You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Valentina Cauda | + 4610 word(s) | 4610 | 2021-08-10 04:20:55 | | | |
| 2 | Vivi Li | Meta information modification | 4610 | 2021-08-24 04:55:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cauda, V. Extracellular Vesicles in Cancer Nanomedicine. Encyclopedia. Available online: https://encyclopedia.pub/entry/13476 (accessed on 23 December 2025).
Cauda V. Extracellular Vesicles in Cancer Nanomedicine. Encyclopedia. Available at: https://encyclopedia.pub/entry/13476. Accessed December 23, 2025.
Cauda, Valentina. "Extracellular Vesicles in Cancer Nanomedicine" Encyclopedia, https://encyclopedia.pub/entry/13476 (accessed December 23, 2025).
Cauda, V. (2021, August 23). Extracellular Vesicles in Cancer Nanomedicine. In Encyclopedia. https://encyclopedia.pub/entry/13476
Cauda, Valentina. "Extracellular Vesicles in Cancer Nanomedicine." Encyclopedia. Web. 23 August, 2021.
Copy Citation
Fast diagnosis and more efficient therapies for cancer surely represent one of the huge tasks for the worldwide researchers’ and clinicians’ community. In the last two decades, our understanding of the biology and molecular pathology of cancer mechanisms, coupled with the continuous development of the material science and technological compounds, have successfully improved nanomedicine applications in oncology.
extracellular vesicles
exosomes
chemico-physical functionalization
loading
cancer
nanomedicine
translational medicine
nanotechnology: bioengineering
1. Introduction
The latest literature reports underline that extracellular vesicles (EVs), released by prokaryotic and eukaryotes cells into the extracellular surroundings, are the main drivers of the intracellular communication, not only in physiological but also under pathological conditions [1][2][3][4][5][6][7][8][9].
The International Society for Extracellular Vesicles (ISEV) defines EVs generally as lipid bilayer-delimited particles released from cells and unable to replicate [10]. Agreement has not yet been reached on the specific markers for defining EVs subtypes, such as exosomes and ectosomes, originated from the endosome and the plasma membrane, respectively. Researchers are advised to contemplate the use of operational terms for EV subtype definition, referring to EVs’ physical characteristics such as size (<100 nm for “small EVs”, and > 200 nm for “medium/large EVs”), density, biochemical composition (tetraspanin/Annexin presence, etc.) and reference to condition or tissue/cell biogenesis (podocyte EVs, cardiosomes and prosatosomes, large oncosomes, apoptotic bodies) [10][11][12]. More in general, referring to their dimension and biogenesis’ mechanisms, EVs can be grouped into three broad categories: apoptotic bodies, ectosomes and exosomes [13][14].
Apoptotic bodies (ApoBDs) are typically 1–5 μm EVs released as cells’ blebs during the apoptotic process. They contain cytoplasm, organelles and often also nuclear fragment, lipids, proteins [15] and a high amounts of phosphatidylserine [16].
Ectosomes and exosomes formation rests on confined microdomains assembled in the plasma membrane for ectosomes and in the endocytic membrane system for exosomes [17]. Ectosomes (100–500 nm diameter) are larger than exosomes (30–150 nm diameter) and both their cargoes and membranes composition partially differ from each other. Exosomes originate from the endosomal compartment inside multivesicular bodies and they are released by the fusion with the plasma membrane. Exosomes’ membranes are rich in tetraspanins (CD9, CD63, CD81, CD82 and CD151) [18], sphingomyelin, cholesterol [19] and adhesion molecule (ICAM-1), while the ectosomes’ ones are characterized by plentiful of glycoproteins, receptors and metallo proteinases [17][20].
Oncosomes are exceptionally large ectosomes, typical of advanced cancers containing active molecules involved in the metabolic pathways promoting tumoral cell survival and growth [21].
Starting from the key role that the tumor microenvironment plays in cancer establishment and progression, it is easy to understand how the EVs have an active part in influencing processes as pre-metastatic niche development, oncogenic transfer, and immune modulation [22][23].
Tumor-derived EVs, by carrying chemokines, are able to induce white blood cells’ chemotactic response [24]. Tumor-derived exosomes promote inflammation compromising natural immunity and reprogramming T cells [25], while ApoBDs join in the horizontal oncogenes transfer thanks to the nuclear material that comes out from the dying cells by which they were produced [26].
Since EVs have an active role in the tumoral intercellular communication and signal transduction systems, it spontaneously comes out to consider their applications as biomarkers and therapeutic agents in oncology.
It actually results very interesting to observe how an advanced Web of Science search (carried out on the 26th September 2019 at the all databases level) for the terms ‘extracellular vesicles cancer’ and ‘extracellular vesicles cancer nanomedicine’ has clearly shown an incredible increase in the number of publications in the last five years (Figure 1). A further more detailed analysis was carried out on these results and considered the percentages of the papers’ distribution in the various research areas. It revealed that, by adding the term ‘nanomedicine’ to the query, the percentage of papers in the section ‘Science technology other topics’ increases from the 25% to the 85%, thus demonstrating the current interdisciplinary research trend of this topic.
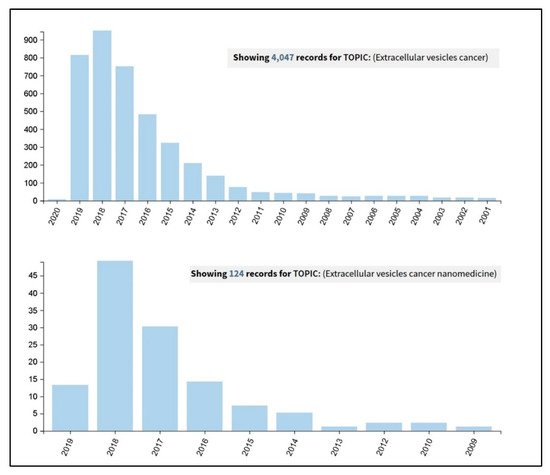
Figure 1. Results coming out from a Web of Science search carried out on the 26th September 2019, at the all databases level, for the terms ‘extracellular vesicles cancer’ (upper panel) and ‘extracellular vesicles cancer nanomedicine’ (lower panel).
Current trends refer to EVs as successfully non-invasive diagnostic and prognostic biomarkers: actually their membrane proteins, their lipid fingerprint (reflecting the protein and lipidic content of the parent cells at the moment of their formation) and micro RNA load can be easily screened in blood, urine and in other biological fluids [20][26][27].
Regarding EVs’ application as cancer therapeutics, it basically differs from conventional approaches, i.e., molecular targeting drugs and chemotherapy. Referring to native EVs, a huge number of in vivo and in vitro studies have been reported [28][29][30][31][32][33][34]. In details, three main approaches in cancer treatment through native EVs can be identified: the inhibition of EVs production [35][36][37], the eradication of circulating EVs, and finally the reduction of EV uptake [38][39].
EVs are usually biocompatible, low immunogenic and non-cytotoxic, with a high loading ability, long life span in circulation and the capacity to cross barriers, i.e., the cytoplasmic and the blood brain barriers, making them suitable for drug delivery applications [40][41]. Furthermore, EVs are internalized 10 times more than liposomes of similar size in cancer cells, showing a higher specificity towards tumoral cells [42] and, thanks to their dimensions, they can also exploit the enhanced retention and permeability effect to accumulate in the cancerous tissues and reach easily the bulk of a solid tumor [43]. The research on EVs is making great strides in cancer medicine and there are already 136 clinical trials on exosomes and 36 on EVs listed on “www.clinicaltrials.gov” both for therapy and diagnosis. Given these premises, EVs can be considered promising tools for the development of new engineered devices for therapeutic and diagnostic applications. Starting from scalable, reproducible and well standardized EVs isolation procedures, it is possible to obtain highly purified products ready for further microscopic, immunological characterizations or for cryopreservation treatments able to guarantee the stability and integrity necessary for long-term storage or subsequent modifications. Otherwise, these modifications can be carried out directly by engineering the parent cells before the isolation, to obtain already loaded or functionalized EVs (Figure 2).
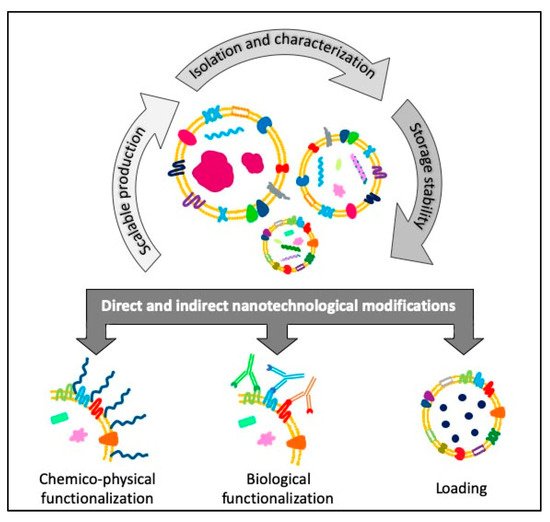
Figure 2. Schematic view of the flow of the different steps concerning the direct and indirect engineering of extracellular vesicles (EVs) for cancer diagnosis and therapy applications.
2. EVs’ Indirect Nanotechnological Modification through Parent Cells’ Engineering
A frequently applied method to modify EVs in vitro, i.e., loading cargo molecules or accomplishing membrane functionalization, is through the engineering of parent cells. Cell engineering methods, such as genetic and metabolic modification and exogenous delivery, can alter the surface expression and cargo content of newly-produced EVs and thus enhance their biocompatibility, targeting and therapeutic abilities [44].
2.1. Indirect Surface Functionalization
EVs’ membrane is a complex structure constituted by phospholipids and membrane proteins. Since the membrane is the first point of contact with the cell, tuning its composition strongly improves the targeting ability and enhances the therapeutic ability of EVs [45].
This approach can be employed for the non-invasive monitoring of EVs in vivo exploiting the fluorescence of some binding molecules. Molecular imaging allows a quantification of the EVs biodistribution and, eventually, a therapeutic effect over the time. For instance, pancreatic cell lines that stably expresses the green fluorescent protein (GFP) linked to CD63 can produce exosomes consistently positive to GFP [46][47][48]. Another effective labelling strategy for EVs is the incorporation of an azido-sugar in the glycans through a combined metabolic glycan labelling click chemistry reaction. Tetraacetylated N-azidoacetyl-D-mannosamine (Ac4ManNAz) is placed in culture with the parent cells, spontaneously incorporated into glycans and uniformly redistributed on their EVs. The azido-EVs are then labeled with azadibenzylcyclooctyne (ADIBO)-fluorescent dyes by a bioorthogonal click reaction [49]. Exploiting the principle of bioluminescence for tracking EVs, in vivo Gaussian Luciferase (Gluc) is linked to a transmembrane domain of a platelet-derived growth factor receptor [50][51], or a lactadherin [52][53]. Gluc is the only naturally-produced luciferase that can emit flash of bioluminescence in the presence of oxygen as cofactor for the reaction. After the engineering of parent cells with Gluc, the produced EVs are extracted and, when administered systemically, they can be tracked in vivo thanks to their bioluminescence [50][51]. The cellular transgene expression into the parent cell allows the expression of the candidate protein or peptide in the released EVs. The coding sequence of the desired ligand is inserted by a gene transfer vector (i.e., lentivirus) between the signal peptide and the N-terminus of the mature peptide of a transmembrane protein. In this way, the parent cells can generate EVs with the peptide of interest on their surface [44]. The candidate protein or peptide, after the transfection in the parent cells, fuses with EVs membrane proteins such as Lamp2b and tetraspanins CD63 and CD9 [43], thus the produced EVs display the just-engineered molecule on their surface. For instance, dendritic cells can be engineered to express a protein composed by Lamp2b and αv integrin-specific iRGD peptide in order to secrete iRGD peptide-EVs. This functionalization considerably increases the delivery of doxorubicin to αv integrin-positive breast cancer cells in vitro [54]. The transfection can occur by using plasmid vectors. A plasmid vector encoding streptavidin (which binds to biotin with high affinity) and lactadherin (an exosome-tropic protein) fusion protein allow to obtain streptavidin-lactadherin-modified exosomes that are mixed with the biotinylated pH-sensitive fusogenic GALA peptide exerting a lytic activity in acid environment [55]. Lentiviral vector bearing LAMP2b-Designed ankyrin repeat protein (DARPin) G3 chimeric gene or herpes simplex virus with plasmid pACgp67B-HER2m, containing the anti-human epidermal growth factor receptor 2 (HER2) scFv (ML39) antibody DNA sequence, are used to engineer HEK-293T cells. EVs isolated from transfected cells can bind specifically to HER2/Neu in adenocarcinoma cell lines [56][57]. Human carcinoembryonic antigen or human HER2/neu can be also inserted into the mouse lactadherin expression plasmid p6mLC1C2 and transfected into dendritic cells, enhancing the production of functionalized EVs to target breast cancer cells [58]. Similarly, prostate-specific antigen and prostatic acid phosphatase linked to the C1C2 domain of lactadherin produce EVs that specifically target prostate cancer cells [59]. In another study, an anti-epidermal growth factor receptor (EGFR) nanobodies with anchor signal peptide glycosylphosphatidylinositol (GPI) fusion protein are transfected to parent cells in order to generate EVs with this functionalization. These EVs show a significantly improved targeting ability towards EGFR-positive tumor cells [60].
An alternative strategy is the hydrophobic insertion used to functionalize the EVs’ membranes by exploiting the phospholipid composition of plasma membranes. Amphiphilic molecule DSPE-PEG, FDA approved for medical applications, can self-assemble in the phospholipid bilayer [61]. Based on this consideration, if DSPE-PEG is bound to the molecule of interest, it can be incorporated inside the cell membrane, making it overexpresses the molecule on its surface and producing EVs with the desired functionalization. The most frequently used molecules are biotin and folate: the first one binds selectively with streptavidin, used for further functionalization, and the second one targets specific cancer cells [62][63][64][65]. In addition to folate, also other binding sites can be created on EVs using this approach, for example by adding the RGD sequence or sulfhydryl groups [66].
A summary of EVs ‘surface functionalization nanotechnological modification through parent cells’ engineering with the related bibliographic references is reported in Table 1.
Table 1. EVs’ Surface functionalization by parent cells’ engineering.
| EVs Type | Nanotechnological Modification | Application | Reference |
|---|---|---|---|
| Exosomes from breast cancer | Ac4ManNAz labeled with ADIBO-fluorescent dyes | Breast cancer imaging | [49] |
| Exosomes from melanoma | Gaussian Luciferase | Biodistribution and tumor targeting | [53] |
| Exosomes from HEK 293T | [50][51] | ||
| Exosomes from melanoma | [52] | ||
| Exosomes from HEK 293T | Alexa Fluor 680-Streptavidin | Biodistribution and tumor targeting | [51] |
| Exosomes from different cell lines | GFP | Monitoring and tracking of exosomes uptake in different types of cancer | [46][47][48][67] |
| EVs from HEK 293T | Palmitoylation signal genetically fused in-frame to the N terminus of enhanced green fluorescence protein (EGFP) and tandem dimer Tomato (tdTomato) | Monitoring the uptake by cancer cells | [68] |
| Exosomes from macrophage | Arginyl–glycyl–aspartic acid (RGD)-functionalized DSPE-PEG (DSPE-PEG-RGD), sulfhydryl-functionalized DSPE-PEG (DSPE-PEG-SH) and folic acid | Targeting Hela cells | [66] |
| EVs from squamous cell carcinoma | DSPE-PEG-Biotin and folate | Targeting breast cancer for diagnosis and therapy | [63] |
| Exosomes from HUVEC | DSPE-PEG-biotin | Targeting hepatocellular carcinoma | [65] |
| EVs from macrophage | DSPE-PEG-Biotin and folate | Targeting Hela cells | [62] |
| EVs from HUVEC | DSPE-PEG-Biotin | Targeting melanoma | [64] |
| Exosomes from HEK 293T | DARPins | Targeting HER-2 over-expressing cancer cells (breast, ovarian and gastric cancers) | [56] |
| Exosomes from HEK 293 | Anti-HER2 scFv antibody (ML39) | Inhibit the growth of HER2 positive breast cancer | [57] |
| Exosomes from murine melanoma | Streptavidin-lactadherin fusion protein linked with biotinylated pH-sensitive fusogenic GALA peptide | Cancer immunotherapy | [55] |
| Extracellular vesicles from murine neural stem cells | Anti-EGFR fused to GPI anchor signal peptides | Targeting of Hela cells | [60] |
| Exosomes from dendritic cells | Lamp2b fused with iRGD (CRGDKGPDC) targeting peptide for αv integrin | Targeting breast cancer | [54] |
| Exosomes from dendritic cells | Carcinoembryonic antigen or HER2 linked to the C1C2 domain of lactadherin | Targeting breast cancer | [58] |
| Exosomes from HEK 293F cells | Prostate-specific antigen, and prostatic acid phosphatase linked to the C1C2 domain of lactadherin |
Targeting prostate cancer | [59] |
| Exosomes from fibrosarcoma cells | Chicken egg ovalbumin by fusing it to the C1C2 domain of the lactadherin | In vivo fibrosarcoma. More efficient antitumor immune response | [69][70] |
| Exosomes from dendritic cell line | C1C2 domain of lactadherin is fused to soluble proteins or extracellular domain of membrane proteins | Generate antibodies against tumor biomarkers | [71] |
| Transmembrane protein HLA-A2 a |
Acronym legend: a HLA-A2: Human leukocyte antigen A2.
2.2. Indirect Loading
Genetically engineered parent cells allow the production of pre-loaded EVs. This approach enhances the loading efficiency of molecules inside the EVs compared to the post-isolation techniques, minimizing the impairment of the structures or of the biological activity of both cargoes and carriers [72]. Some reports demonstrate the successful internalization of miRNA, siRNA [73][74] and nanoparticles [75] inside EVs produced from engineered parental cells. Furthermore, cells can be transfected with short RNA-encoding plasmid DNA (pDNA) in order to generate EVs enriched with target RNA [76][77]. The efficiency of cargo uptake inside EVs strongly depends on its high concentration inside the parent cells, because only a small amount is released as packed in the EVs [45]. Loading proteins inside the EVs can be accomplished by transfecting the parent cell with a vector containing the gene which codifies the specific protein. Proteins encoded by the transfected gene are synthesized by the cells and then secreted enveloped in EVs. Despite the apparent simplicity of this approach, many aspects need to be considered. The expression of cytotoxic proteins can inhibit the growth of the parent cells or induce their apoptosis. Furthermore, impaired biological reactions and interactions can obstacle the production of EVs ability [40].
Viruses are often used for the transfection of genetic materials or molecules inside the parent cells in vitro. Different kind of viruses are employed, but the most used is lentivirus because of its transfection ability and safety. Generally, the transfection of parent cells has the aim to overexpress a particular therapeutic or anticancer molecule in order to secrete it as enveloped inside EVs. For example, EVs-enriched human MUC1 (hMUC1) injected intra-dermally suppress the growth of hMUC1-expressing tumor [78]. Similarly, tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), a widely tested anticancer protein, causes the apoptosis of transformed or tumoral cells, but not of the normal ones. Due to its therapeutic efficacy, it has been encapsulated in EVs to overcome the shortcomings of a poor pharmacokinetic profile and the tumor resistance to drug [79][80]. Target proteins can also be delivered inside the parent cell by fusion with constitutive proteins of EVs, such as CD63, to improve the specificity of the protein loaded inside EVs [81]. Nef/E7 DNA vector expressing Nef exosome-anchoring protein combined with HPV-E7 is delivered to parent cells to make them able to generate immunogenic EVs containing the Nef-E7 fusion protein to elicit an efficient anti-E7 cytotoxic T lymphocyte immune response for cancer therapy [82]. Another strategy to incorporate proteins of interest inside EVs is pseudotyping, which packages viral RNAs or DNAs with the envelope proteins from another virus. The G glycoprotein of the vesicular stomatitis virus glycoprotein (VSVG) is frequently used for this purpose because of its efficacy in transduction and broad tropism. The selected protein is fused with VSVG and transfected into different parent cell lines [83]. This method can be further developed by adding to VSVG cell-recognizing peptides for targeting or engineered therapeutic antibodies, such as anti-CD19 chimeric antigen receptors, that target specific suppressors of cytotoxic T cells for cancer therapy [84]. A novel method, called EXPLORs (exosomes for protein loading via optically reversible protein-protein interactions), allows the loading of cargo proteins inside EVs through endogenous biogenesis processes, delivering soluble proteins into the cytosol via controlled, reversible protein-protein interactions. For this purpose, a photoreceptor cryptochrome 2 (CRY2) and CRY-interacting basic-helix-loop-helix 1 (CIB1) protein module, which regulates the floral initiation of Arabidopsis thaliana via blue light-dependent phosphorylation, are selected. Then, a transient docking of CRY2-conjugated cargo proteins is induced by introducing CIBN (a truncated version of CIB1) conjugated with an exosome-associated tetraspanin protein CD9 and by blue light illumination. After the release of the EVs with the cargo proteins linked to tetraspanins from the parent cell, they can be detached from CD9-conjugated CIBN by the removal of the illumination source, releasing them into the intraluminal space of the EVs [85].
The strategies described above to load EVs with proteins by engineering of the parent cells can be applied also in the case of nucleic acids. For instance, to reverse the chemoresistance to cisplatin-refractory gastric cancer, human embryonic kidney 293T (HEK-293T) cells are transfected with anti-miR-214 and the produced vesicles are administered systemically in combination with cisplatin, injected intraperitoneally, to overcome the in vitro and in vivo drug-resistance [86]. EVs produced by miR-134 or anti-miR-21 transfected mammary carcinoma cells have the ability to reduce the cellular proliferation and migration and to enhance the apoptosis in breast cancers [76][87]. miR-122 is essential to tune the chemosensitivity of hepatocellular carcinoma cells. Its effective delivery is accomplished by transfecting adipose-derived mesenchymal stem cells in order to produce EVs already loaded with miR-122 [88]. EVs from mesenchymal stem cells transfected with miR-146b expressing plasmid silence the EGFR and significantly decrease glioma growth [89], while EVs loaded with miR-143 inhibit the migration ability of osteosarcoma cells [90]. Mesenchymal stem cells can be loaded with anti-miR-9 to produce anti-miR-9 EVs. Anti-miR-9 delivered to cancer cells can reverse the expression of P-glycoprotein, involved in the chemoresistance, to enhance the efficacy of the temozolomide in otherwise resistant glioblastomas [91]. HEK-293T cell line can be genetically engineered to overexpress a suicide gene mRNA and protein-cytosine deaminase fused to uracil phosphoribosyltransferase in their microvesicles. They can transfer the therapeutic mRNA/protein to schwannoma cancer cells, achieving the inhibition of tumor growth [92]. Prostate cancer cell line is incubated with spherical nucleic acids (SNA), which are a new type of therapeutic agent composed by a core of gold nanoparticle with a dense shell of highly oriented nucleotides. The secreted EVs display a potent gene knockdown, when internalized in cancer cell, due to the presence of the anti-miR-21 [75]. EVs overexpressing hepatocyte growth factor (HGF) siRNA drastically reduced HGF and vascular endothelial growth factor (VEGF) expression in gastric cancer [93]. EVs delivery of siRNA against RAD51 and RAD52 causes an inhibition of proliferation and a massive reproductive cell death in human breast cancer cells [94].
The previously described method EXPLOR can be also used for the encapsulation of peptides inside cells, in particular of miR-21 sponges inside HEK-293T cells. The EVs produced are then loaded with this nucleic acid, which is an inhibitor of miR-21, overexpressed in most cancer types, and reduces the tumor progression and metastasis. After the collection of EVs loaded with miR-21 sponges, EVs are functionalized with cholesterol-AS411 aptamers exploiting the interaction with lipids of EVs’ membrane. The expression of AS1411 on EVs allows the targeting of leukemia cells for the interaction with nucleolin, overexpressed by these cancer lines. miR-21 sponges can inhibit miR-21 functions, triggering leukemia cells’ apoptosis [95].
Engineering the donor cells in order to make them produce already loaded EVs is possible also in the case of chemotherapeutic drugs and nanoparticles, as resumed in Table 2. For example, mesenchymal stromal cells are cultured for 24 h with paclitaxel and, after a change of media, cells are left to produce EVs with Paclitaxel for 48 h. These EVs can be used in the treatment of human pancreatic adenocarcinoma and they demonstrate a strong antiproliferative activity [96]. A melanoma cell line is engineered to produced EVs loaded with both survivin T34A and gemcitabine. Loaded EVs are collected and administered to pancreatic adenocarcinoma cells. The presence of survivin-T34A, which targets and inhibits survivin, an inhibitor of apoptosis, enhances the toxic effect of the Gemcitabine with lower dosages [97]. Different cell lines are incubated with methotrexate or doxorubicin and then irradiated with ultraviolet light to induce cells apoptosis. The produced ApoBDs, as delivery vehicles of chemotherapeutic drugs, exert a strong cytotoxic effect and inhibit the drug efflux from cancer cells [98]. A hybrid approach between drugs and nanoparticles involves the co-incubation of macrophages with both iron oxide NPs and a photosensitizer called m-THPC. The produced EVs containing both the two cargoes stabilize the strong hydrophobic photosensitizer drug and are injected into a mouse model. The drug allows the photodynamic therapy on cancer cells, while nanoparticles, responsive to magnetic fields, can be tracked with magnetic resonance imaging and used for hyperthermia treatments [99]. A further experiment, carried out by the previous research groups, besides the iron oxide nanoparticles, includes also a chemotherapeutic agent (doxorubicin), tissue-plasminogen activator (t-PA) and two photosensitizers (disulfonated tetraphenylchlorin-TPCS2a and 5,10,15,20-tetra(m-hydroxyphenyl)chlorin-mTHPC) to better enhance the antitumor ability of the produced EVs [100]. The delivery of compounds to parent cells can be difficult, especially in presence of hydrophobic molecules. For this reason, in the case of the hydrophobic photosensitizer zinc phthalocyanine, it is encapsulated in liposomes and they are used to treat the parent cells. The hydrophobic compound is secreted from the parent cells by incorporation in the EVs and then transferred to adjacent cells. This approach allows to significantly penetrates spheroids and in vivo solid tumors, enhancing the efficacy of the therapy [101]. The same procedure can be followed also for other molecule, both hydrophobic or hydrophilic, including fluorophores such as 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindodicarbocyanine perchlorate (DiD) and carboxy-fluorescein, drugs (paclitaxel and tirapazamine), lipids and bio-orthogonal chemicals [102]. A similar approach is used also to incorporate nanoparticles inside EVs. Hollow-gold nanoparticles were shielded with a PEG functionalization and then incubated with human placental mesenchymal stem cells. After the uptake, the cells produced EVs loaded with hollow-gold nanoparticles. These EVs allowed to track the cell-cell communication and also perform the optical hyperthermia for cancer therapy [103].
Table 2. Nanotechnological modification of EVs’ loading through parent cell engineering.
| EVs Type | Nanotechnological Modification | Application | Reference |
|---|---|---|---|
| Human placental mesenchymal stem cells | Hollow gold NPs | Hyperthermia therapy against different type of cancer | [103] |
| Exosomes from hepatocellular carcinoma | Porous silicon NPs loaded with doxorubicin | Decreased the expression of multidrug-resistant protein P-glycoprotein | [104] |
| EVs from mesenchymal stem cells | SPIONs | Therapy against leukemia | [105] |
| EVs from HUVEC | Iron oxide NPs and clinical photosensitizer (Foscan) | Phototoxicity against prostate adenocarcinoma cells | [106] |
| Extracellular vesicles from human macrophages | Iron oxide nanoparticles and m-THPC photosensitizer | Theranostic approach against cervical and prostate cancer | [99] |
| Microvesicles from different cancer cell lines | A hydrophobic photosensitizer zinc phthalocyanine encapsulated in liposomes | Photodynamic therapy for different cancer cell lines | [101] |
| Microvesicles from human macrophages | Doxorubicin, tissue-plasminogen activator and two photosensitizers | Targeting and therapy of ovarian and prostate cancers | [100] |
| Exosomes from mesenchyme stromal cells | Paclitaxel | Treatment of pancreatic cancer | [96] |
| Exosomes from melanoma cell line | Survivin-T34A and Gemcitabine | Treatment of pancreatic adenocarcinoma | [97] |
| Apoptotic bodies from tumoral cells | Doxorubicin or Metotrexate | Tumor cells killing with reduce side effects | [98] NCT01854866 |
| Exosomes from breast cancer | Curcumin | Reverse inhibition of NK cell tumor cytotoxicity in breast cancer | [107] |
| Exosomes from HEK 293 | P53 gene | Transfer p53 gene to p53-deficient cells | [108] |
| Exosomes from HEK 293T | miR-21 sponges | Therapy for leukemia cells | [95] |
| Extracellular vesicles from breast cancer | Anti-miR-21 | Theranostic method for breast cancer | [76] |
| Exosomes from HEK 293T | Inhibitor of miR-214 | Reverse chemoresistance to cisplatin in gastric cancer | [86] |
| Exosomes from prostate cancer cells | Anti-miR-21 spherical nucleic acid | Prostate cancer | [75] |
| Exosomes from mammary carcinomas | miR-134 | Increase sensitivity of breast cancers to chemotherapeutic drugs | [87] |
| Exosomes from mesenchyme stem cells | miR-122 | Increase sensitivity of hepatocellular carcinoma to chemotherapeutic drugs | [88] |
| Exosomes from mesenchymal stem cells | miR-143 | Inhibit migration of osteosarcoma cells | [90] |
| Exosomes from mesenchymal stem cells | anti-miR-9 | Increase sensitivity of glioblastoma multiforme to chemotherapeutic drugs | [91] |
| Exosomes from mesenchyme stem cells | miR-146b | Inhibit glioma growth | [89] |
| Microvesicles from HEK 293T | Suicide gene mRNA and protein-cytosine deaminase fused to uracil phosphoribosyltransferase | Inhibit schwannoma tumor growth | [92] |
| Exosomes from HEK 293T | HGF siRNA | Inhibition of tumor growth and angiogenesis in gastric cancer | [93] |
| Exosomes from breast cancer cells | RAD51 and RAD52 siRNA | Gene therapy against breast cancer | [94] |
| Extracellular vesicles from mesenchymal stem cells | TNF-related apoptosis-inducing ligand (TRAIL) | Lung, breast, kidney cancer, pleural mesothelioma and neuroblastoma | [79] |
| Exosomes from chronic myelogenous leukemia cells | TNF-related apoptosis-inducing ligand (TRAIL) | Enhance apoptosis in lymphoma | [80] |
| Exosomes from HEK 293T | VSVG | Glioblastoma and liver cancer cells | [83] |
| Exosomes from lymphoblast | Nef-E7 fusion protein | T lymphocytes immune response | [82] |
| Exosomes from two mouse cell lines | Human MUC1 tumor antigen | Generate immune response against tumor | [78] |
| Microvesicles from different cancer cell lines | DiD, carboxyfluorescein, paclitaxel, tirapazamine encapsulated in fusogenic liposomes | The same cancer cell lines used to produce microvesicles | [102] |
The engineering of parent cells can also be addressed to obtain EVs loaded with molecules, such as drugs or nucleic acids, and with a specific surface functionalization (as summarized in Table 3). HEK-293T cells engineered to express Lamp2b protein, fused with a fragment of interleukin 3 (IL-3), and then incubated with Imatinib or BCR-ABL siRNA, can produce EVs loaded with the desired cargoes and expressing the IL-3 fragment on their surface. The IL-3 receptor is overexpressed in chronic myeloid leukemia and acute myeloid leukemia blasts and almost absent in hematopoietic stem cells. Exploiting this characteristic, IL-3 expressing EVs can target these cancerous cells and overcome the drug resistance to imatinib or deliver functional BCR-ABL siRNA towards imatinib-resistant cells [73]. The cell line used above can be also transfected with pDisplay vector encoding GE11 peptide or EGF, and with let-7a miRNA. The harvested EVs are functionalized with the peptide on their surface and loaded with the miRNA. Then, EVs are injected intravenously and their surface functionalization allows the specific targeting of EGFR-expressing cancer tissues, such as breast cancer. The tumor suppressor let-7a is delivered to the tumor and reduce the expression of RAS and HMGA2 inhibiting the malignant growth of cancer cells [74]. In another study, adeno-associated virus (AAV) is used as viral vector for transfection. It is broadly used for gene therapy in human, thanks to its safety profile, but it has some limitations, such as off-target gene delivery (to liver for example) and low transfection of target cells. For this reason, by transfecting the parent cells with AAV, capsids associate with the membrane and the interior part of the newly-produced EVs (called vexosomes). Harvested vexosomes show to be more resistant to anti-AAV antibodies if compared to naked AAV and they can efficiently transduce cells, enhancing gene transfer. Furthermore, parent cells are also engineered to express a transmembrane receptor on the microvesicle surface, i.e., biotin acceptor peptide-transmembrane domain (BAP-TM) receptor, allowing the specific targeting of glioblastoma cells [109]. Gene engineering method is applied to HEK-293T cell line to functionalize the CD9 tetraspanin with the RNA-binding protein HuR and then, they are modified with miR-155 or the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 system. The produced EVs are effectively enriched by the above mentioned RNAs and in future these nanoconstructs need to be evaluated in some diseases such as liver cancer [110].
Table 3. EVs’ indirect modifications through combined loading and surface parent cells engineering.
| EVs Type | Nanotechnological Modification | Application | Reference |
|---|---|---|---|
| Exosomes from HEK 293T | Functionalization: CD9-HuR Load: miR-155 or CRISPR/Cas9 |
Targeting and therapy of liver cancer | [110] |
| Exosomes from HEK 293T | Functionalization: Lamp2b, fused to a fragment of IL-3 Load: Imatinib or BCR-ABL siRNA |
Inhibition of chronic myeloid leukemia growth | [73] |
| Exosomes from HEK 293T | Functionalization: GE 11 peptide Load: let-7a miRNA |
Targeting and therapy of EGFR-expressing cancer tissues | [74] |
| Exosomes from HEK 293T cells | Functionalization: BAP-TM receptor and biotin ligase BirA Load: viral capside |
Gene therapy against glioma | [109] |
References
- D’Anca, M.; Fenoglio, C.; Serpente, M.; Arosio, B.; Cesari, M.; Scarpini, E.A.; Galimberti, D. Exosome determinants of physiological aging and age-related neurodegenerative diseases. Front. Aging Neurosci. 2019, 11, 232.
- Giusti, I.; Di Francesco, M.; D’Ascenzo, S.; Palmerini, M.G.; Macchiarelli, G.; Carta, G.; Dolo, V. Ovarian cancer-derived extracellular vesicles affect normal human fibroblast behavior. Cancer Biol. Ther. 2018, 19, 722–734.
- Ilaria, G.; Marianna Di, F.; Vincenza, D. Extracellular vesicles in glioblastoma: Role in biological processes and in therapeutic applications. Curr. Cancer Drug Targets 2017, 17, 221–235.
- Palumbo, P.; Lombardi, F.; Augello, F.R.; Giusti, I.; Luzzi, S.; Dolo, V.; Cifone, M.G.; Cinque, B. NOS2 inhibitor 1400W induces autophagic flux and influences extracellular vesicle profile in human glioblastoma U87MG cell line. Int. J. Mol. Sci. 2019, 20, 3010.
- Rome, S.; Forterre, A.; Mizgier, M.L.; Bouzakri, K. Skeletal muscle-released extracellular vesicles: State of the art. Front. Physiol. 2019, 10, 929.
- Ruivo, C.F.; Adem, B.; Silva, M.; Melo, S.A. The biology of cancer exosomes: Insights and new perspectives. Cancer Res. 2017, 77, 6480.
- Rybak, K.; Robatzek, S. Functions of extracellular vesicles in immunity and virulence. Plant Physiol. 2019, 179, 1236–1247.
- Yamamoto, T.; Kosaka, N.; Ochiya, T. Latest advances in extracellular vesicles: From bench to bedside. Sci. Technol. Adv. Mater. 2019, 20, 746–757.
- Yang, J.; Dang, G.; Lü, S.; Liu, H.; Ma, X.; Han, L.; Deng, J.; Miao, Y.; Li, X.; Shao, F.; et al. T-cell-derived extracellular vesicles regulate B-cell IgG production via pyruvate kinase muscle isozyme 2. FASEB J. 2019, 33.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
- Witwer, K.W.; Théry, C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell. Vesicles 2019, 8, 1648167.
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular vesicle heterogeneity: Subpopulations, isolation techniques, and diverse functions in cancer progression. Front. Immunol. 2018, 9, 738.
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213.
- Caruso, S.; Poon, I.K.H. Apoptotic cell-derived extracellular vesicles: More than just debris. Front. Immunol. 2018, 9, 1486.
- Hauser, P.; Wang, S.; Didenko, V.V. Apoptotic bodies: Selective detection in extracellular vesicles. In Signal Transduction Immunohistochemistry: Methods and Protocols; Kalyuzhny, A.E., Ed.; Springer New York: New York, NY, USA, 2017; pp. 193–200.
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics 2018, 10, 218.
- Meldolesi, J. Exosomes and ectosomes in intercellular communication. Curr. Biol. 2018, 28, R435–R444.
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579.
- Kalra, H.; Drummen, G.P.C.; Mathivanan, S. Focus on extracellular vesicles: Introducing the next small big thing. Int. J. Mol. Sci. 2016, 17, 170.
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome theranostics: Biology and translational medicine. Theranostics 2018, 8, 237–255.
- Jaiswal, R.; Sedger, L.M. Intercellular vesicular transfer by exosomes, microparticles and oncosomes—Implications for cancer biology and treatments. Front. Oncol. 2019, 9, 125.
- Maacha, S.; Bhat, A.A.; Jimenez, L.; Raza, A.; Haris, M.; Uddin, S.; Grivel, J.-C. Extracellular vesicles-mediated intercellular communication: Roles in the tumor microenvironment and anti-cancer drug resistance. Mol. Cancer 2019, 18, 55.
- Han, L.; Lam, E.W.F.; Sun, Y. Extracellular vesicles in the tumor microenvironment: Old stories, but new tales. Mol. Cancer 2019, 18, 59.
- Sung, B.H.; Weaver, A.M. Exosome secretion promotes chemotaxis of cancer cells. Cell Adh. Migr. 2017, 11, 187–195.
- Gao, L.; Wang, L.; Dai, T.; Jin, K.; Zhang, Z.; Wang, S.; Xie, F.; Fang, P.; Yang, B.; Huang, H.; et al. Tumor-derived exosomes antagonize innate antiviral immunity. Nat. Immunol. 2018, 19, 233–245.
- Choi, D.; Lee, T.H.; Spinelli, C.; Chennakrishnaiah, S.; D’Asti, E.; Rak, J. Extracellular vesicle communication pathways as regulatory targets of oncogenic transformation. Semin. Cell Dev. Biol. 2017, 67, 11–22.
- Tirinato, L.; Pagliari, F.; Limongi, T.; Marini, M.; Falqui, A.; Seco, J.; Candeloro, P.; Liberale, C.; Di Fabrizio, E. An overview of lipid droplets in cancer and cancer stem cells. Stem Cells Int. 2017, 2017, 1656053.
- Romagnoli, G.G.; Zelante, B.B.; Toniolo, P.A.; Migliori, I.K.; Barbuto, J.A.M. Dendritic cell-derived exosomes may be a tool for cancer immunotherapy by converting tumor cells into immunogenic targets. Front. Immunol. 2015, 5.
- Pitt, J.M.; André, F.; Amigorena, S.; Soria, J.-C.; Eggermont, A.; Kroemer, G.; Zitvogel, L. Dendritic cell-derived exosomes for cancer therapy. J. Clin. Investig. 2016, 126, 1224–1232.
- Markov, O.; Oshchepkova, A.; Mironova, N. Immunotherapy based on dendritic cell-targeted/-derived extracellular vesicles—A novel strategy for enhancement of the anti-tumor immune response. Front. Pharmacol. 2019, 10, 1152.
- Munich, S.; Sobo-Vujanovic, A.; Buchser, W.J.; Beer-Stolz, D.; Vujanovic, N.L. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology 2012, 1, 1074–1083.
- Viaud, S.; Théry, C.; Ploix, S.; Tursz, T.; Lapierre, V.; Lantz, O.; Zitvogel, L.; Chaput, N. Dendritic cell-derived exosomes for cancer immunotherapy: What’s next? Cancer Res. 2010, 70, 1281.
- Zhang, B.; Yin, Y.; Lai, R.C.; Lim, S.K. Immunotherapeutic potential of extracellular vesicles. Front. Immunol. 2014, 5, 518.
- Syn, N.L.; Wang, L.; Chow, E.K.-H.; Lim, C.T.; Goh, B.-C. Exosomes in cancer nanomedicine and immunotherapy: Prospects and challenges. Trends Biotechnol. 2017, 35, 665–676.
- Yokoi, A.; Yoshioka, Y.; Yamamoto, Y.; Ishikawa, M.; Ikeda, S.-I.; Kato, T.; Kiyono, T.; Takeshita, F.; Kajiyama, H.; Kikkawa, F.; et al. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat. Commun. 2017, 8, 14470.
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30.
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685.
- Khawar, M.B.; Abbasi, M.H.; Siddique, Z.; Arif, A.; Sheikh, N. An update on novel therapeutic warfronts of extracellular vesicles (EVs) in cancer treatment: Where we are standing right now and where to go in the future. Oxid. Med. Cell Longev. 2019, 2019, 9702562.
- Kosaka, N.; Yoshioka, Y.; Fujita, Y.; Ochiya, T. Versatile roles of extracellular vesicles in cancer. J. Clin. Investig. 2016, 126, 1163–1172.
- Liu, C.; Su, C. Design strategies and application progress of therapeutic exosomes. Theranostics 2019, 9, 1015–1028.
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61.
- Smyth, T.J.; Redzic, J.S.; Graner, M.W.; Anchordoquy, T.J. Examination of the specificity of tumor cell derived exosomes with tumor cells in vitro. Biochim. Biophys. Acta 2014, 1838, 2954–2965.
- Wang, J.; Zheng, Y.; Zhao, M. Exosome-based cancer therapy: Implication for targeting cancer stem cells. Front. Pharmacol. 2017, 7, 533.
- Mentkowski, K.I.; Snitzer, J.D.; Rusnak, S.; Lang, J.K. Therapeutic potential of engineered extracellular vesicles. AAPS J. 2018, 20, 50.
- Kim, H.; Kim, D.; Nam, H.; Moon, S.; Kwon, Y.J.; Lee, J.B. Engineered extracellular vesicles and their mimetics for clinical translation. Methods 2019, 19, 30221-x.
- Zomer, A.; Maynard, C.; Verweij, F.J.; Kamermans, A.; Schäfer, R.; Beerling, E.; Schiffelers, R.M.; de Wit, E.; Berenguer, J.; Ellenbroek, S.I.J.; et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 2015, 161, 1046–1057.
- Hoffman, R.M. Stromal-cell and cancer-cell exosomes leading the metastatic exodus for the promised niche. Breast Cancer Res. 2013, 15, 310.
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177.
- Lee, T.S.; Kim, Y.; Zhang, W.; Song, I.H.; Tung, C.-H. Facile metabolic glycan labeling strategy for exosome tracking. Biochim. Biophys. Acta 2018, 1862, 1091–1100.
- Lai, C.P.; Tannous, B.A.; Breakefield, X.O. Noninvasive in vivo monitoring of extracellular vesicles. In Bioluminescent Imaging: Methods and Protocols; Badr, C.E., Ed.; Humana Press: Totowa, NJ, USA, 2014; pp. 249–258.
- Lai, C.P.; Mardini, O.; Ericsson, M.; Prabhakar, S.; Maguire, C.; Chen, J.W.; Tannous, B.A.; Breakefield, X.O. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 2014, 8, 483–494.
- Takahashi, Y.; Nishikawa, M.; Shinotsuka, H.; Matsui, Y.; Ohara, S.; Imai, T.; Takakura, Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J. Biotechnol. 2013, 165, 77–84.
- Imai, T.; Takahashi, Y.; Nishikawa, M.; Kato, K.; Morishita, M.; Yamashita, T.; Matsumoto, A.; Charoenviriyakul, C.; Takakura, Y. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J. Extracell. Vesicles 2015, 4, 26238.
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390.
- Morishita, M.; Takahashi, Y.; Nishikawa, M.; Ariizumi, R.; Takakura, Y. Enhanced class I tumor antigen presentation via cytosolic delivery of exosomal cargos by tumor-cell-derived exosomes displaying a pH-sensitive fusogenic peptide. Mol. Pharmacol. 2017, 14, 4079–4086.
- Limoni, S.K.; Moghadam, M.F.; Moazzeni, S.M.; Gomari, H.; Salimi, F. Engineered exosomes for targeted transfer of siRNA to HER2 positive breast cancer cells. Appl. Biochem. Biotechnol. 2019, 187, 352–364.
- Wang, J.-H.; Forterre, A.V.; Zhao, J.; Frimannsson, D.O.; Delcayre, A.; Antes, T.J.; Efron, B.; Jeffrey, S.S.; Pegram, M.D.; Matin, A.C. Anti-HER2 scFv-directed extracellular vesicle-mediated mRNA-based gene delivery inhibits growth of HER2-positive human breast tumor xenografts by prodrug activation. Mol. Cancer Ther. 2018, 17, 1133–1142.
- Hartman, Z.C.; Wei, J.; Glass, O.K.; Guo, H.; Lei, G.; Yang, X.-Y.; Osada, T.; Hobeika, A.; Delcayre, A.; Le Pecq, J.-B.; et al. Increasing vaccine potency through exosome antigen targeting. Vaccine 2011, 29, 9361–9367.
- Rountree, R.B.; Mandl, S.J.; Nachtwey, J.M.; Dalpozzo, K.; Do, L.; Lombardo, J.R.; Schoonmaker, P.L.; Brinkmann, K.; Dirmeier, U.; Laus, R.; et al. Exosome targeting of tumor antigens expressed by cancer vaccines can improve antigen immunogenicity and therapeutic efficacy. Cancer Res. 2011, 71, 5235.
- Kooijmans, S.A.A.; Aleza, C.G.; Roffler, S.R.; van Solinge, W.W.; Vader, P.; Schiffelers, R.M. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles 2016, 5, 31053.
- Yamamoto, T.; Teramura, Y.; Itagaki, T.; Arima, Y.; Iwata, H. Interaction of poly(ethylene glycol)-conjugated phospholipids with supported lipid membranes and their influence on protein adsorption. Sci. Technol. Adv. Mater. 2016, 17, 677–684.
- Zhang, W.; Yu, Z.-L.; Wu, M.; Ren, J.-G.; Xia, H.-F.; Sa, G.-L.; Zhu, J.-Y.; Pang, D.-W.; Zhao, Y.-F.; Chen, G. Magnetic and folate functionalization enables rapid isolation and enhanced tumor-targeting of cell-derived microvesicles. ACS Nano 2017, 11, 277–290.
- Zhu, L.; Dong, D.; Yu, Z.-L.; Zhao, Y.-F.; Pang, D.-W.; Zhang, Z.-L. Folate-engineered microvesicles for enhanced target and synergistic therapy toward breast cancer. ACS Appl. Mater. Interfaces 2017, 9, 5100–5108.
- Chen, G.; Zhu, J.-Y.; Zhang, Z.-L.; Zhang, W.; Ren, J.-G.; Wu, M.; Hong, Z.-Y.; Lv, C.; Pang, D.-W.; Zhao, Y.-F. Transformation of cell-derived microparticles into quantum-dot-labeled nanovectors for antitumor siRNA delivery. Angew. Chem. Int. Ed. 2015, 54, 1036–1040.
- Wang, J.; Li, W.; Zhang, L.; Ban, L.; Chen, P.; Du, W.; Feng, X.; Liu, B.-F. Chemically edited exosomes with dual ligand purified by microfluidic device for active targeted drug delivery to tumor cells. ACS Appl. Mater. Interfaces 2017, 9, 27441–27452.
- Wang, J.; Dong, Y.; Li, Y.; Li, W.; Cheng, K.; Qian, Y.; Xu, G.; Zhang, X.; Hu, L.; Chen, P.; et al. Designer exosomes for active targeted chemo-photothermal synergistic tumor therapy. Adv. Funct. Mater. 2018, 28, 1707360.
- Suetsugu, A.; Honma, K.; Saji, S.; Moriwaki, H.; Ochiya, T.; Hoffman, R.M. Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Adv. Drug Deliv. Rev. 2013, 65, 383–390.
- Lai, C.P.; Kim, E.Y.; Badr, C.E.; Weissleder, R.; Mempel, T.R.; Tannous, B.A.; Breakefield, X.O. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat. Commun. 2015, 6, 7029.
- Zeelenberg, I.S.; Ostrowski, M.; Krumeich, S.; Bobrie, A.; Jancic, C.; Boissonnas, A.; Delcayre, A.; Le Pecq, J.-B.; Combadière, B.; Amigorena, S.; et al. Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res. 2008, 68, 1228.
- Zeelenberg, I.S.; van Maren, W.W.C.; Boissonnas, A.; Van Hout-Kuijer, M.A.; Den Brok, M.H.M.G.M.; Wagenaars, J.A.L.; van der Schaaf, A.; Jansen, E.J.R.; Amigorena, S.; Théry, C.; et al. Antigen localization controls T cell-mediated tumor immunity. J. Immunol. 2011, 187, 1281.
- Delcayre, A.; Estelles, A.; Sperinde, J.; Roulon, T.; Paz, P.; Aguilar, B.; Villanueva, J.; Khine, S.; Le Pecq, J.-B. Exosome display technology: Applications to the development of new diagnostics and therapeutics. Blood Cells Mol. Dis. 2005, 35, 158–168.
- Armstrong, J.P.; Holme, M.N.; Stevens, M.M. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano 2017, 11, 69–83.
- Bellavia, D.; Raimondo, S.; Calabrese, G.; Forte, S.; Cristaldi, M.; Patinella, A.; Memeo, L.; Manno, M.; Raccosta, S.; Diana, P.; et al. Interleukin 3-receptor targeted exosomes inhibit in vitro and in vivo Chronic Myelogenous Leukemia cell growth. Theranostics 2017, 7, 1333–1345.
- Ohno, S.-I.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013, 21, 185–191.
- Alhasan, A.H.; Patel, P.C.; Choi, C.H.J.; Mirkin, C.A. Exosome encased spherical nucleic acid gold nanoparticle conjugates as potent microRNA regulation agents. Small 2014, 10, 186–192.
- Bose, R.J.C.; Uday Kumar, S.; Zeng, Y.; Afjei, R.; Robinson, E.; Lau, K.; Bermudez, A.; Habte, F.; Pitteri, S.J.; Sinclair, R.; et al. Tumor cell-derived extracellular vesicle-coated nanocarriers: An efficient theranostic platform for the cancer-specific delivery of Anti-miR-21 and imaging agents. ACS Nano 2018, 12, 10817–10832.
- Tao, S.-C.; Yuan, T.; Zhang, Y.-L.; Yin, W.-J.; Guo, S.-C.; Zhang, C.-Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017, 7, 180–195.
- Cho, J.-A.; Yeo, D.-J.; Son, H.-Y.; Kim, H.-W.; Jung, D.-S.; Ko, J.-K.; Koh, J.S.; Kim, Y.-N.; Kim, C.-W. Exosomes: A new delivery system for tumor antigens in cancer immunotherapy. Int. J. Cancer 2005, 114, 613–622.
- Yuan, Z.; Kolluri, K.K.; Gowers, K.H.C.; Janes, S.M. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J. Extracell. Vesicles 2017, 6, 1265291.
- Rivoltini, L.; Chiodoni, C.; Squarcina, P.; Tortoreto, M.; Villa, A.; Vergani, B.; Bürdek, M.; Botti, L.; Arioli, I.; Cova, A.; et al. TNF-related apoptosis-inducing ligand (TRAIL)-armed exosomes deliver proapoptotic signals to tumor site. Clin. Cancer Res. 2016, 22, 3499.
- Yang, Y.; Hong, Y.; Cho, E.; Kim, G.B.; Kim, I.-S. Extracellular vesicles as a platform for membrane-associated therapeutic protein delivery. J. Extracell. Vesicles 2018, 7, 1440131.
- Di Bonito, P.; Chiozzini, C.; Arenaccio, C.; Anticoli, S.; Manfredi, F.; Olivetta, E.; Ferrantelli, F.; Falcone, E.; Ruggieri, A.; Federico, M. Antitumor HPV E7-specific CTL activity elicited by in vivo engineered exosomes produced through DNA inoculation. Int. J. Nanomed. 2017, 12, 4579–4591.
- Meyer, C.; Losacco, J.; Stickney, Z.; Li, L.; Marriott, G.; Lu, B. Pseudotyping exosomes for enhanced protein delivery in mammalian cells. Int. J. Nanomed. 2017, 12, 3153–3170.
- Kochenderfer, J.N.; Rosenberg, S.A. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat. Rev. Clin. Oncol. 2013, 10, 267–276.
- Yim, N.; Ryu, S.-W.; Choi, K.; Lee, K.R.; Lee, S.; Choi, H.; Kim, J.; Shaker, M.R.; Sun, W.; Park, J.-H.; et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein–protein interaction module. Nat. Commun. 2016, 7, 12277.
- Wang, X.; Zhang, H.; Bai, M.; Ning, T.; Ge, S.; Deng, T.; Liu, R.; Zhang, L.; Ying, G.; Ba, Y. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer. Mol. Ther. 2018, 26, 774–783.
- O’Brien, K.; Lowry, M.C.; Corcoran, C.; Martinez, V.G.; Daly, M.; Rani, S.; Gallagher, W.M.; Radomski, M.W.; MacLeod, R.A.F.; O’Driscoll, L. MiR-134 in extracellular vesicles reduces triple-negative breast cancer aggression and increases drug sensitivity. Oncotarget 2015, 6, 32774–32789.
- Lou, G.; Song, X.; Yang, F.; Wu, S.; Wang, J.; Chen, Z.; Liu, Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015, 8, 122.
- Katakowski, M.; Buller, B.; Zheng, X.; Lu, Y.; Rogers, T.; Osobamiro, O.; Shu, W.; Jiang, F.; Chopp, M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013, 335, 201–204.
- Shimbo, K.; Miyaki, S.; Ishitobi, H.; Kato, Y.; Kubo, T.; Shimose, S.; Ochi, M. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem. Biophys. Res. Commun. 2014, 445, 381–387.
- Munoz, J.L.; Bliss, S.A.; Greco, S.J.; Ramkissoon, S.H.; Ligon, K.L.; Rameshwar, P. Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol. Ther. Nucleic Acids 2013, 2, e126.
- Mizrak, A.; Bolukbasi, M.F.; Ozdener, G.B.; Brenner, G.J.; Madlener, S.; Erkan, E.P.; Ströbel, T.; Breakefield, X.O.; Saydam, O. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol. Ther. 2013, 21, 101–108.
- Zhang, H.; Wang, Y.; Bai, M.; Wang, J.; Zhu, K.; Liu, R.; Ge, S.; Li, J.; Ning, T.; Deng, T.; et al. Exosomes serve as nanoparticles to suppress tumor growth and angiogenesis in gastric cancer by delivering hepatocyte growth factor siRNA. Cancer Sci. 2018, 109, 629–641.
- Shtam, T.A.; Kovalev, R.A.; Varfolomeeva, E.Y.; Makarov, E.M.; Kil, Y.V.; Filatov, M.V. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun. Signal. 2013, 11, 88.
- Huang, L.; Gu, N.; Zhang, X.-E.; Wang, D.-B. Light-inducible exosome-based vehicle for endogenous RNA loading and delivery to leukemia cells. Adv. Funct. Mater. 2019, 29, 1807189.
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270.
- Aspe, J.R.; Diaz Osterman, C.J.; Jutzy, J.M.S.; Deshields, S.; Whang, S.; Wall, N.R. Enhancement of Gemcitabine sensitivity in pancreatic adenocarcinoma by novel exosome-mediated delivery of the Survivin-T34A mutant. J. Extracell. Vesicles 2014, 3.
- Tang, K.; Zhang, Y.; Zhang, H.; Xu, P.; Liu, J.; Ma, J.; Lv, M.; Li, D.; Katirai, F.; Shen, G.-X.; et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat. Commun. 2012, 3, 1282.
- Silva, A.K.A.; Kolosnjaj-Tabi, J.; Bonneau, S.; Marangon, I.; Boggetto, N.; Aubertin, K.; Clément, O.; Bureau, M.F.; Luciani, N.; Gazeau, F.; et al. Magnetic and photoresponsive theranosomes: Translating cell-released vesicles into smart nanovectors for cancer therapy. ACS Nano 2013, 7, 4954–4966.
- Silva, A.K.A.; Luciani, N.; Gazeau, F.; Aubertin, K.; Bonneau, S.; Chauvierre, C.; Letourneur, D.; Wilhelm, C. Combining magnetic nanoparticles with cell derived microvesicles for drug loading and targeting. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 645–655.
- Lee, J.; Kim, J.; Jeong, M.; Lee, H.; Goh, U.; Kim, H.; Kim, B.; Park, J.-H. Liposome-based engineering of cells to package hydrophobic compounds in membrane vesicles for tumor penetration. Nano Lett. 2015, 15, 2938–2944.
- Lee, J.; Lee, H.; Goh, U.; Kim, J.; Jeong, M.; Lee, J.; Park, J.-H. Cellular engineering with membrane fusogenic liposomes to produce functionalized extracellular vesicles. ACS Appl. Mater. Interfaces 2016, 8, 6790–6795.
- Sancho-Albero, M.; Navascués, N.; Mendoza, G.; Sebastián, V.; Arruebo, M.; Martín-Duque, P.; Santamaría, J. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J. Nanobiotechnol. 2019, 17, 16.
- Yong, T.; Zhang, X.; Bie, N.; Zhang, H.; Zhang, X.; Li, F.; Hakeem, A.; Hu, J.; Gan, L.; Santos, H.A.; et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019, 10, 3838.
- Mulens-Arias, V.; Nicolas-Boluda, A.; Brun, A.; Gazeau, F. Theranostic iron oxide nanoparticle cargo defines extracellular vesicle-dependent modulation of macrophage activation and migratory behavior. Adv. Biosyst. 2018, 2.
- Piffoux, M.; Silva, A.K.A.; Lugagne, J.-B.; Hersen, P.; Wilhelm, C.; Gazeau, F. Extracellular vesicle production loaded with nanoparticles and drugs in a trade-off between loading, yield and purity: Towards a personalized drug delivery system. Adv. Biosyst. 2017, 1, 1700044.
- Zhang, H.-G.; Kim, H.; Liu, C.; Yu, S.; Wang, J.; Grizzle, W.E.; Kimberly, R.P.; Barnes, S. Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochim. Biophys. Acta 2007, 1773, 1116–1123.
- Burdakov, V.S.; Kovalev, R.A.; Pantina, R.A.; Varfolomeeva, E.Y.; Makarov, E.M.; Filatov, M.V. Exosomes transfer p53 between cells and can suppress growth and proliferation of p53-negative cells. Cell Tissue Biol. 2018, 12, 20–26.
- Maguire, C.A.; Balaj, L.; Sivaraman, S.; Crommentuijn, M.H.W.; Ericsson, M.; Mincheva-Nilsson, L.; Baranov, V.; Gianni, D.; Tannous, B.A.; Sena-Esteves, M.; et al. Microvesicle-associated AAV vector as a novel gene delivery system. Mol. Ther. 2012, 20, 960–971.
- Li, Z.; Zhou, X.; Wei, M.; Gao, X.; Zhao, L.; Shi, R.; Sun, W.; Duan, Y.; Yang, G.; Yuan, L. In vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019, 19, 19–28.
More
Information
Subjects:
Medicine, General & Internal
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
889
Revisions:
2 times
(View History)
Update Date:
24 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




