis, C. glabrata, and C. auris, also associate with extracellular synthesis and high rich polysaccharides contents [38].
Both C. albicans and C. glabrata can form biofilms on abiotic substrates, especially medical devices including catheters and implanted materials [26][27]. Microbial biofilms can form in nature but also inside an infected host. Recently, there has been an increased relevance of microbial biofilms in human diseases, with an estimated 65% of all human infections being of biofilm aetiology [39]. Biofilm formation is another pathogenic mechanism observed in C. albicans with high biofilm mass, densely packed with pseudohyphae. However, C. glabrata produces sparse biofilm (less weight) with yeast cells. Thus, it is an essential pathogenic mechanism for its survival [40] (Figure 2).
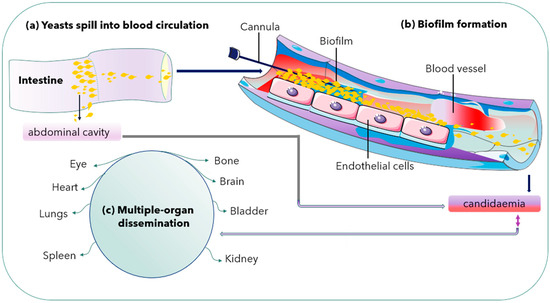
Figure 2. Biofilm formation in a blood vessel and dissemination into multiple organs. Double arrow shows either way dissemination of C. glabrata cells.
2.4. Presence of a Stable Cell Wall
The fungal cell wall is the primary contact site for host-pathogen interaction [41]. The fungal cell wall consists of complex biomolecule structures made up of polysaccharides, proteins, and lipids. The composition is dynamic, responding to changes in the local environment [25][42]. Candida cell wall consists of an inner layer of polysaccharides (chitin, 1,3-β-glucans, and 1,6-β-glucans). An outer layer of proteins glycosylated with mannan constitutes the pathogen-associated molecular patterns (PAMPs). The PAMPs are recognised by specific innate immune receptors known as pathogen recognition receptors (PRRs) [20]. The cell wall is dynamic and necessary to maintain the osmotic pressure exertion and morphology during vegetative growth. Other environmentally induced developmental changes such as sporulation, sexual reproduction, or pseudohyphae growth are often necessary for survival and growth. The fungal cell wall comprises three significant polysaccharides: glucans, mannoproteins, and chitin [43]. Moreover, the findings of Srivastava et al. [44] showed that cysteine abundance is common in fungal extracellular membranes (CFEM) domain-harbouring cell wall structural protein, CgCcw14, and a putative haemolysin, CgMam3. They are vital for the maintenance of intracellular iron content, adherence to epithelial cells, and virulence.
Genetic mutations confer susceptibility to patients against Candida species [20]. Candida glabrata has well-characterised genes, including ACE2 (CgACE2), a transcription factor that serves as a negative regulator of virulence. It was studied in an invasive infection of an immunocompromised mice model. The evolved (Evo) strain is another hyper-virulent C. glabrata strain with a single nucleotide mutation in the chitin synthase gene CHS2. Both mutants have enhanced virulence. Moreover, they stimulate inflammatory response factors, such as tumour necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6). Thus, the ace2 mutant and Evo strain exhibit a clumpy pseudohypha-like structure [25]. Other strains with enhanced virulence characters include a strain with the PDR1 gain-of-function mutation, a strain with mitochondrial dysfunction, and the anp1 and mnn2 glycosylation mutants [25].
2.5. Novel Hybrid Iron Regulation and Acquisition Strategies
Candida glabrata requires iron as an essential micronutrient for its growth during infection. Thus, it is necessary to strategize the mechanism for its acquisition for disease establishment [45]. Among the known iron uptake mechanisms in fungi are siderophore-interceded uptake of Fe3+, reductive iron procurement, and haemoglobin/haem uptake. All these frameworks are operational in C. glabrata except for the receptor-interceded haem uptake [9]. The underscore tight regulation of all processes involving iron in the organism, including uptake, distribution, utilisation, and storage. Candida glabrata has high-affinity iron uptake mechanisms as critical virulence determinants.
While Saccharomyces cerevisiae is a non-pathogenic yeast belonging to whole-genome duplication clade (WGD), having significant similarities with pathogenic C. glabrata [3], it is poorly understood whether the different pathogenic clades, including CTG, may use common infection strategies or lineage-specific mechanisms or both combinations for pathogenicity [3][45]. C. glabrata combines the iron regulation network properties of both pathogenic and non-pathogenic fungi (S. cerevisiae). Candida glabrata, such as S. cerevisiae, uses the Aft1 gene as the primary positive regulator during the sub-optimal iron condition. At the same time, Cth2 degrades mRNAs encoding iron-requiring enzymes. However, it contrasts with S. cerevisiae in that it requires Sef1 ortholog for total growth under iron-limited conditions. The iron homeostasis mechanisms in C. glabrata is still unknown. Candida glabrata showed host-specific iron acquisition mechanisms by utilising siderophores and haemoglobin as a source of iron and haemolysin. It also uses cell wall structural protein to maintain iron homoeostasis [44].
2.6. Adaptation to Various Environmental Conditions
Yeast cells within their natural habitat make many metabolic adjustments in response to changes in extracellular environmental nutrients. Such changes result in gene expression, which are either upregulated or downregulated depending on the environmental requirements [46]. Adaptation of gene expression through transcription regulation is a significant mechanism in fungal response to rapidly changing environmental conditions [47]. The response was first described in Saccharomyces cerevisiae and is referred to as general stress response or environmental stress response (ESR). Genome-wide environmental stress response (ESR) expression profile of C. glabrata is coordinated by Msn2 which is the main transcriptional response activator. Transcription factors Msn2 and Msn4 are crucial for resistance to various stresses in C. glabrata [48]. Activation of Msn2 and Msn4 in the cells causes their rapid accumulation in the nucleus and recruitment to chromatin. Msn2 has separate functional domains for nuclear import (nuclear localization signal, NLS), nuclear export (nuclear export signal, NES), and DNA binding. The stress conditions including disturbed cellular integrity, osmostress, elevated temperature, and the presence of antifungal drug resistance are commonly observed in clinical isolates [22].
During phagocytosis, the internalised microbes become lysed in lysosomes—a specialised compartment in which oxidative and non-oxidative mechanisms kill and degrade the internalised microbes [21]. Candida glabrata lacks hyphal formation and phagosomal extrusions to escape the phagocytic cells attack contrary to C. albicans [49][50]. In Cryptococcus neoformans, the produced capsules inhibit phagocytosis by macrophages and prevent the killings of the already internalised cells [51]. The less aggressive mechanism helps in an autophagy process by mobilising its intracellular resources for metabolism and survival during prolonged starvation [49][50] Evidence suggests that growth in the presence of alternative carbon sources affects the phagocytosis of Candida species. C. glabrata has high-stress resistance. Perhaps its enhanced sustenance during starvation allows it to survive and replicate inside the immune system cells (macrophages). The C. glabrata are engulfed during bloodstream circulation [13][18]. Chew et al. [52] revealed that the ICL1 gene helps promote the growth and prolonged survival of C. glabrata during macrophage engulfment. Thus, C. glabrata shows a unique immune system evasion mechanism and survives after cellular engulfment despite the antifungal presence. Perhaps through concealment within intracellular niches [21][28]. Lactate-grown C. glabrata cells, for example, resist killing by macrophages and have developed distinct tactics for intracellular survival killing and escaping phagocytosis [53]. Following extended division, the macrophages rupture, and yeast cells escape and disseminate into the blood system for further spread [13] (Figure 3).
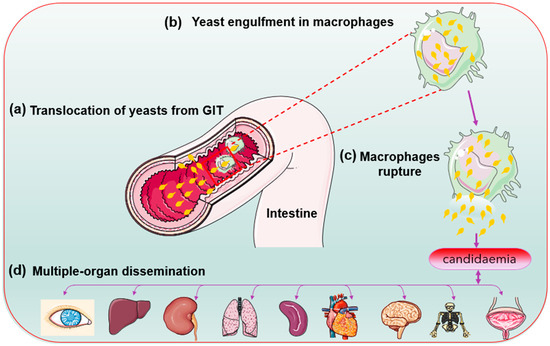
Figure 3. Candida glabrata cells (yellow) replication inside the macrophage cells before organ dissemination.
Successful clearance of pathogens depends on phagocytes’ rapid actions of the innate immune system, such as macrophages, dendritic cells, and neutrophils [21]. The primary factor aiding the persistence of C. glabrata is its less aggressive nature to stimulate the strong reaction of the host immune system [24]. Because of the low host cell damage, C. glabrata cells elicit a cytokine profile significantly different from that of C. albicans. Consequently, C. glabrata is associated with mononuclear cell proliferation (macrophages). In contrast, neutrophil emergence becomes typical of C. albicans [8]. Despite the medical importance of C. glabrata, it is less lethal because it provokes a low inflammatory immune response. The systemic mouse infection models indicated that even at high inocula doses of intravenous infection [21]. Furthermore, the upregulation of Trx1p as a stress-response protein exerts defences to C. glabrata against oxidative stress [54]. Considering the role of dimorphism as a factor for pathogenicity in some Candida species, C. glabrata is exceptional; it does not germinate into hyphae yet is virulent [55].
2.7. Replicative Ageing
Candida glabrata as occur in S. cerevisiae, C. albicans, and C. neoformans show a replicative ageing, a process where original mother cells progressively age, producing asymmetric mitotic divisions resulting in phenotypically distinct daughter cells [16]. It can also contribute to the microevolution of pathogens in a specific host [56]. A mother cell can only produce a specific number of buds during mitotic division. The total number of buds that a mother cell produces before the division ceases and dies is the designated replicative life span (RLS). Each cycle of bud formation by a mother cell represents one generation [57]. Several studies showed that replicative ageing in many fungal pathogens leads to significant changes that affect the fungal resistance to phagocytic clearance and antifungal therapy [57]. The phenotypic changes in the daughter cells due to ageing are not genetically inherited. The old cells only emerge because of neutrophil pressure in the environment that favour the killing of young fungal cells and the promotion of the persistence of old cells [57]. Thus, for the pathogen, this form of adaptation is advantageous, as it avoids the risk of random permanent mutations and instead assures that all adaptive changes are easily reversed in the daughter cells that are borne from asymmetric budding. Aged cells exhibit different lipid composition that leads to the emergence of azole resistance. The replicative age allows the transition from commensalism to a pathogenic state. The intimate association between C. glabrata and a mammalian host may result in resilience and high-stress tolerance. The host becomes vulnerable to invasive diseases during neutropenic or immunocompromised states [56].
Candida glabrata can shift from a commensal to pathogenic state due to the pressure of neutrophils. Bouklas et al. [56] reported a controlled depletion in studies of C. glabrata in the murine models. The findings indicated that ageing leads to remodelling of the cell wall and that neutrophils selection controls generational distribution within the C. glabrata population. The in vivo study by Bhattacharya et al. [58] viewed that the neutrophils cells in the host selectively kill younger cells, leaving the old yeast cells to accumulate. Perhaps, the ageing C. glabrata mother cells’ large cell sizes and thicker cell walls contribute to their better resistance to neutrophil killings than the young daughter cells.
3. Drug-Resistance Mechanisms of Candida glabrata
The emergence of antifungal resistance becomes a problem in clinical medicine, significantly when associated with Candida species. Knowledge of C. glabrata infection symptoms is essential because Candida species commonly share indices of suspicion of the disease. C. glabrata among the non-albicans Candida species can acquire drug resistance. Moreover, it can develop secondary resistance to other available antifungal classes, resulting in poor treatment outcomes. It is a well-known fact that both C. krusei and some C. glabrata have intrinsic resistance to fluconazole. In such a situation, proper diagnosis is essential to justify appropriate treatment [59].
The incidence of candidemia caused by fluconazole-resistant strains and derivatives is high [60]. Azole drugs are among the four classes of antifungals commonly used in clinical practice to treat cancer, AIDS, patients on chemotherapy, and bone marrow transplant patients with fungal infections [61]. The most prevalent Candida species, C. albicans and C. glabrata differ significantly in response to antifungal therapy [62]. Fluconazole is extensively prescribed and administered because of its availability for oral administration, has low toxicity, and is less expensive. However, the extensive use of fluconazole has led to the increasing emergence of resistant isolates [63][64].
Candida glabrata infections are complicated to treat due to their inherent resistance to antifungals, especially against azoles [53]. Sardi et al. [65] viewed that C. glabrata has intrinsic antifungal resistance, especially to fluconazole. Arendrup and Patterson [66] argued that C. glabrata developed acquired resistance to antifungal drugs through prolonged exposure. Moreover, Jensen et al. [67] supported the view that prolonged administration of antifungal drugs for treatment and prevention is the primary cause of the emergence of resistant strains. The frequency and relatively high mortality rates of these infections are generally associated with pathogenic yeast capacity to efficiently develop multiple drug resistance (MDR).
Moreover, C. glabrata shows multi-drug-resistant capacity at an alarming rate. The genomes of C. glabrata can accumulate gene mutations that result in phenotypic resistance to antifungals after exposure to multiple drugs [68]. For example, mutations in the MSH2 gene, encoding a DNA mismatch repair protein, occur in C. glabrata. Its effects have been found in clinical isolates to facilitate the selection of resistance to azoles, echinocandins, and polyenes in vitro [1]. On a general note, the published in vitro data have shown that deoxycholate amphotericin B (dAmB) and echinocandins such as caspofungin or micafungin demonstrated high activity against C. albicans and C. glabrata growing in biofilms settings [69].
References
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026.
- Won, E.J.; Shin, J.H.; Choi, M.J.; Lee, W.G.; Park, Y.-J.; Uh, Y.; Kim, S.-Y.; Lee, M.-K.; Kim, S.H.; Shin, M.G.; et al. Antifungal Susceptibilities of Bloodstream Isolates of Candida Species from Nine Hospitals in Korea: Application of New Antifungal Breakpoints and Relationship to Antifungal Usage. PLoS ONE 2015, 10, e0118770.
- Timmermans, B.; De Las Peñas, A.; Castaño, I.; Van Dijck, P. Adhesins in Candida glabrata. J. Fungi 2018, 4, 60.
- Norimatsu, Y.; Morii, D.; Kogure, A.; Hamanaka, T.; Kuwano, Y.; Yokozawa, T.; Oda, T. A case of breakthrough Candida parapsilosis fungemia during micafungin therapy for a Candida glabrata bloodstream infection. Med. Mycol. Case Rep. 2017, 16, 1–3.
- Vanhee, L.M.E.; Meersseman, W.; Lagrou, K.; Maertens, J.; Nelis, H.J.; Coenye, T. Rapid and Direct Quantification of Viable Candida Species in Whole Blood by Use of Immunomagnetic Separation and Solid-Phase Cytometry. J. Clin. Microbiol. 2010, 48, 1126–1131.
- Lamagni, M.T.L.; Evans, B.G.; Shigematsu, M.; Johnson, E.M. Emerging trends in the epidemiology of invasive mycoses in England and Wales (1990–1999). Epidemiol. Infect. 2001, 126, 397–414.
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36, 288–305.
- Kounatidis, I.; Ames, L.; Mistry, R.; Ho, H.-L.; Haynes, K.; Ligoxygakis, P. A Host-Pathogen Interaction Screen Identifies ada2 as a Mediator of Candida glabrata Defenses against Reactive Oxygen Species. G3 Genes Genomes Genet. 2018, 8, 1637–1647.
- Kumar, K.; Askari, F.; Sahu, M.S.; Kaur, R. Candida glabrata: A Lot More Than Meets the Eye. Microorganisms 2019, 7, 39.
- Ahmad, A.; Husain, A.; Khan, S.; Mujeeb, M.; Bhandari, A. Design, synthesis, molecular properties and antimicrobial activities of some novel 2(3H) pyrrolone derivatives. J. Saudi Chem. Soc. 2015, 19, 340–346.
- McCarty, T.P.; Pappas, P.G. Invasive Candidiasis. Infect. Dis. Clin. N. Am. 2016, 30, 103–124.
- Pfaller, M.A.; Diekema, D. Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 2007, 20, 133–163.
- Galocha, M.; Pais, P.; Cavalheiro, M.; Pereira, D.; Viana, R.; Teixeira, M.C. Divergent approaches to virulence in C. Albicans and C. Glabrata: Two sides of the same coin. Int. J. Mol. Sci. 2019, 20, 2345.
- Pérez-Torrado, R.; Querol, A. Saccharomyces cerevisiae show low levels of traversal across the human blood brain barrier in vitro. F1000Research 2017, 6, 944.
- Zhang, L.; Zhou, S.; Pan, A.; Li, J.; Liu, B. Surveillance of antifungal susceptibilities in clinical isolates of Candida species at 36 hospitals in China from 2009 to 2013. Int. J. Infect. Dis. 2015, 33, 1–4.
- Kaur, R.; Domergue, R.; Zupancic, M.L.; Cormack, B.P. A yeast by any other name: Candida glabrata and its interaction with the host. Curr. Opin. Microbiol. 2005, 8, 378–384.
- Singh, R.; Parija, S. Candida parapsilosis: An emerging fungal pathogen. Indian J. Med. Res. 2012, 136, 671–673.
- Gabaldón, T.; Carreté, L. The birth of a deadly yeast: Tracing the evolutionary emergence of virulence traits in Candida gla-brata. FEMS Yeast Res. 2016, 16, 1–9.
- Moreira, A.; Silva, S.; Botelho, C.; Sampaio, P.; Pais, C.; Henriques, M. Candida bracarensis: Evaluation of Virulence Factors and its Tolerance to Amphotericin B and Fluconazole. Mycopathologia 2015, 180, 305–315.
- Davidson, L.; Netea, M.G.; Kullberg, B.J. Patient Susceptibility to Candidiasis—A Potential for Adjunctive Immunotherapy. J. Fungi 2018, 4, 9.
- Kasper, L.; Seider, K.; Gerwien, F.; Allert, S.; Brunke, S.; Schwarzmüller, T.; Ames, L.; Zubiria-Barrera, C.; Mansour, M.K.; Becken, U.; et al. Identification of Candida glabrata Genes Involved in pH Modulation and Modification of the Phagosomal Environment in Macrophages. PLoS ONE 2014, 9, e96015.
- Schwarzmüller, T.; Ma, B.; Hiller, E.; Istel, F.; Tscherner, M.; Brunke, S.; Ames, L.; Firon, A.; Green, B.; Cabral, V.; et al. Systematic Phenotyping of a Large-Scale Candida glabrata Deletion Collection Reveals Novel Antifungal Tolerance Genes. PLoS Pathog. 2014, 10, e1004211.
- Linde, J.; Duggan, S.; Weber, M.; Horn, F.; Sieber, P.; Hellwig, D.; Riege, K.; Marz, M.; Martin, R.; Guthke, R.; et al. Defining the transcriptomic landscape of Candida glabrata by RNA-Seq. Nucleic Acids Res. 2015, 43, 1392–1406.
- Rapala-Kozik, M.; Bochenska, O.; Zajac, D.; Karkowska-Kuleta, J.; Gogol, M.; Zawrotniak, M.; Kozik, A. Extracellular proteinases of Candida species pathogenic yeasts. Mol. Oral Microbiol. 2018, 33, 113–124.
- Shang-Jie, Y.; Ya-Lin Chang, Y.-L.C. Deletion of ADA2 Increases Antifungal Drug Susceptibility and Virulence in Candida glabrata. Antimicrob. Agents Chemother. 2018, 62, e01924-17.
- Kawai, A.; Yamagishi, Y.; Mikamo, H. In vitro efficacy of liposomal amphotericin B, micafungin and fluconazole against non-albicans Candida species biofilms. J. Infect. Chemother. 2015, 21, 647–653.
- Brunke, S.; Hube, B. Two unlike cousins: Candida albicans and C. glabrata infection strategies. Cell Microbiol. 2013, 15, 701–708.
- Skrzypek, M.S.; Binkley, J.; Binkley, G.; Miyasato, S.R.; Simison, M.; Sherlock, G. The Candida Genome Database (CGD): Incorporation of Assembly 22, systematic identifiers and visualization of high throughput sequencing data. Nucleic Acids Res. 2017, 45, D592–D596.
- Schaller, M.; Borelli, C.; Korting, H.C.; Hube, B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses 2005, 48, 365–377.
- Kumar, V.; Latha, R.; Vedhagiri, K.; Sathiamoorthi, T.; Jayarani, G.; Sasikala, R.; Selvin, J.; Natarajaseenivasan, K. Phospholipase C, proteinase and hemolytic activities of Candida spp. isolated from pulmonary tuberculosis patients. J. Mycol. Med. 2009, 19, 3–10.
- Nahas, J.V.; Iosue, C.L.; Shaik, N.F.; Selhorst, K.; He, B.Z.; Wykoff, D.D. Dynamic changes in yeast phosphatase families allow for specialization in phosphate and thiamine starvation. G3 Genes Genomes Genet. 2018, 8, 2333–2343.
- Figueiredo-Carvalho, M.H.; Ramos, L.; Barbedo, L.; Chaves, A.L.D.S.; Muramoto, I.A.; Dos Santos, A.L.S.; Almeida-Paes, R.; Zancopé-Oliveira, R.M. First description of Candida nivariensis in Brazil: Antifungal susceptibility profile and potential virulence attributes. Mem. Inst. Oswaldo Cruz 2016, 111, 51–58.
- Kühbacher, A.; Burger-Kentischer, A.; Rupp, S. Interaction of Candida Species with the Skin. Microorganisms 2017, 5, 32.
- Hoyer, L.L.; Green, C.B.; Oh, S.; Zhao, X. Discovering the secrets of the Candida albicans agglutinin-like sequence (ALS) gene family a sticky pursuit. Med. Mycol. 2008, 46, 1–15.
- De Groot, P.W.J.; Kraneveld, E.A.; Qing, Y.Y.; Dekker, H.L.; Groß, U.; Crielaard, W.; de Koster, C.G.; Bader, O.; Klis, F.M.; Weig, M. The cell wall of the human pathogen Candida glabrata: Differential incorporation of novel adhesin-like wall proteins. Eukaryot. Cell 2008, 7, 1951–1964.
- Kohlenberg, A.; Struelens, M.J.; Monnet, D.L.; Plachouras, D.; The Candida Auris Survey Collaborative Group. Candida auris: Epidemiological situation, laboratory capacity and preparedness in European Union and European economic area countries, 2013 to 2017. Eurosurveillance 2018, 23, 13.
- Tumbarello, M.; Posteraro, B.; Trecarichi, E.M.; Fiori, B.; Rossi, M.; Porta, R.; de Gaetano Donati, K.; La Sorda, M.; Spanu, T.; Fadda, G.; et al. Biofilm production by Candida species and in-adequate antifungal therapy as predictors of mortality for patients with candidemia. J. Clin. Microbiol. 2007, 45, 1843–1850.
- Nett, J.E.; Andes, D.R. Contributions of the Biofilm Matrix to Candida Pathogenesis. J. Fungi 2020, 6, 21.
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500.
- Santos, P.S.; Lana, D.F.D.; Mezzari, A. Candida auris: Emergence and Epidemiology of a Highly Pathogenic Yeast. Clin. Biomed. Res. 2017, 37, 247–254.
- Charlet, R.; Pruvost, Y.; Tumba, G.; Istel, F.; Poulain, D.; Kuchler, K.; Sendid, B.; Jawhara, S. Remodeling of the Candida glabrata cell wall in the gastrointestinal tract affects the gut microbiota and the immune response. Sci. Rep. 2018, 8, 1–12.
- Netea, M.G.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Kullberg, B.-J.; Van De Veerdonk, F.L. Immune defence against Candida fungal infections. Nat. Rev. Immunol. 2015, 15, 630–642.
- Molina, M.; Gil, C.; Pla, J.; Arroyo, J.; Nombela, C. Protein Localisation Approaches for Understanding Yeast Cell Wall Biogenesis. Histol. Stud. Yeast 2000, 612, 601–612.
- Srivastava, V.K.; Suneetha, K.J.; Kaur, R. A systematic analysis reveals an essential role for high-affinity iron uptake system, haemolysin and CFEM domain-containing protein in iron homoeostasis and virulence in Candida glabrata. Biochem. J. 2014, 463, 103–114.
- Gerwien, F.; Safyan, A.; Wisgott, S.; Hille, F.; Kaemmer, P.; Linde, J.; Brunke, S.; Kasper, L.; Hube, B. A Novel Hybrid Iron Regulation Network Combines Features from Pathogenic and Nonpathogenic Yeasts. mBio 2016, 7, e01782-16.
- De Wever, V.; Reiter, W.; Ballarini, A.; Ammerer, G.; Brocard, C. A dual role for PP1 in shaping the Msn2-dependent transcrip-tional response to glucose starvation. EMBO J. 2005, 24, 4115–4123.
- Roetzer, A.; Gregori, C.; Jennings, A.M.; Quintin, J.; Ferrandon, D.; Butler, G.; Kuchler, K.; Ammerer, G.; Schüller, C. Candida glabrata environmental stress response involves Saccharomyces cerevisiae Msn2/4 orthologous transcription factors. Mol. Microbiol. 2008, 69, 603–620.
- Wu, J.; Chen, X.; Cai, L.; Tang, L.; Liu, L. Transcription factors Asg1p and Hal9p regulate pH homeostasis in Candida glabrata. Front. Microbiol. 2015, 6, 843.
- Yih, C.S.; Than, L.; Lung, T. Candida glabrata: Niche adaptation and mechanisms of survival. In Updates in Medical Microbiology; Universiti Putra Malaysia Press: Selangor, Malaysia, 2012.
- Roetzer, A.; Gratz, N.; Kovarik, P.; Schüller, C. Autophagy supports Candida glabrata survival during phagocytosis. Cell. Microbiol. 2010, 12, 199–216.
- Del Poeta, M. Role of phagocytosis in the virulence of Cryptococcus neoformans. Eukaryot. Cell. 2004, 3, 1067–1075.
- Chew, S.Y.; Ho, K.L.; Cheah, Y.K.; Ng, T.S.; Sandai, D.; Brown, A.J.P.; Than, L.T.L. Glyoxylate cycle gene ICL1 is essential for the metabolic flexibility and virulence of Candida glabrata. Sci. Rep. 2019, 9, 1–11.
- Mota, S.; Alves, R.; Carneiro, C.; Silva, S.; Brown, A.J.; Istel, F.; Kuchler, K.; Sampaio, P.; Casal, M.; Henriques, M.; et al. Candida glabrata susceptibility to antifungals and phagocytosis is modulated by acetate. Front. Microbiol. 2015, 6, 919.
- Jayampath Seneviratne, C.; Wang, Y.; Jin, L.; Abiko, Y.; Samaranayake, L.P. Proteomics of drug resistance in Candida glabrata biofilms. Proteomics 2010, 10, 1444–1454.
- Gonçalves Dos Santos, M.T.P.; Benito, M.J.; Córdoba, M.D.G.; Alvarenga, N.; de Herrera, S.R.-M.S. Yeast community in traditional Portuguese Serpa cheese by culture-dependent and -independent DNA approaches. Int. J. Food Microbiol. 2017, 262, 63–70.
- Bouklas, T.; Alonso-Crisóstomo, L.; Székely, T.; Navarro, E.D.; Orner, E.P.; Smith, K.; Munshi, M.A.; Del Poeta, M.; Balazsi, G.; Fries, B.C. Generational distribution of a Candida glabrata population: Resilient old cells prevail, while younger cells dominate in the vulnerable host. PLoS Pathog. 2017, 13, e1006355.
- Bhattacharya, S.; Bouklas, T.; Fries, B.C. Replicative Aging in Pathogenic Fungi. J. Fungi 2020, 7, 6.
- Bhattacharya, S.A.; Fries, B.C. Enhanced Efflux Pump Activity in Old Candida glabrata Cells. Antimicrob. Agents Chemother. 2018, 62, e02227-17.
- Alastruey-Izquierdo, A.; Melhem, M.S.C.; Bonfietti, L.X.; Rodriguez-Tudela, J.L. Susceptibility Test for Fungi: Clinical and Laboratorial Correlations in Medical Mycology. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 57–64.
- Dadar, M.; Tiwari, R.; Karthik, K.; Chakraborty, S.; Shahali, Y.; Dhama, K. Candida albicans—Biology, molecular characterization, pathogenicity, and advances in diagnosis and control—An update. Microb. Pathog. 2018, 117, 128–138.
- Costa, C.; Ribeiro, J.; Miranda, I.M.; Silva-Dias, A.; Cavalheiro, M.; Costa-de-Oliveira, S.; Rodrigues, A.G.; Teixeira, M.C. Clotrimazole drug resistance in Candida glabrata clinical isolates correlates with increased expression of the drug: H+ antiporters CgAqr1, CgTpo1_1, CgTpo3, and CgQdr2. Front. Microbiol. 2016, 7, 1–11.
- Irinyi, L.; Lackner, M.; de Hoog, G.S.; Meyer, W. DNA barcoding of fungi causing infections in humans and animals. Fungal Biol. 2016, 120, 125–136.
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species. Front. Microbiol. 2017, 7, 2173.
- Yoo, J.I.; Choi, C.W.; Lee, K.M.; Lee, Y.S. Gene Expression and Identification Related to Fluconazole Resistance of Candida glabrata Strains. Osong Public Health Res. Perspect. 2010, 1, 36–41.
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Giannini, M.J.M. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24.
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216, S445–S451.
- Jensen, R.H.; Johansen, H.K.; Søes, L.M.; Lemming, L.E.; Rosenvinge, F.S.; Nielsen, L.; Olesen, B.; Kristensen, L.; Dzajic, E.; Astvad, K.; et al. Posttreatment Antifungal Resistance among Colonizing Candida Isolates in Candidemia Patients: Results from a Systematic Multicenter Study. Antimicrob. Agents Chemother. 2016, 60, 1500–1508.
- Biswas, C.; Chen, S.-A.; Halliday, C.; Kennedy, K.; Playford, E.; Marriott, D.; Slavin, M.; Sorrell, T.; Sintchenko, V. Identification of genetic markers of resistance to echinocandins, azoles and 5-fluorocytosine in Candida glabrata by next-generation sequencing: A feasibility study. Clin. Microbiol. Infect. 2017, 23, 676.e7–676.e10.
- Basas, J.; Palau, M.; Gomis, X.; Almirante, B.; Gavaldà, J. Efficacy of liposomal amphotericin B and anidulafungin using an an-tifungal lock technique (ALT) for catheter-related Candida albicans and Candida glabrata infections in an experimental model. PLoS ONE 2019, 14, e0212426.
More
Information
Subjects:
Microbiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
30 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




