
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Annabel Fernandes | + 1641 word(s) | 1641 | 2021-08-18 10:01:07 |
Video Upload Options
Complex wastewater matrices present a major environmental concern. Besides the biodegradable organics, they may contain a great variety of toxic chemicals, heavy metals, and other xenobiotics. The electrochemically activated persulfate process, an efficient way to generate sulfate radicals, has been widely applied to the degradation of such complex effluents with very good results.
1. Introduction
Increasing industrialization and urbanization results in the generation of large volumes of wastewaters, which represent a major environmental concern, due to their possible release in the environment without the appropriate treatment. Depending on its origin, municipal, livestock, refinery, industrial or food-processing activities, among others, wastewaters can present, in its composition, biodegradable organics, a great variety of toxic chemicals, heavy metals, and other xenobiotics [1]. Most of these compounds are persistent and adversely affect human health and aquatic biota, imparting genotoxicity, endocrine disruption, and bioaccumulation, which makes it imperative to treat the wastewaters before their discharge into water courses [1].
Conventional wastewater treatments often lack any capability to treat complex wastewaters that contain a mixture of refractory and non-biodegradable compounds. Thus, in the past years, several studies have been focused on the development of new technologies that enable the efficient treatment of these complex wastewater matrices to comply with the discharge regulations. Among the studied technologies, advanced oxidation processes (AOPs) have gained increasing attention, due to their good performance in the removal of recalcitrant pollutants. Traditionally, AOPs are based on the in situ generation of the non-selective strong oxidant hydroxyl radical (HO • ), with a redox potential of 1.8–2.7 V, which unselectively promotes the partial or complete mineralization of a wide range of organic compounds [2]. However, more recently, sulfate radical-based AOPs have emerged, as a sub-category of the AOPs, where the generation of the sulfate radical (SO 4•− ) is promoted, alone or combined with hydroxyl radicals [3][4].
Among the different SO 4− generation processes, the electrochemical ones, usually designated as electro-persulfate processes, were extensively investigated and considered the most efficient for the formation of sulfate radicals [5]. There are several studies reporting the application of electro-persulfate processes in the treatment of wastewaters with recalcitrant properties, with very promising results, making this technology a possible solution for the remediation of complex wastewater matrices.
2. Fundamentals of the Electro-Persulfate Processes
A scheme of the main reactions involved in the persulfate electrochemical production and activation is presented in Figure 1 .
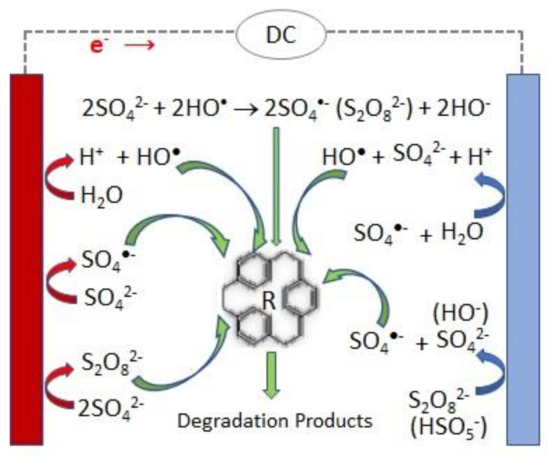
Attending that persulfate can be electrochemically generated from sulfate ions (Equation (3)), it has been suggested that PS can be regenerated at the anode after completion of the sulfate radical reactions with water, organics, etc., thus enabling a perpetual source of sulfate radicals [6][7].
The combination of electrochemical activation with other PS activation methods has been widely studied with the aim of enhance the PS activation efficiency. Besides electrochemical activation, PS can be activated by transition metals, non-metal catalysts, UV or visible radiation, ultrasound (US), microwave, alkaline medium, and heat. Each one of these methods has advantages and shortcomings for the PS activation [4]. So, the combination of different activation methods can make full use of their advantages and overcome the drawbacks.
The PS activation through US irradiation (Equations (21) and (22)), often referred to as a type of heat activation, is a result of extreme temperature and pressure rises, due to cavitation [8][9]. One of the main advantages of coupling ultrasound with electrochemical PS activation, besides the additional persulfate activation, is that ultrasonic irradiation accelerates the mass transfer rate of persulfate anions in solution towards the cathode, by acoustic streams [9].
Though less applied in hybrid persulfate electro-activation processes, traditional heat is one of the simplest methods for PS activation [10]. The energy input by the high temperature (>50 °C) causes the cleavage of the persulfate peroxide bond and sulfate radicals are formed according to Equations (21) and (22) [4]. Although heat is effective in PS activation, the energy demand is too high, which makes this method unfeasible for remediation processes on a large scale [4]. The introduction of an electrochemically assisted persulfate activation enhances the sulfate radical production and reduces the energy consumption of the heat-activated process [10].
3. Treatment of Complex Wastewater Matrices by Electro-Persulfate Processes
In the work developed by Bashir and co-workers [11], when treating a palm oil mill wastewater, COD removal increased when pH decreased from 5 to 3. According to the authors, at lower pH, the oxygen evolution overpotential increases, decreasing the competition between the organic matter oxidation and oxygen evolution reaction.
Iron concentration is another key factor in the combined electrolytic and metal activation of persulfate. According to Zhang et al. [12], the increase in iron ion dosage enhances the available Fe 2+ concentration, resulting in an increased PDS activation. In the study performed by these authors, during the treatment of a SLL, COD removal by oxidation increased from 33.5% to 40.1%, when Fe 2+ concentration rose from 7.81 to 15.6 mM. However, when Fe 2+ concentration was further increased to 31.2 mM, the COD removal by oxidation decreased to 23.1%, which was explained by the scavenging reaction between Fe 2+ and SO 4•− , promoted by the excess amount of Fe 2+ in solution (Equation (34)). Despite this, the authors observed that the increase in iron dosage increases the production of ferric-oxyhydroxides during the neutralization stage, which favored the COD removal by coagulation. Similar results and conclusions were attained in the study performed by Cui et al. [13], where an increase in Fe 3+ dosage, from 3.75 to 15 mM, resulted in an increase in COD removal from 21% to 55%, with a variation in COD removal by oxidation from 18% to 28% and a variation in COD removal by coagulation from 3% to 27%.
Electro-persulfate processes involving co-activation by ultrasound radiation were applied in the treatment of petrochemical [9][14] and textile [15][16] wastewaters. Ahmadi et al. [14] assessed the influence of different variables, such as pH, applied voltage, and US power, in a US-assisted electro-Fenton process for the treatment of saline petrochemical wastewater. At the optimized operational conditions, the effect of PDS concentration on the COD removal was studied. The authors have reported that an increase in PDS concentration, from 0 to 0.75 mM, resulted in an increase in COD removal from 80.2% to 91.7%. However, a further increase in PDS concentration to 1 mM resulted in a slight decrease in COD removal. In this combined process, both the continuous production of iron ions in the anode, and the application of US radiation, are responsible for the PDS activation and sulfate radical formation (Equations (14) and (22), respectively). Therefore, the initial increase in PDS concentration leads to the formation of more sulfate radicals to partake in wastewater treatment and COD removal. However, with excess PDS ions, an adverse effect starts to occur, as they act as sulfate radical scavengers, hindering the process and decreasing its efficiency (Equation (26)).
Xue and collaborators [10] developed a heat-assisted electro/PDS/Fe 2+ process for the treatment of a landfill leachate nanofiltration concentrate. The process was carried in a H-type reactor, divided into two chambers by a proton exchange membrane. A two-stage procedure was employed, consisting in 15 min of anodic reaction, followed by 105 min of cathodic reaction. At the optimized experimental conditions, the combined heat/electro/PDS/Fe 2+ process resulted in a COD removal of 87%, which was considerably higher than the COD removal attained by the heat/PDS process (43%). The influence of PDS concentration (37.5–150 mM), applied current (40–160 mA), and Fe 3+ dosage (3.75–15 mM) were assessed. For PDS and Fe 3+ concentrations of 75 and 15 mM, respectively, at an applied current of 80 mA, the raise of the temperature from 60 °C to 80 °C resulted in an increased COD removal from ~50% to 87%. However, no significant increase in COD removal was observed when the temperature was further raised to 90 °C.
4. Major Challenges and Future Prospects
Current density/potential is another parameter of key importance in the electrolytic activation of persulfate, since it directly affects persulfate activation and regeneration, sulfate and hydroxyl radicals formation and current efficiency, thus being highly reflected in the treatment cost. So, a proper steadiness must be attained prior to the process scale-up.
Still, mass transfer limitations have been reported in processes with single electrolytic persulfate activation, since the reactions mainly occur at the electrodes surface, increasing treatment time and energy consumption. To overcome this drawback, PS electrochemical activation has been combined with in bulk PS activation methods. Although the PS activation efficiency is usually enhanced by the combination of electrochemical activation with other PS activation methods, there are some aspects that need to be taking into account when considering a hybrid PS electro-activation process. Another possibility to solve the problem raised by the limitation in mass transfer is to improve reactor configuration and, recently, reactive electrochemical membranes are being utilized as a flow-through electrode. These electrochemical membranes significantly increase the active surface area, while enhancing pollutants mass transport by convection [17][18][19].
The addition of persulfate leads to a significant sulfate concentration, which can be a problem in the treated effluent. Although electro-persulfate processes may enable persulfate regeneration, thus reducing the amount required for the effective treatment of the effluents, final sulfate content in the treated effluents must be properly addressed prior to make it a proper industrial offer.
Finally, the problem associated with all electrochemical processes is the energy consumption. Besides the integration of electrochemical persulfate activation with other activation methods, which decreases the treatment costs, the integration with different treatment technologies and the use of renewable energy sources, to power the electrochemical system, are possible solutions to overcome this drawback. In fact, with the many green energy options available nowadays, energy consumption must become a minor drawback in the near future.
References
- Shrivastava, P.; Naoghare, P.K.; Gandhi, D.; Devi, S.S.; Krishnamurthi, K.; Bafana, A.; Kashyap, S.M.; Chakrabarti, T. Application of cell-based assays for toxicity characterization of complex wastewater matrices: Possible applications in wastewater recycle and reuse. Ecotoxicol. Environ. Saf. 2017, 142, 555–566.
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B 2015, 166–167, 603–643.
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083.
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517.
- Zhi, D.; Lin, Y.; Jiang, L.; Zhou, Y.; Huang, A.; Yang, J.; Luo, L. Remediation of persistent organic pollutants in aqueous systems by electrochemical activation of persulfates: A review. J. Environ. Manag. 2020, 260, 110125.
- Matzek, L.W.; Tipton, M.J.; Farmer, A.T.; Steen, A.D.; Carter, K.E. Understanding electrochemically activated persulfate and its application to ciprofloxacin abatement. Environ. Sci. Technol. 2018, 52, 5875–5883.
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188.
- Darsinou, B.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Mantzavinos, D. Sono-activated persulfate oxidation of bisphenol A: Kinetics, pathways and the controversial role of temperature. Chem. Eng. J. 2015, 280, 623–633.
- Yousefi, N.; Pourfadakari, S.; Esmaeili, S.; Babaei, A.A. Mineralization of high saline petrochemical wastewater using Sonoelectro-activated persulfate: Degradation mechanisms and reaction kinetics. Microchem. J. 2019, 147, 1075–1082.
- Xue, W.; Cui, Y.; Liu, Z.; Yang, S.; Li, J.; Guo, X. Treatment of landfill leachate nanofiltration concentrate after ultrafiltration by electrochemically assisted heat activation of peroxydisulfate. Sep. Purif. Technol. 2020, 231, 115928.
- Bashir, M.; Wei, C.; Aun, N.; Amr, S. Electro persulphate oxidation for polishing of biologically treated palm oil mill effluent (POME). J. Environ. Manag. 2017, 193, 458–469.
- Zhang, H.; Wang, Z.; Liu, C.; Guo, Y.; Shan, N.; Meng, C.; Sun, L. Removal of COD from landfill leachate by an electro/Fe2+/peroxydisulfate process. Chem. Eng. J. 2014, 250, 76–82.
- Cui, Y.H.; Xue, W.J.; Yang, S.Q.; Tu, J.L.; Guo, X.L.; Liu, Z.Q. Electrochemical/peroxydisulfate/Fe3+ treatment of landfill leachate nanofiltration concentrate after ultrafiltration. Chem. Eng. J. 2018, 353, 208–217.
- Ahmadi, M.; Haghighifard, N.J.; Soltani, R.D.C.; Tobeishi, M.; Jorfi, S. Treatment of a saline petrochemical wastewater containing recalcitrant organics using electro-Fenton process: Persulfate and ultrasonic intensification. Desalin. Water Treat. 2019, 169, 241–250.
- Johin, J.; Nidheesh, P.V.; Sivasankar, T. Sono-electro-chemical treatment of Reactive Black 5 dye and real textile effluent using MnSO4/Na2S2O8 electrolytes. Arab. J. Sci. Eng. 2019, 44, 9987–9996.
- Jorfi, S.; Ghaedrahmat, Z. Evaluating the efficiency of advanced oxidation processes for textile wastewater treatment: Electro-kinetic, sonochemical and persulfate. Environ. Prog. Sustain. Energy 2020, 40, 1–7.
- Trellu, C.; Chaplin, B.P.; Coetsier, C.; Esmilaire, R.; Cerneaux, S.; Causserand, C.; Cretin, M. Electro-oxidation of organic pollutants by reactive electrochemical membranes. Chemosphere 2018, 208, 159–175.
- Chaplin, B.P. The prospect of electrochemical technologies advancing worldwide water treatment. Acc. Chem. Res. 2019, 52, 596–604.
- Chuah, C.Y.; Lee, J.; Bae, T.-H. Graphene-based membranes for H2 separation: Recent progress and future perspective. Membranes 2020, 10, 336.





