
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Regina Kratzer | + 2191 word(s) | 2191 | 2021-07-24 10:05:40 | | | |
| 2 | Lindsay Dong | -2 word(s) | 2189 | 2021-08-26 09:34:57 | | |
Video Upload Options
Food Ingredients and Nutraceuticals from Microalgae: Main Product Classes and Biotechnological Production Microalgal products are an emerging class of food, feed, and nutraceuticals. They include dewatered or dried biomass, isolated pigments, and extracted fat. The oil, protein, and antioxidant-rich microalgal biomass is used as a feed and food supplement formulated as pastes, powders, tablets, capsules, or flakes designed for daily use. Pigments such as astaxanthin (red), lutein (yellow), chlorophyll (green), or phycocyanin (bright blue) are natural food dyes used as isolated pigments or pigment-rich biomass. Algal fat extracted from certain marine microalgae represents a vegetarian source of n-3-fatty acids (eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), γ-linolenic acid (GLA)). Gaining an overview of the production of microalgal products is a time-consuming task.
1. Introduction
Microalgae are microscopic photosynthetic organisms that are found in marine, as well as in freshwater, environments. These unicellular organisms have a size ranging from a few to several hundred micrometers, depending on class and species. Most microalgae have photosynthetic mechanisms similar to land-based plants but generally more efficient in the photoautotrophic conversion of solar energy into biomass. This is since the cellular structure of microalgae is less sophisticated, and the normal environment is aqueous with easy access to CO2 and further nutrients [1].
Microalgae are a resource for food and feed with high potential. Currently, the most cultured species are Arthrospira platensis and Arthrospira maxima, Chlorella vulgaris, Dunaliella salina, and Haematococcus pluvialis, which can be grown photoautotrophically with sunlight as an energy source. The carbon source is CO2, which can be supplied by gassing or supplementing the water with soluble carbonates. Heterotrophic cultivation on organic carbon sources such as sugars is used for the production of n-3 fatty acids with marine organisms (Crypthecodinium cohnii, Schizochytrium sp. TC 002, and Ulkenia sp. strain TC 010) in fermenters excluding sunlight [2]. For feed for fish aquaculture, the main cultivated species are: Chlorella vulgaris, Isochrysis galbana, Pavlova sp., Phaeodactylum tricornutum, Chaetoceros sp., Nannochloropsis oceanica, Skeletonema sp., Thalassiosira sp., Haematococcus pluvialis, and Tetraselmis suecica [3][4][5]. However, the technology to produce microalgae is still immature. Research and development have been done in recent years and continue on cultivation systems. A leap in the development of microalgae technology is required; on a practical level, the scale of production needs to increase with a concomitant decrease in the cost of production [1]. Microalgae are cultivated in a wide range of different cultivation systems that can be placed outdoors or indoors. Cultivation systems range from open shallow raceway ponds to closed photobioreactors. The systems mostly used on a large scale and on a commercial basis are open systems [6].
2. Microalgae Production
2.1. Microalgae Cultivation
2.1.1. Illumination
Low cell densities and moderate growth rates are the major obstacles towards a broad commercial use of microalgal products. The most important cultivation parameters are light and nutrient supply. Decisive factors of light supply are light intensity, spectral range, and photoperiod.
2.1.2. Diverse Growth Conditions
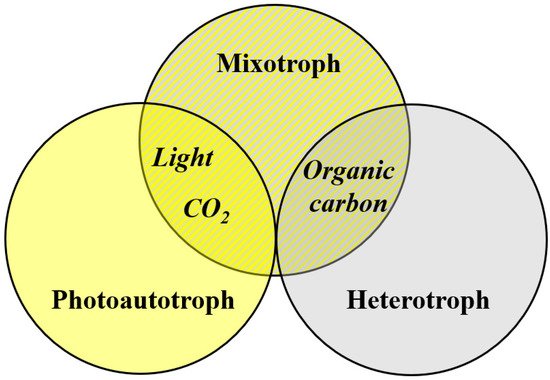
2.2. Cultivation Systems
2.2.1. Open Ponds
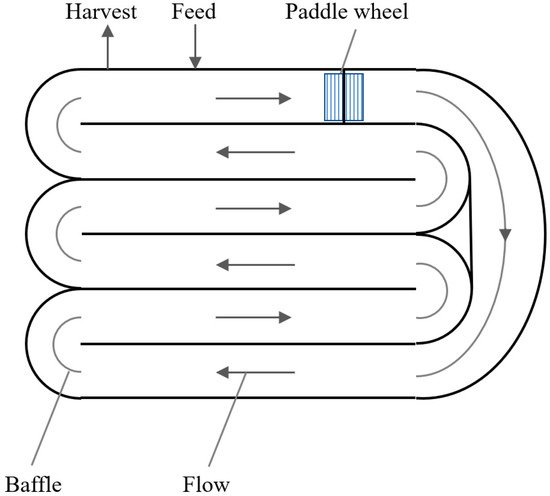
Classical algae cultivation uses open ponds in natural habitats or shallow artificial ponds often mixed by a rotating arm or by paddle wheels (Figure 2).
2.2.2. Closed Photobioreactors
PBRs facilitate increased biomass and product concentrations. PBRs protect the algal culture against (microbial) contaminations, provide nutrients, illumination, pH and temperature control, and outgassing of the produced oxygen. Mixing and illumination are again the most important parameters. Optimal cultivation conditions provide gentle mixing so that cells, carbon dioxide, and other essential nutrients are evenly circulated. Cell movement facilitates constant illumination and prevents cell growth on vessel walls (see also Figure 3). The simplest PBRs consist of transparent plastic bags or plastic columns operated as bubble columns and airlift reactors (also referred to as vertical tubular reactors) (Figure 3).

2.3. Contaminations
A major bottleneck in the industrial production of products from algae is that algae cultures are prone to contaminations. To avoid contamination, closed systems can be used; however, in open systems (large scale), contamination is unavoidable. These are either organisms that graze upon and feed on the algae or undesired algae strains that take over the cultivation media. Contaminations can lead to culture crashes and product loss, and also the formation of toxins [12][13]. Therefore, effective strategies to avoid, repress, monitor, and control contaminants without interfering with the growth of the target microalgae are required. Grazers and contaminants experienced (especially in open-pond cultivations) are mainly insect larvae, protozoans (ciliates, amoeba, dinoflagellates), viruses, fungi, bacteria, and other algae strains [14].
2.4. Downstream Processing
Similar to the majority of bioprocesses, downstream processing constitutes a considerable cost factor. Specifically, the cost for the harvest of microalgae was calculated as 20–30% of the total production cost of cheap bulk products such as biodiesel [15].
3. Rentability of Microalgae Production
One of the major concerns relates to the rentability of microalgal products. Key challenges are the high production costs of products made up of commercial-scale cultivation, harvesting (dewatering), and finally, product quality assurance. The value of microalgal products spans from exceptionally high market values (e.g., EPA 4600 USD/kg) to products in the middle price segment (e.g., biomass for food and feed 10 to 50 USD/kg) down to fishmeal replacement (fishmeal price is approximately 2 USD/kg) [18]. Several algal-based foods have been launched in the last years: Microalgae biomass is used in the form of powders, tablets, and capsules. Especially in Western countries, the number of snacks and drinks containing microalgae doubled in the past few years. Most new products launched were bakery, meals, and chocolate confectionery [19]. Improvements in capital and operational costs shall enable economic production of low-value food and feed products going down to fishmeal replacement in the future economy [18][20].
4. Nutritional Composition of Microalgae
The composition of the microalgae is similar to other bacteria. Usually, they contain high amounts of protein, with all essential amino acids present. Especially the microalgal species Arthrospira platensis, Chlorella vulgaris, Dunaliella salina, Haematococcus pluvialis, and Scenedesmus obliquus show a very high content of protein with values higher than soybean, corn, and wheat (Table 2). Polyunsaturated fatty acids are occurring in significant amounts, including linoleic acid, γ-linolenic acid (GLA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and arachidonic acid (Table 3).
| Species | Protein (wt%) |
Carbohydrate (wt%) |
Lipid (wt%) |
|---|---|---|---|
| Nannochloropsis sp. | 29–32 | 9–36 | 15–18 |
| Nannochloropsis oceanica | 29 | 32–39 | 19–24 |
| Botryococcus braunii | 70 | – | – |
| Arthrospira platensis | 53–70 | 12–24 | 6–20 |
| Chlorella vulgaris | 49–55 | 7–42 | 3–36 |
| Haematococcus pluvialis | 48 | 27 | 15 |
| Isochrysis galbana | 27 | 17 | 17 |
| Dunaliella salina | 57 | 32 | 6 |
| Scenedesmus obliquus | 50–56 | 10–17 | 12–14 |
| Porphyridium cruentum | 28–39 | 40–57 | 9–14 |
| Species | LA | ALA | GLA | STA | AA | EPA | DPA | DHA |
|---|---|---|---|---|---|---|---|---|
| Chlorella vulgaris | 3–10 | 2–21 | 15–24 | Traces | Traces | 3 | 3 | 21 |
| Arthrospira platensis | 10–21 | 1 | 1–25 | 1 | 0.3–0.4 | 2–3 | - | 3 |
| Isochrysis galbana | 1 | 0.5 | 0.5 | 1 | 1 | 2 | Traces | 19 |
| Scenedesmus obliquus | 1–2 | 4–22 | 0.1–4 | 1–3 | 0.1–0.2 | - | - | - |
| Haematococcus pluvialis | 4 | 15 | 0.1 | - | - | - | - | - |
| Porphyridium cruentum | 0.4–25 | - | - | - | 1–35 | 1–27 | - | 6.1 |
| Nannochloropsis oceanica | 0.6–0.8 | - | - | - | 1.4–1.9 | 8.4–11 | - | - |
5. Food Ingredients and Nutraceuticals from Microalgae
5.1. Microalgae Used as a Food Ingredient
When using microalgae as a food ingredient, some limitations are obvious. This is primarily because of the intensive green color that is added to the recipes and a fishy aroma [24]. As the flavor is one of the biggest disadvantages of algae-containing food, a lot of work was invested in masking this flavor by using intensive spices [25] or by using the aroma of the microalgae to bring the typical aroma of seafood out. A commercial product [26][27] was developed that shows microalgae can be used as an ingredient in foods with a typical seafood aroma. Many products from algae are commercialized directly as biomass. These are sold as nutritional supplements since the microalgae, mostly Arthrospira platensis, contain high amounts of protein and n-3 fatty acids. Other products derived from Haematococcus pluvialis are used for supplementing the carotenoids astaxanthin and lutein. Since microalgae are also known for having high concentrations of n-3 fatty acids (mainly Chlorella vulgaris and Arthrospira platensis containing γ-linolenic acid, eicosapentaenoic acid, docosapentaenoic acid, docosahexaenoic acid, Table 2), extracts are developed that might be used to upgrade foods with these fatty acids.
5.2. Isolated Products from Microalgae for Enrichment of Foods
5.2.1. n-3 Fatty Acids
Some of the cyanobacteria are known for their high content of n-3 fatty acids (Table 3). Since the alimentary supply of n-3 fatty acids is generally limited due to limited availability of fish oils, a series of efforts are made to increase the content of these fatty acids in foods. From a nutritional point of view, the uptake of n-3 fatty acids from microalgal oil is comparable to fish oil with the possible advantage of a better supply with carotenoids [28]. In addition to adding the n-3 fatty acids directly to the food, it is possible to enrich animal products by supplying the unsaturated fatty acids with the feed. This was shown, for example, by Lemahieu and a co-worker [29], who could increase the DHA content of eggs when feeding Isochrysis galbana to the chicken. Polyunsaturated fatty acids (PUFAs), particularly the n-3 fatty acids having five (EPA) or six (DHA double bonds, are prone to oxidation due to the lability of the hydrogens linked to the methylene groups between the double bonds. These PUFAs require stabilization against oxidation. Shen and co-workers could show that a combination of octyl gallate with tea polyphenol palmitate showed the most effective protection [30].
5.2.2. Carotene and Other Carotenoids
5.3. Microalgae as Food Supplements
5.4. Toxicological Relevance of Microalgal Products
References
- Habib, M.A.B.; Parvin, M.; Huntington, T.C.; Hasan, M.R. A Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish; FAO Food and Agriculture Organization of the United Nations: Rome, Italy, 2008.
- Karnaouri, A.; Chalima, A.; Kalogiannis, K.G.; Varamogianni-Mamatsi, D.; Lappas, A.; Topakas, E. Utilization of lignocellulosic biomass towards the production of omega-3 fatty acids by the heterotrophic marine microalga Crypthecodinium cohnii. Biores. Technol. 2020, 303, 122899.
- Dolganyuk, V.; Andreeva, A.; Budenkova, E.; Sukhikh, S.; Babich, O.; Ivanova, S.; Prosekov, A.; Ulrikh, E. Study of morphological features and determination of the fatty acid composition of the microalgae lipid complex. Biomolecules 2020, 10, 1571.
- Patil, V.; Källqvist, T.; Olsen, E.; Vogt, G.; Gislerød, H.R. Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquacult. Int. 2007, 15, 1–9.
- Bhattacharjya, R.; Marella, T.K.; Tiwari, A.; Saxena, A.; Singh, P.K.; Mishra, B. Bioprospecting of marine diatoms Thalassiosira, Skeletonema and Chaetoceros for lipids and other value-added products. Biores. Technol. 2020, 318, 24073.
- Koller, M. Design of closed photobioreactors for algal cultivation. In Algal Biorefineries; Prokop, A., Bajpai, R.K., Zappi, M.E., Eds.; Springer: Heidelberg, Germany, 2015; pp. 133–186.
- Morales-Sánchez, D.; Martinez-Rodriguez, O.A.; Martinez, A. Heterotrophic cultivation of microalgae: Production of metabolites of commercial interest. J. Chem. Technol. Biotechnol. 2017, 92, 925–936.
- Hu, J.; Nagarajan, D.; Zhang, Q.; Chang, J.-S.; Lee, D.J. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol. Adv. 2018, 36, 54–67.
- Daneshvar, E.; Ok, Y.S.; Tavakoli, S.; Sarkar, B.; Shaheen, S.M.; Hong, H.; Luo, Y.; Rinklebe, J.; Song, H.; Bhatnagar, A. Insight into upstream processing of microalgae: A review. Biores. Technol. 2021, 329, 124870.
- Li, T.; Zheng, Y.; Yu, L.; Chen, S. Mixotrophic cultivation of a Chlorella sorokiniana strain for enhanced biomass and lipid production. Biomass Bioenergy 2014, 66, 204–213.
- Wen, X.; Wang, Z.; Ding, Y.; Geng, Y.; Li, Y. Enhancing the production of astaxanthin by mixotrophic cultivation of Haematococcus pluvialis in open raceway ponds. Aquac. Int. 2020, 28, 625–638.
- Ger, K.A.; Urrutia-Corderob, P.; Frost, P.C.; Hansson, L.-A.; Sarnelle, O.; Wilson, A.E.; Lürling, M. The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 2016, 54, 128–144.
- Jang, M.-H.; Ha, K.; Joo, G.-J.; Takamura, N. Toxin production of cyanobacteria is increased by exposure to zooplankton. Freshw. Biol. 2003, 48, 1540–1550.
- Karuppasamy, S.; Musale, A.S.; Soni, S.; Bhadra, B.; Gujarathi, N.; Sundaram, M.; Sapre, A.; Dasgupta, S.; Kumar, C. Integrated grazer management mediated by chemicals for sustainable cultivation of algae in open ponds. Algal Res. 2018, 35, 39–448.
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C.M. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500.
- Monte, J.; Sá, M.; Galinha, C.F.; Costa, L.; Hoekstrad, H.; Brazinha, C.; Crespo, J.G. Harvesting of Dunaliella salina by membrane filtration at pilot scale. Sep. Purif. Technol. 2018, 190, 252–260.
- Desmorieux, H.; Hernandez, F. Biochemical and physical criteria of Spirulina after different drying processes. In Proceedings of the 14th International Drying Symposium (IDS), São Paulo City, Brazil, 22–25 August 2004; pp. 900–907.
- Walsh, M.J.; Van Doren, L.G.; Shete, N.; Prakash, A.; Salim, U. Financial tradeoffs of energy and food uses of algal biomass under stochastic conditions. Appl. Energy 2018, 210, 591–603.
- Nova, P.; Martins, A.P.; Teixeira, C.; Abreu, H.; Silva, J.G.; Machado Silva, A.; Freitas, A.C.; Gomes, A.M. Foods with microalgae and seaweeds fostering consumers health: A review on scientific and market innovations. J. Appl. Phycol. 2020, 32, 1789–1802.
- Enzing, C.; Ploeg, M.; Barbosa, M.; Sijtsma, L. Microalgae-based products for the food and feed sector: An outlook for Europe. In EUR—Scientific and Technical Research Series; Vigani, M., Parisi, C., Cerezo, E.R., Eds.; European Commission, EUR 26255—Joint Research Centre—Institute for Prospective Technological Studies, Publications Office: Luxembourg, 2014.
- Guerra, I.; Pereira, H.; Costa, M.; Silva, J.T.; Santos, T.; Varela, J.; Mateus, M.; Silva, J. Operation regimes: A comparison based on Nannochloropsis oceanica biomass and lipid productivity. Energies 2021, 14, 1542.
- Kartik, A.; Akhil, D.; Lakshmi, D.; Gopinath, K.P.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. A critical review on production of biopolymers from algae biomass and their applications. Biores. Technol. 2021, 329, 124868.
- Chacon-Lee, T.L.; Gonzalez-Marino, G.E. Microalgae for “healthy” foods—Possibilities and challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675.
- Lafarga, T. Effect of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019, 41, 101566.
- da Silva, S.P.; Ferreira do Valle, A.; Perrone, D. Microencapsulated Spirulina maxima biomass as an ingredient for the production of nutritionally enriched and sensorially well-accepted vegan biscuits. LWT Food Sci. Technol. 2021, 142, 110997.
- Mantecón, L.; Moyano, R.; Cameánc, A.M.; Jos, A. Safety assessment of a lyophilized biomass of Tetraselmis chuii (TetraSOD®) in a 90 day feeding study. Food Chem. Toxicol. 2019, 133, 110810.
- Plankton Marino. Available online: https://www.planctonmarino.com (accessed on 28 May 2021).
- Ryckebosch, E.; Bruneel, C.; Termote-Verhalle, R.; Goiris, K.; Muylaert, K.; Foubert, I. Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fatty acids as an alternative for fish oil. Food Chem. 2014, 160, 393–400.
- Lemahieu, C.; Bruneel, C.; Ryckebosch, E.; Muylaert, K.; Buyse, J.; Foubert, I. Impact of different omega-3 polyunsaturated fatty acid (n-3 PUFA) sources (flaxseed, Isochrysis galbana, fish oil and DHA Gold) on n-3 LC-PUFA enrichment (efficiency) in the egg yolk. J. Funct. Foods 2015, 19, 821–827.
- Shen, Y.; Guo, C.; Lu, T.; Ding, X.-Y.; Zhao, M.-T.; Zhang, M.; Liu, H.-L.; Song, L.; Zhou, D.-Y. Effects of gallic acid alkyl esters and their combinations with other antioxidants on oxidative stability of DHA algae oil. Food Res. Int. 2021, 143, 110280.
- de Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Carotenoids from marine microalgae: A valuable natural source for the prevention of chronic diseases. Mar. Drugs 2015, 13, 5128–5155.
- Plaza, M.; Cifuentes, A.; Ibanez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39.
- Katircioglu, H.; Akin, B.S.; Atici, T. Microalgal toxin(s): Characteristics and importance. Afr. J. Biotechnol. 2004, 3, 667–674.
- Heussner, A.H.; Mazija, L.; Fastner, J.; Dietrich, D.R. Toxin content and cytotoxicity of algal dietary supplements. Toxicol. Appl. Pharmacol. 2012, 265, 263–271.




