
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ireneusz Majsterek | + 1875 word(s) | 1875 | 2021-08-08 02:23:43 |
Video Upload Options
According to the epigenetic hypothesis, a number of environmental stimuli can, through miRNAs, influence the phenotype of an organism. Discovering the phenomena of miRNAs regulation enables understanding of AMD pathomechanism. The characteristic profile of changes in miRNA expression levels may be helpful in early diagnosis. However, the role of miRNAs as biomarkers is not yet clear.
1. Introduction
Age-related macular degeneration (AMD) is a progressive, neurodegenerative disease that affects the macula of the retina. The incidence of AMD was estimated to be approximately 170 million people worldwide in 2016, but this number is predicted to increase to 288,000,000 in 2040, thus becoming the third leading cause of vision loss worldwide [1]. AMD is a slow and progressive disease that attacks retinal cells in the macula, the region of the eye responsible for central vision. The macula, which is at the center of retina, is particularly prone to age-related degenerative changes. The retinal pigment epithelium RPE, adjacent to Bruch’s membrane, has many important functions, including light absorption, phagocytosis of photoreceptor outer segments, heat exchange, vitamin A metabolism, retinal outer blood barrier, and maintenance of choriocapillaris.
AMD is a disease affecting four components of the visual organ: photoreceptors, retinal pigment epithelium (RPE), Bruch’s membrane, and choroidal capillaries. This process results in the destabilization of four key physiological functions: homeostasis/stress response, extracellular remodeling, complement-related inflammation, and phagocytosis. Disruption of these functions leads to distortion of Bruch’s membrane, the RPE, and the subretinal space. The pigmentary abnormalities in the RPE, drusen and Bruch’s membrane, and the molecular changes associated with this disease, are believed to be caused by inflammation, angiogenesis, and increased oxidation of cellular components, which play a central role in the pathogenesis and progression of AMD [2].
Although the etiology of AMD is still not well understood, the disease is divided into early and late stages. The early stage is characterized by the accumulation of lipofuscin and drusen deposits between Bruch’s membrane and the RPE, the specialized cells that form the outer blood–retinal barrier. Detection of vision deterioration in early stage of AMD is important for both prevention and treatment.
Poor antioxidant capacity, and the resulting overproduction of free radicals, is thought to be a major factor affecting RPE in the pathophysiology of AMD. This theory is underpinned by both environmental and genetic factors. The macula lives in an environment of high oxidative stress. Normal antioxidant capacity is influenced by the activity of enzymatic and non-enzymatic defense mechanisms, as well as the efficiency of DNA repair. DNA repair efficiency depends on the expression of genes encoding DNA repair proteins, which is mainly regulated by the interaction of regulatory proteins and specific sequences regulatory proteins and specific sequences in DNA repair genes. Changes in miRNA expression profile occur during organ development, aging, or cell death. The expression of miRNAs is also altered in the pathophysiology of complex diseases such as inflammation or neurodegenerative diseases including AMD. Furthermore, in addition to its role in regulating gene expression, miRNAs released into body fluids have emerged as potential blood serum biomarkers. Some researchers have reported that mRNA transcription factors are sensitive to redox status [3][4].
2. AMD—Enzymatic Antioxidant Capacity
Although the macula lives in an environment of high oxidative stress caused by the presence of many sources of reactive oxygen species (ROS), it contains many enzymatic and non-enzymatic antioxidants in the photoreceptor and RPE cells. Macular pigment is a natural barrier that protects the central part of the retina from oxidative damage. It is formed by two dihydrocarotenoids: lutein and zeaxanthin. In addition, the antioxidant defense includes various enzymes including superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx), and non-enzymatic antioxidants such as glutathione, albumin, bilirubin, uric acid, vitamin A, C, E, lipoic acid, carotenoids, plant polyphenols, zinc and selenium, among others.
Antioxidant enzymes reduce lipid hydroperoxide and hydrogen peroxide (H 2O 2) levels, thus playing an important role in preventing lipid peroxidation and maintaining cell membrane structure and function.
The copper-zinc superoxide dismutase (CuZnSOD) plays a key role in the removal of superoxide anion and protects cells from oxidative injury caused by free radicals. Located in the cytosol and mitochondria, SOD catalytically converts the superoxide anion (O 2˙ − ) to O 2 and H 2O 2 in the presence of metal ion cofactors such as copper (Cu), zinc (Zn) or manganese (Mn). It catalyzes the one-electron dismutation of the superoxide anion. The human SOD1 gene, located on chromosome 21q22.11, is highly polymorphic. Few studies have examined the relationships between SOD1 polymorphisms with AMD. A study conducted in a Polish AMD population [5] reported that a polymorphism in the SOD1 gene protected against AMD. Many works implicate SOD2 polymorphisms as potential determinants of AMD susceptibility. SOD2, a mitochondrial enzyme, has manganese (Mn) in its reactive center and active site, which functions as a metal cofactor. The enzyme scavenges toxic superoxide anions formed as a byproduct of oxidative phosphorylation, which enables the removal of mitochondrial ROS and provides protection against cell death. The SOD2 gene (6q25) encodes MnSOD and has several single nucleotide polymorphisms (SNPs), among which is rs4880 (C/T) (also designated as C47T, Ala16Val, Ala-9Val or A16V). The T allele of this SNP (rs4880-T) has been linked to changes in MnSOD activity due to modification of the mitochondrial target sequence, which may play a role in neurodegenerative processes including primary open angle glaucoma, AMD [6].
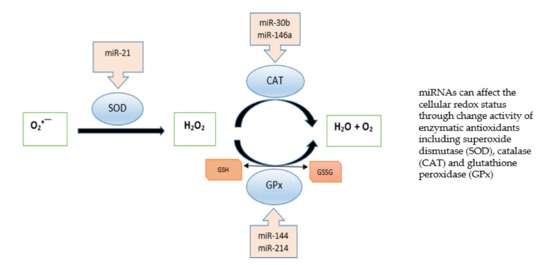
Moreover, recent studies indicate that miRNAs play an essential role in ROS generation by targeting genes associated with antioxidant response. Gene silencing by miRNAs can induce changes in antioxidant enzymes, leading to a complex interplay between redox imbalance by free radicals and miRNAs in modulating cellular redox homeostasis. The transcription, biogenesis and translocation of miRNAs, as well as their function, are connected with oxidative stress, and miRNAs can regulate the expression of redox markers such as cellular antioxidant status. Figure 1 shows miRNAs that affect enzymatic antioxidants. Expression analyses indicate that Cu/Zn SOD is downregulated by miRNAs in human bronchial cells [7], and the suppression of miR-146a was found to effectively restore catalase expression [8]. miR-30b downregulated CAT expression in human retinal pigment epithelial cell line [9]. Furthermore, dysregulation of miR-214 affected glutathione peroxidase activity: miR-214 overexpression increased GPx activity and reduced oxidative stress in an in vitro diabetic nephropathy model [10]. In contrast, miR-144 suppressed GPx expression in sickle cell disease [11].
3. AMD—miRNA Expression and Diagnostic Markers
Alterations in the expression profile of miRNAs have been successively demonstrated in many diseases, including AMD. Differences in miRNA gene expression can be accounted for by the localization of genes in disease-related regions, changes in miRNA processing mechanisms, and epigenetic mechanisms.
miRNAs play key roles in regulating pathological processes involved in AMD development, including angiogenesis, the imbalance of complement activation, inflammation and oxidative stress. In addition to vascular endothelial growth factor (VEGF), other growth factors disrupt angiogenesis and vascular balance and may also serve as significant markers in AMD: platelet-derived growth factor (PDGF), fibroblast growth factors (FGFs), placental growth factor (PlGF), hepatocyte growth factor (HGF), fibroblast growth factor-2 (FGF-2), pigment epithelium-derived growth factor (PEDF), and angiopoietins (ANGPTs).
Previous studies have shown a link between oxidative stress and inflammation. It has been indicated that oxidative stress induces inflammation during the pathological process of AMD [12]. Pathological oxidative damage contributes to protein, lipid and DNA damage and mitochondrial dysfunction, and generates toxic free radicals and high concentrations of harmful compounds such as AGEs and MDA; it also induces pro-inflammatory responses, and promotes the recruitment of macrophages, which release pro-inflammatory and pro-angiogenic mediators [13]. Many pro-inflammatory cytokines and chemokines, including IL-1, IL-6, IL-8, TNF, INF-γ, MCP-1, have been shown to accelerate AMD progression [14].
miR-21 has a very vital function in the regulation of angiogenesis, tumor growth and metastasis. It demonstrates very high expression in endothelial cells and is believed to be involved in endothelial cell differentiation, migration and angiogenesis [15]. Samples from patients with wet AMD showed downregulation of miR-21 and involvement in regulation of blood vessel growth, as indicated by its elevated expression in retinal endothelial cells [16][17].
4. Conclusions
References
- Xiayan, X.; Jing, W.; Xiaoning, Y.; Yelei, T.; Xiajing, T.; Xingchao, S. Regional differences in the global burden of age-related macular degeneration. BMC Public Health 2020, 20, 410–418.
- Shaw, P.X.; Stiles, T.; Douglas, C.; Ho, D.; Fan, W.; Du, H.; Xiao, X. Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol. Sci. 2016, 3, 196–221.
- Johnston, H.; Dickinson, P.; Ivens, A.; Buck, A.H.; Levine, R.D.; Remacle, F.; Campbell, C.J. Intracellular redox potential is correlated with miRNA expression in MCF7 cells under hypoxic conditions. Proc. Natl. Acad. Sci. USA 2019, 116, 19753–19759.
- Konovalova, J.; Gerasymchuk, D.; Parkkinen, I.; Chmielarz, P.; Domanskyi, A. Interplay between MicroRNAs and oxidative stress in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 6065.
- Mrowicka, M.; Mrowicki, J.; Szaflik, J.P.; Szaflik, M.; Ulinska, M.; Szaflik, J.; Majsterek, I. Analysis of antioxidative factors related to AMD risk development in the polish patients. Acta Ophthalmol. 2017, 95, 530–536.
- Yeye, C.; Hezheng, Z. Role of two common SNPs of superoxide dismutase 2 gene in the development of primary open angle glaucoma. Biomed. Res. 2017, 28, 7503–7508.
- Zhang, X.; Ng, W.L.; Wang, P.; Tian, L.L.; Werner, E.; Wang, H.; Doetsch, P.; Wang, Y. MicroRNA-21 modulates the levels of reactive oxygen species by targeting SOD3 and TNFα. Canc. Res. 2012, 72, 4707–4713.
- Wang, Q.; Chen, W.; Bai, L.; Chen, W.; Padilla, M.T.; Lin, A.S.; Shi, S.; Wang, X.; Lin, Y. Receptor-interacting protein 1 increases chemoresistance by maintaining inhibitor of apoptosis protein levels and reducing reactive oxygen species through a microRNA-146a-mediated catalase pathway. J. Biol. Chem. 2014, 289, 5654–5663.
- Haque, R.; Chun, E.; Howell, J.C.; Sengupta, T.; Chen, D.; Kim, H. MicroRNA-30b-Mediated Regulation of Catalase Expression in Human ARPE-19 Cells. PLoS ONE 2012, 7, e42542.
- Yang, S.; Fei, X.; Lu, Y.; Xu, B.; Ma, Y.; Wan, H. miRNA-214 suppresses oxidative stress in diabetic nephropathy via the ROS/Akt/mTOR signaling pathway and uncoupling protein 2. Exp. Ther. Med. 2019, 7, 3530–3538.
- Sangokoya, C.; Telen, M.; Chi, J. microRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood 2010, 116, 4338–4348.
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The Impact of Oxidative Stress and Inflammation on RPE Degeneration in Non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218.
- Wei, T.; Jingling, Z.; Shigeo, Y.; Bing, J.; Yedi, Z. The Role of Inflammation in Age-Related Macular Degeneration. Int. J. Biol. Sci. 2020, 16, 2989–3001.
- Knickelbein, J.E.; Chan, C.C.; Sen, H.N.; Ferris, F.L.; Nussenblatt, R.B. Inflammatory mechanisms of age-related macular degeneration. Int. Ophthalmol. Clin. 2015, 55, 63–78.
- Krzywińska, O.; Bracha, M.; Jeanniere, C.; Recchia, E.; Kędziora Kornatowska, K.; Kozakiewicz, M. Meta-Analysis of the Potential Role of miRNA-21 in Cardiovascular System Function Monitoring. BioMed Res. Int. 2020, 2020, 4525410.
- Ulańczyk, Z.; Sobuś, A.; Łuczkowska, K.; Grabowicz, A.; Mozolewska-Piotrowska, K.; Safranow, K.; Kawa, M.P.; Pałucha, A.; Krawczyk, M.; Sikora, P.; et al. Associations of microRNAs, angiogenesis-regulating factors and CFH Y402H polymorphism–an attempt to search for systemic biomarkers in age-related macular degeneration. Int. J. Mol. Sci. 2019, 20, 5750.
- Ertekin, S.; Yildirim, O.; Dinc, E.; Ayaz, L.; Fidanci, S.B.; Tamer, L. Evaluation of circulating miRNAs in wet age-related macular degeneration. Mol. Vis. 2014, 20, 1057–1066.
- Litwińska, Z.; Sobuś, A.; Łuczkowska, K.; Grabowicz, A.; Mozolewska-Piotrowska, K.; Safranow, K.; Kawa, M.P.; Machaliński, B.; Machalińska, A. The interplay between systemic inflammatory factors and microRNAs in age-related macular degeneration. Front. Aging Neurosci. 2019, 11, 286–298.
- Ménard, C.; Rezende, F.A.; Miloudi, K.; Wilson, A.; Tétreault, N.; Hardy, P.; SanGiovanni, J.P.; de Guire, V.; Sapieha, P. MicroRNA signatures in vitreous humour and plasma of patients with exudative AMD. Oncotarget 2016, 7, 19171–19184.
- Wang, Q.; Bozack, S.N.; Yan, Y.; Boulton, M.E.; Grant, M.B.; Busik, J.V. Regulation of retinal inflammation by rhythmic expression of MiR-146a in diabetic retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3986–3994.
- Harris, T.A.; Yamakuchi, M.; Ferlito, M.; Mendell, J.T.; Lowenstein, C.J. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. USA 2008, 105, 1516–1521.
- Pogue, A.I.; Lukiw, W.J. Up-regulated pro-inflammatory microRNAs (miRNAs) in Alzheimer’s disease and age-related macular degeneration (AMD). Cell. Mol. Neurobiol. 2018, 38, 1021–1031.
- Liu, C.-H.; Sun, Y.; Li, J.; Gong, Y.; Tian, K.T.; Evans, L.P.; Morss, P.C.; Fredrick, T.W.; Saba, N.J.; Chen, J. Endothelial microRNA-150 is an intrinsic suppressor of pathologic ocular neovascularization. Proc. Natl. Acad. Sci. USA 2015, 112, 12163–12168.
- Lin, J.B.; Moolani, H.V.; Sene, A.; Sidhu, R.; Kell, K.; Lin, J.B.; Dong, Z.; Ban, N.; Ory, D.S.; Apte, R.S. Macrophage microRNA-150 promotes pathological angiogenesis as seen in age-related macular degeneration. JCI Insight 2018, 3, e120157.
- Yan, L.; Lee, S.; Lazzaro, D.R.; Aranda, J.; Grant, M.B.; Chaqour, B. Single and Compound Knock-outs of MicroRNA (miRNA)-155 and Its Angiogenic Gene Target CCN1 in Mice Alter Vascular and Neovascular Growth in the Retina via Resident Microglia. J. Biol. Chem. 2015, 290, 23264–23281.
- Lukiw, W.J.; Surjyadipta, B.; Dua, P.; Alexandrov, P.N. Original Article Common micro RNAs (miRNAs) target complement factor H (CFH) regulation in Alzheimer’s disease (AD) and in age-related macular degeneration (AMD). Int. J. Biochem. Mol. Biol. 2012, 3, 105–116.
- Pan, C.; Yan, X.; Li, H.; Huang, L.; Yin, M.; Yang, Y.; Gao, R.; Hong, L.; Ma, Y.; Shi, C.; et al. Systematic literature review and clinical validation of circulating microRNAs as diagnostic biomarkers for colorectal cancer. Oncotarget 2017, 8, 68317–68328.
- Berber, P.; Grassmann, F.; Kiel, C.; Weber, B.H. An Eye on Age-Related Macular Degeneration: The Role of MicroRNAs in Disease Pathology. Mol. Diagn. Ther. 2017, 21, 31–43.




